Abstract
Purpose
The poor tissue penetration of visible light has been a major barrier for optical imaging, photoactivatable conversions, and photodynamic therapy for in vivo targets with depths beyond 10 mm. In this report, as a proof-of-concept, we demonstrated that a positron emission tomography (PET) radiotracer, 2-deoxy-2-[18F]fluoro-d-glucose (18FDG), could be used as an alternative light source for photoactivation.
Procedures
We utilized 18FDG, which is a metabolic activity-based PET probe, as a source of light to photoactivate caged luciferin in a breast cancer animal model expressing luciferase.
Results
Bioluminescence produced from luciferin allowed for the real-time monitoring of Cherenkov radiation-promoted uncaging of the substrate.
Conclusion
The proposed method may provide a very important option for in vivo photoactivation, in particular for activation of photosensitizers for photodynamic therapy and eventually for combining radioisotope therapy and photodynamic therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photoactivatable conversions such as photosynthesis in plants, photoswitchable chemical reactions, photoactivatable probes for imaging, and photosensitization in photodynamic therapy exist throughout nature and are exploited in many laboratory techniques. However, the poor tissue penetrating ability of light undoubtedly limits its broad application in vivo [1–3]. To address light penetration issues, long wavelength light sources are often used, but this can come as a tradeoff with excitation losses due to poor overlap with absorption spectra. Ideally, incident light for these applications would exhibit both high-depth penetration and a high spectral overlap with photoactive molecule’s absorbance spectrum. An emerging way to address depth is to “move” the light source from outside of the tissue to the depth where it is co-localized with photoactive agents. Importantly, placement of an excitation “light source” in vivo can be accomplished noninvasively with Cherenkov radiation.
Recently, there has been growing interest in the use of photons from Cherenkov radiation for optical imaging [4–12] and for excitation of quantum dots and fluorophores in vivo [6, 8]. Charged particles such as β+ and β− which are generated from radioactive isotope decay can result in visible light with a broad energy range (ca. 6.1 to 1.23 eV, 200–1,000 nm). As a charged particle travels, it can polarize the molecules of its medium into a high-energy (excited) state. When the polarized molecules relax back to the ground state, they emit light in the form of radiation luminescence. The spectrum of radiation luminescence consists of continuous wavelengths throughout the ultraviolet and visible spectrum, with the intensity distribution inversely proportional to the square of the wavelength [4, 5]. The use of this light for in vivo optical imaging has, to date, been limited to the lower energy and intensity portion of the continuous spectrum. To the best of our knowledge, harnessing the higher intensity portion of the spectrum (200–400 nm) has not yet been demonstrated.
Photoactivatable conversions, such as photosynthesis in plants, photoswitchable chemical reactions, photoactivatable probes for imaging, and photosensitization in photodynamic therapy are widely observed in nature and have been applied in many chemical biology applications [13]. We hypothesized that a radioactive isotope that produces charged particles and thus Cherenkov radiation could be used as an “internal light source” to photoactivate caged compounds in vivo. The radiation luminescence in the higher energy and intensity portion of the spectrum could be considered as an alternative for external ultraviolet light, which is necessary for photoactivation (uncaging; Fig. 1a). Recognizing that many medicinal applications of caged drugs have been impeded by the poor penetrating ability of the light source [13–16], we feel that this method could provide a critical step forward for in vivo diagnostics and therapy. Since radioactive isotopes can be tuned and localized to tissues within the body, the proposed photoactivation reaction should have no depth limitation. Obviously, the distribution of an administered radioisotope within tissue will be an issue, but one that is likely controllable through chemical modifications. We anticipate that the use of Cherenkov radiation could have potential clinical value for therapies and diagnostics associated with many forms of photoactivatable conversions.
A new method for uncaging photoactivateable compounds. a The illustration of the activation processes of caged active molecules. UV light can be used for uncaging but exhibits limited tissue penetration for in vivo applications. Charged particles from radioactive decay such as β+ or β− produce radiation luminescence capable of affecting the transformation. In principal, this approach has limitless tissue-penetrating capability. b The release of luciferin by uncaging reaction from DMNP-luciferin with UV 365 nm irradiation or with radiation luminescence generated from 18FDG.
In this proof-of-concept study, we utilized 2-deoxy-2-[18F]fluoro-d-glucose (18FDG), which is a metabolic activity-based positron emission tomography (PET) probe [17–19], as a source of light to photoactivate caged luciferin in a breast cancer animal model expressing luciferase. Bioluminescence produced from luciferin allowed for the real-time monitoring of Cherenkov radiation-promoted uncaging (Fig. 1a).
In this report, we used luciferin 1-(4,5-dimethoxy-2-nitrophenyl) ethyl ester (DMNP-luciferin) to demonstrate the in vitro and in vitro feasibilities of photoactivation with 18FDG. The 1-(2-nitrophenyl)ethyl functional group has been used widely as a caging group for various biological molecules [13–15]. To uncage/photoactivate 1-(2-nitrophenyl)ethyl-bearing molecules, the covalent bond between the active molecule and (2-nitrophenyl)ethyl group has to be cleaved by light (Fig. 1b). Normally, irradiation with 365 nm (ultraviolet) light is used to photocleave the bond and thus release the active form of a compound [13–15]. In this report, we demonstrated that the ester bond could be cleaved by 18FDG.
Materials and Methods
18FDG-Promoted Uncaging in Solution
DMNP-luciferin (25.0 μg, 50.0 nmol, Invitrogen, Molecular Probes) was suspended in distilled water (1.0 mL) and treated with 18FDG (500 μCi, IBA Molecular) in water (0.1 mL) in the dark. The resulting suspension was incubated in the dark at room temperature for 12 h. LC–MS analysis of the sample was accomplished using a C18 reversed-phase column on a HP 1100 LC/MSD LC-MS spectrometer.
For light-promoted uncaging, a distilled water solution of DMNP-luciferin (25.0 μg, 50.0 nmol) was irradiated with UV 365 nm light (UVGL-58 lamp, 6 W). The solution in a 1.5-mL Eppendorf tube was placed directly under the lamp and irradiated for 5 min. The delivered UV dose was ∼1.8 kJ/m2. After the UV treatment, LC–MS was recorded.
Cell Imaging
Luciferase-expressing breast adenocarcinoma cells (MDA-MB-231-luc-D3H1, Caliper) were seeded in a 96-well black clear-bottom plate (2,000 cells/well). Cells were incubated with a 100-μl solution from each of the three treatment groups (n = 4 wells/group) as follows: (1) a solution (1.0 mL) of 18FDG (500 μCi) and DMNP-luciferin (25.0 μg; for this cell study, before adding to cell media, the solution was kept for 24 h to deconvolute the signal from the radiation luminescence of 18FDG itself); (2) a solution (1.0 mL) of UV 365 nm irradiated DMNP-luciferin (25.0 μg); (3) a solution (1.0 mL) of DMNP-luciferin (25.0 μg) alone. Wells with the above-listed solutions without cells served as controls. The plate was incubated at 37°C for 5 min and then imaged using IVIS Spectrum (Caliper, Hopkinton MA). The imaging parameters were the following: block excitation; open emission filter; Bin = 8, FOV = C, f = 1, and exposure time = 120 s. The quantification was based on photon radiance (photon per second per square centimeter per steradian).
Animal Imaging
Animal Preparation
Nude mice (nu/nu, Massachusetts General Hospital Radiation Oncology breeding facilities) were injected with 2 × 106 breast adenocarcinoma cells (MDA-MB-231-luc-D3H1, Caliper) in the mammary fat pad. Tumors were allowed to grow for 3 weeks.
Imaging Procedure
An IVIS Spectrum imaging system was used to record the images (exposure time, 120 s; bin = 8, f = 1, FOV = D). Tumor-bearing mice were divided into five groups. In the first group (f = 5), each animal received an i.p. injection of luciferin (0.5 mg/mouse, Caliper, Hopkinton MA). Imaging was performed before injection and at 2, 10, 20, 30, 40, and 60 min post-injection. In the second group (f = 6), each animal received an i.p. injection of DMNP-luciferin (0.5 mg/mouse). Images were acquired before injection and at 10, 30, 60, 90, and 120 min post-injection. The third group of mice (f = 3) was injected i.p. with DMNP-luciferin and the whole bodies were irradiated with 365 nm UV lamp (UVGL-58, 6 W) for 3 min (UV dose was ∼10.8 kJ/m2) before each image acquisition [20] of the five imaging time points. Images were acquired before injection and at 10, 30, 60, 90, and 120 min after injection. The fourth group of mice (f = 5) was injected with an aqueous solution of 18FDG (400 μCi, 100 μL, i.p.). Images were acquired before injection and 10, 20, 30, 40, 50, 70, 100, 130, and 160 min post-injection (in Fig. 4b, imaging was started at 40 min after 18FDG injection). The fifth group of mice (f = 6) was first injected with an aqueous solution of 18FDG (400 μCi, IBA Molecular, 100 μL, i.p.). Images were acquired before injection and at 10, 20, 30, 40 min post-injection. Next, the same animals were injected with DMNP-luciferin (0.5 mg/mouse, i.p.). Images were acquired at 50, 70, 100, 130, and 160 min post-18FDG injection (i.e., 10, 30, 60, 90, and 120 min post-DMNP-luciferin injection).
Image Analysis
A region of interest (ROI) was selected to encircle the tumor in each mouse. The analysis was performed using ROIs of the same size. The quantification was based on photon radiance (photon per second per square centimeter per steradian).
Results
Photoactivation of Caged Luciferin with 18FDG in Solution
In this report, we first tested whether DMNP-luciferin [21] could be converted to its active form, luciferin, by using 18FDG (as a source of Cherenkov radiation luminescence) instead of 365 nm UV light (Fig. 1b). Indeed, incubation of an aqueous 18FDG solution (400 μCi in 1.0 mL) with DMNP-luciferin in the dark promoted the ester bond cleavage as revealed by LC–MS analysis (Fig. 2a, b), with a yield of about 25%. Positive control study where 365 nm UV irradiation was used to promote the cleavage to release luciferin yielded similar results (SI Fig. 1), with a yield of about 20%.
LCMS characterization of luciferin and the products of 18FDG (radiation luminescence) promoted uncaging of DMNP-luciferin. a LCMS data from analysis of d-luciferin standard (Caliper). Chromatogram has two major peaks, corresponding to a luciferin water addition product (retention time, 3.7 min) and luciferin (retention time, 5.0 min). The parent ion observed as major species. b Chromatogram of the products of 18FDG-promoted uncaging reaction of DMNP-luciferin. Peak 1 corresponds to luciferin hydrate; peak 2—the released luciferin; peak 3—DMNP-luciferin.
Photoactivation of Caged Luciferin with 18FDG in Cells
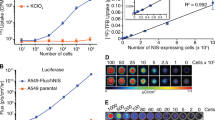
To verify that the product of DMNP-luciferin uncaged by radiation luminescence could serve as an active substrate for luciferase, we used luciferase-expressing breast adenocarcinoma cells (MDA-MB-231-luc-D3H1). First, we confirmed that DMNP-luciferin alone and the products of DMNP-luciferin uncaging by UV 365 nm light and radiation luminescence (via 18FDG) had no significant signal without the cells (Fig. 3a, three lower rows). To image the bioluminescence of the uncaged luciferin with cells, we treated DMNP-luciferin with 0.5 mCi 18FDG for 24 h to ensure the complete decay of 18F before adding to the cell culture media. This was necessary to avoid the direct luminescence signal from 18FDG itself (the half-life of FDG is 109.8 min). Then, the solution was applied to the cells (Fig. 3a, top row). Cells treated with DMNP-luciferin irradiated with UV 365 nm light (Fig. 3a, second row from the top) and cells treated with DMNP-luciferin alone (Fig. 3a, third row from the top) were used as the positive and negative controls, respectively. We found that the signal from the cells treated with the product uncaged by 18FDG incubation was significantly higher than that from DMNP-luciferin-treated cells (Fig. 3b) and was similar to the treatment with the product of UV 365 nm irradiation, suggesting that radiation luminescence-promoted uncaging released an active form of luciferin from caged DMNP-luciferin. Signal from the control wells (Fig. 3a, three lower rows) was negligible (<10% of the positive control).
Bioluminescence imaging of MDA-MB-231-luc-D3H1 cells (Caliper). a Rows 1–3 cells were treated with the products of 18FDG-promoted (radiation luminescence) uncaging of DMNP-luciferin, UV 365 nm irradiated DMNP-luciferin, and DMNP-luciferin (25.0 μg) alone. Rows 4–6 blank controls of the three solutions described above without cells. b Quantitative analysis of the image in (a). Radiation-luminescence-promoted uncaging and UV-light-promoted uncaging showed significantly higher bioluminescence signal than that of DMNP-luciferin alone.
Photoactivation of Caged Luciferin with 18FDG Radiation Luminescence In Vivo
In this study, we hypothesized that 18FDG could be used in vivo for uncaging/photoactivation of a substrate in a model system. To test this hypothesis, we used mice bearing luciferase-expressing breast tumors. The experimental group of animals was first injected with 18FDG (0.4 mCi, i.p.) followed by DMNP-luciferin at 40 min after 18FDG administration (Fig. 4a). This time point was selected to allow for 18FDG accumulation, which normally reaches peak levels in tumors at approximately 1 h post-injection [17]. For this group, the total signal in tumor site was the sum of radiation luminescence of 18FDG and bioluminescence of luciferin released by uncaging (Fig. 4a). Four control groups were used to support the findings from this study. The first control group included animals injected with luciferin as a positive control, which showed the expected BLI signal (SI Fig. 2). The second control group was injected with only 18FDG to measure the signal from the direct radiation luminescence of 18FDG (Fig. 4b). The third control group was injected with DMNP-luciferin and then subjected to ex vivo irradiation using 365 nm UV light (Fig. 4c). The final control group was treated with only DMNP-luciferin to test the inherent background signal (Fig. 4d).
Luminescence imaging of tumor-bearing mice subjected to different treatments regimens. The tumor in each group was marked with red circle in the first image. a Mice were first treated with 18FDG and then with DMNP-luciferin 40 min later. Images are from 0 min (pre-injection), 10, 30, 60, 90, and 120 min post-injection of DMNP-luciferin. b Mice were injected with 18FDG only. For comparison purposes, images shown here were obtained 40 min after 18FDG injection (for the whole-time 18FDG course, see SI Fig. 3). c Mice were treated with DMNP-luciferin and irradiated with UV 365 nm light for 3 min before each image acquisition. d Mice were i.p. injected with DMNP-luciferin only. e Quantitative analysis of the images in (a–d). The signal from the group treated with both 18FDG and DMNP-lucferin (red line) was significantly higher than that of DMNP-luciferin alone group (black line), UV irradiated group (blue line), or 18FDG only group (green line).
Our results indicate that the bioluminescence signal from the tumor of 18FDG-injected animals significantly increased after DMNP-luciferin administration (Fig. 4a). The signal from tumors in the 18FDG-only control group increased gradually until peaking around 40 min. This is consistent with PET imaging results reported by Fueger (SI Fig. 3, full-time course is shown) [17]. The signal in the experimental group (DMNP-luciferin + 18FDG) was 12.5-fold higher of that in the 18FDG-only group and reached its peak at 70 min post-DMNP-luciferin injection (Fig. 4e). Our data suggest that the radiation luminescence signal from 18FDG contributed to only about 8% of the total signal observed in the experimental group. The contribution of background to the signal as determined by the DMNP-luciferin-only control group (Fig. 4d) was approximately 5% of the highest signal observed in the experimental group (Fig. 4e). Taken together, our data suggested that 18FDG, which had accumulated in the tumor, had a remarkable capacity to uncage DMNP-luciferin and thus release the active substrate for luciferase.
As expected, we found that irradiation with 365 nm light could indeed activate caged luciferin (Fig. 4c), similar to previously reported findings [20]. However, the signal in this group was only slightly higher than that of DMNP-luciferin-only group (Fig. 4d) and significantly lower than the signal from the experimental group, indicative of the low penetrating ability of UV light for in vivo uncaging.
Discussion
Although radiation luminescence from charged particles has been used successfully for in vivo optical imaging [4, 5], to the best of our knowledge, its application beyond imaging has not yet been explored. In this proof-of-concept report, we demonstrated that the phenomenon of radiation luminescence could be used for photoactivatable chemical conversion. This suggests that a radioactive isotope could serve as an alternative “light source,” which could be used to overcome the tissue penetrating inadequacy of conventional light sources. We believe that our approach will open new directions for various light-promoted conversions, particularly for in vivo applications.
It has been shown recently that the 400–500-nm portion of the continuous spectrum of radiation luminescence could be used to excite quantum dots in vitro and in vivo [5–8]. However, the exact excitation mechanism of this phenomenon is still not clear. Cherenkov resonance energy transfer [6] to quantum dots and the excitation of quantum dots by radiation luminescence are likely the major contributors. In our experiments, the observed uncaging effect could probably be ascribed to: (1) Cherenkov (i.e., radiation luminescence), (2) direct interaction/polarization by positrons, (3) scintillation light from positron interaction with the media and container. However, it is not possible at this point to experimentally verify this assumption.
The application of the technology that we have described is certainly not limited to photo-uncaging of molecules in vivo with 18FDG. Previously, it was demonstrated that in addition to radionuclides that generate β+ particles (such as 18F and 64Cu), β− emitters such as 131I and 90Y could be used for optical imaging [5]. In this report, we demonstrated that radiation luminescence generated by 18FDG was capable of activating caged luciferin in vivo; thus, a natural extension of this work is the use of other radionuclides, which emit either β+ or β− particles, for the same reaction.
Similarly, this approach could be used for other photoconversions including photodynamic therapy, which we are currently exploring. We expect this could increase the efficacy of such treatments. Most photosensitizers of photodynamic therapy have to be activated using light in the near-infrared range [1–3]. However, porphyrin compounds, the drugs that are most commonly used in photodynamic therapy, exhibit stronger absorption and excitation in the range of 400–600 nm [22–24]. Radiation luminescence could be used to excite molecules for photodynamic therapy in this higher energy portion of the spectrum.
We anticipate a possible synergy between photodynamic therapy and administration of therapeutic radioisotopes given that excitation of photosensitizers could occur by local radiation luminescence. This has not been explored despite the fact that photodynamic and radioisotope therapies are widely used in combination for surface tumors. To date, the synchronized combination of the two has not been practiced widely. We feel that concurrent treatment with a therapeutic radioisotope with unlimited depth penetration and photodynamic therapy using a photosensitizer could be synergistic and could produce clinical benefits not only for surface tumors but also for deep tumors. By extension, proton therapy, which both uses and produces charged particles (such as positrons [25]), could likely be harnessed to excite a photosensitizer in photodynamic therapy, thus enhancing the overall therapeutic efficacy.
Conclusion
In summary, in this proof-of-principle study, we have demonstrated that Cherenkov radiation may have chemical and therapeutic applications beyond radiation luminescence optical imaging. We accomplished this using a straightforward DMNP-luciferin uncaging experiment, which showed that Cherenkov radiation from 18FDG could serve as an internal “light source” for photoactivation. We are currently exploring the application of this finding to new imaging and therapeutic strategies. However, challenges associated with optimization of radiation dose for maximum effect should be considered, especially when the radiation risk may limit the utility of this approach.
References
MacCormack MA (2006) Photodynamic therapy. Adv Dermatol 22:219–258
Celli JP, Spring BQ, Rizvi I et al (2010) Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev 110:2795–2838
Dolmans DE, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3:380–387
Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD (2009) Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol 54:N355–N365
Liu H, Ren G, Miao Z et al (2010) Molecular optical imaging with radioactive probes. PLoS One 5:e9470
Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D (2010) Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PLoS One 5:e13300
Li C, Mitchell GS, Cherry SR (2010) Cerenkov luminescence tomography for small-animal imaging. Opt Lett 35:1109–1111
Liu H, Zhang X, Xing B, Han P, Gambhir SS, Cheng Z (2010) Radiation-luminescence-excited quantum dots for in vivo multiplexed optical imaging. Small 6:1087–1091
Ruggiero A, Holland JP, Lewis JS, Grimm J (2010) Cerenkov luminescence imaging of medical isotopes. J Nucl Med 51:1123–1130
Spinelli AE, D’Ambrosio D, Calderan L, Marengo M, Sbarbati A, Boschi F (2010) Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers. Phys Med Biol 55:483–495
Hu Z, Liang J, Yang W et al (2010) Experimental Cerenkov luminescence tomography of the mouse model with SPECT imaging validation. Opt Express 18:24441–24450
Lewis MA, Kodibagkar VD, Oz OK, Mason RP (2010) On the potential for molecular imaging with Cerenkov luminescence. Opt Lett 35:3889–3891
Mayer G, Heckel A (2006) Biologically active molecules with a “light switch”. Angew Chem Int Ed Engl 45:4900–4921
Adams SR, Tsien RY (1993) Controlling cell chemistry with caged compounds. Annu Rev Physiol 55:755–784
Yu H, Li J, Wu D, Qiu Z, Zhang Y (2010) Chemistry and biological applications of photo-labile organic molecules. Chem Soc Rev 39:464–473
Lee HM, Larson DR, Lawrence DS (2009) Illuminating the chemistry of life: design, synthesis, and applications of “caged” and related photoresponsive compounds. ACS Chem Biol 4:409–427
Fueger BJ, Czernin J, Hildebrandt I et al (2006) Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 47:999–1006
Abouzied MM, Crawford ES, Nabi HA (2005) 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol 33:145–155
Hung JC (2002) Comparison of various requirements of the quality assurance procedures for (18)F-FDG injection. J Nucl Med 43:1495–1506
Shao Q, Jiang T, Ren G, Cheng Z, Xing B (2009) Photoactivable bioluminescent probes for imaging luciferase activity. Chem Commun (Camb) 27:4028–4030
Yang J, Thomason DB (1993) An easily synthesized, photolyzable luciferase substrate for in vivo luciferase activity measurement. Biotechniques 15:848–850
O’Connor AE, Gallagher WM, Byrne AT (2009) Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem Photobiol 85:1053–1074
Berg K, Selbo PK, Weyergang A et al (2005) Porphyrin-related photosensitizers for cancer imaging and therapeutic applications. J Microsc 218:133–147
Kaiser PK (2007) Verteporfin photodynamic therapy and anti-angiogenic drugs: potential for combination therapy in exudative age-related macular degeneration. Curr Med Res Opin 23:477–487
Pshenichnov I, Larionov A, Mishustin I, Greiner W (2007) PET monitoring of cancer therapy with 3He and 12 C beams: a study with the GEANT4 toolkit. Phys Med Biol 52:7295–7312
Conflict of Interest Statement
A.M. declares the payment for lectures outside the submitted work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
SI Fig. 1
The LC–MS spectra of a water solution of DMNP-luciferin irradiated with UV 365 nm light for 5 min. Insert mass spectra of the released luciferin (left) and the unreacted DMNP-luciferin (right). (PDF 27 kb)
SI Fig. 2
a Bioluminescence images of mice bearing luciferase-expressing tumors injected with luciferin. b Quantitative analysis of the images in (a). (PDF 602 kb)
SI Fig. 3
Full-time course optical signal from tumor sites of control mice injected with 18FDG only. (PDF 58 kb)
Rights and permissions
About this article
Cite this article
Ran, C., Zhang, Z., Hooker, J. et al. In Vivo Photoactivation Without “Light”: Use of Cherenkov Radiation to Overcome the Penetration Limit of Light. Mol Imaging Biol 14, 156–162 (2012). https://doi.org/10.1007/s11307-011-0489-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-011-0489-z