Abstract
Purpose
Our previous study using 123I-iodo-benzamide single photon emission computed tomography (SPECT) showed a positive relationship in healthy adults between striatal postsynaptic D2/D3 receptor availability and sleep duration in good sleepers. To further investigate the role of dopamine (DA) in the sleep–wake cycle, we explored the correlation between presynaptic dopamine transporter (DAT) availability and sleep quality in healthy volunteers.
Methods
A total of 83 healthy volunteers (33 males, 50 females; mean age, 34.62 years), including 39 good sleepers and 44 poor sleepers, were recruited. The sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). Striatal DAT availability was determined by 99mTc-TRODAT-1 SPECT, and the DAT availability in the good and poor sleepers was compared. Furthermore, the correlation between PSQI and DAT availability was analyzed.
Results
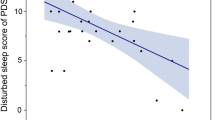
There was no significant difference in DAT availability between the good and poor sleepers. No significant relationship was found between the global score or individual-component PSQI scores and DAT availability in the good sleepers. However, the sleep duration component score in the poor sleepers negatively correlated with DAT availability in the caudate (ρ = −0.31, P = 0.049).
Conclusions
The study demonstrates that healthy poor sleepers, with a lower DAT availability in the caudate, sleep for a shorter length of time. This suggests that a decrease in DA reuptake due to reduced DAT availability causes a shorter sleep duration in poor sleepers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep and wakefulness are regulated by complex neurobiologic mechanisms and several neurotransmitters modulate the sleep–waking state. Growing evidence from animal studies and clinical patient data indicates an important role for dopamine (DA) in sleep regulation [1], specifically, its wake-promoting action. A recent rat study, for example, showed higher extracellular DA levels in the striatum during awake periods compared to sleep periods [2], and dopamine transporter (DAT)-knockout mice have been shown to be hyperactive and have sleep abnormalities [3]. Most human evidence comes from patients with a compromised DA system, such as sleepiness in patients suffering from Parkinson’s disease, which is characterized by loss of dopaminergic neurons [4]. The influence of DA seems to be sustained because DA neuron firing is not significantly altered during the sleep–wake cycle. In addition, as DA receives little interference with other neurotransmitters, it may play the seminal role in the sleep cycle [5].
Currently available evidence suggests that DA is necessary for the maintenance of alert waking [2], and increased DA neuronal discharge activity has been observed in animals during active waking [2]. Amphetamine-like stimulants, the most potent of wake-promoting agents, increase wakefulness by either blocking DA reuptake via DAT, by stimulating DA release, or both [6]. To date, however, only a limited number of studies have examined the role of DA in modulating the sleep–wake cycle in healthy humans. A better understanding of the role of DA in sleep–wake regulation in healthy subjects would be invaluable because sleep disorders are becoming increasingly prevalent and are a public health concern [6]. This knowledge may also contribute to the development of new treatments for insomnia.
Owing to the development of new radiopharmaceuticals, it is now possible to image the DA system in vivo. In our previous study, we observed a correlation between striatal postsynaptic dopamine D2/D3 receptor availability and sleep duration in healthy adults using 123I-iodo-benzamide single photon emission computed tomography (SPECT) [7]. To further investigate the role of DA in the sleep–wake cycle, we explored the correlation between presynaptic DAT availability and sleep quality in healthy volunteers.
Subjects and Methods
Ethics
The ethics committee for human research at the National Cheng Kung University Hospital approved the study protocol. Before any procedure was performed, informed consent was obtained from all volunteers.
Subjects
We recruited healthy adult volunteers from the local community by advertising in a local newspaper. Most of the volunteers had been enrolled in previous studies as healthy controls. The exclusion criteria in this study were: mental illness, pregnancy, any past or present medical or neurological disorder, alcohol or substance abuse, neuropsychological or medical conditions that can alter sleep, use of hypnotics during the previous 2 weeks, and a history of head trauma with loss of consciousness. The presence of mental illness was assessed by a senior psychiatrist using the Chinese version of the Mini International Neuropsychiatric Interview [8]. In addition, all subjects were administered the Chinese version of the Pittsburgh Sleep Quality Index (PSQI) to determine sleep quality [9].
In addition, all subjects underwent brain MRI and those subjects with abnormal brain MRI findings were excluded. Brain SPECT imaging was also performed using 99mTc-[2[[2-[[[3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3,2,1]-oct-2-yl]-methyl](2-mercaptoethyl)amino]ethyl]amino]ethanethiolato(3-)-N2,N2_,S2,S2]oxo-[1R-(exo-exo)] (99mTc-TRODAT-1) SPECT to assess DAT availability. None of the subjects were taking medication at the time of the study. The Chinese version of the PSQI has been documented as a reliable and valid instrument [10]. All participants completed the PSQI and 99mTc-TRODAT-1 SPECT assessment on two consecutive days.
99mTc-TRODAT-1 SPECT
Each subject was intravenously administered 740 MBq (20 mCi) of 99mTc-TRODAT-1 (Institute of Nuclear Energy Research, Lungtan, Taiwan) in a quiet environment at approximately 10 min after the intravenous line was set up. The brain SPECT images were acquired 240 min after the injection using a triple-head gamma camera with ultra-high-resolution fanbeam collimators (Multispect3; Siemens, Hoffman Estates, IL, USA), which yielded an image resolution of approximately 8.5 mm full-width at half maximum. The SPECT data were acquired over a circular 360° rotation in 120 steps, 50 s per step, in a 128 × 128 × 16 matrix. Images were reconstructed by filtered back projection using a Butterworth filter (cut-off frequency, 0.4 Nyquist; power factor, 7) with attenuation correction by the method of Chang [11]. The reconstructed transverse images were realigned parallel to the canthomeatal line. The slice thickness of each transverse image was 2.89 mm.
We used a set of standard region-of-interest (ROI) templates to define the caudate, putamen, striatum (i.e., caudate plus putamen), and the frontal and occipital regions for the measurement of striatal TRODAT-1 binding. The six consecutive transverse slices that best visualized the striatum were combined. An experienced nuclear medicine specialist manually positioned the template on the transverse TRODAT-1 images of each subject without changing the size or shape of the ROIs. The average counts from the frontal and occipital regions were used to determine the nonspecific activity. The specific striatal TRODAT-1 binding (which represents striatal DAT availability) was calculated as the average count in the striatal ROI (including the caudate, putamen, and striatum) minus the average count from the frontal and occipital regions divided by the average count in the frontal and occipital regions.
Chinese Version of the Pittsburgh Sleep Quality Index
The PSQI is a widely used, self-administered questionnaire for evaluating sleep quality during the previous month. The sum of seven component scores (including subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, use of sleep medications, and daytime dysfunction) yield a global PSQI score ranging from 0 to 21, with higher scores indicating poorer sleep quality. A global PSQI score higher than five has a diagnostic sensitivity of 90% and specificity of 87% in distinguishing good from poor sleepers [9]. We adopted the PSQI because of its high test–retest reliability [12], lack of first-night effect, high correlation with sleep log data [12], and validated values for determining sleep duration, sleep-onset latency, and sleep efficiency [13]. The internal consistency (Cronbach’s alpha) of the PSQI for this sample was 0.60 in our institution.
Statistical Analysis
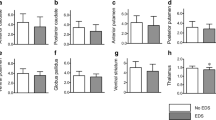
To analyze the differences between good and poor sleepers in terms of age, PSQI score, and striatal DAT availability (including the caudate, putamen, and striatum), the Student’s t test was employed. Chi-square analyses were used to compare the differences in the distribution of gender and educational level between the good and poor sleepers. According to the results of our previous study [14], body mass index (BMI), rather than age, should be considered as a covariate when measuring striatal DAG availability. Therefore, partial correlations between PSQI score and striatal DAT availability (including the caudate, putamen, and striatum) in the good and poor sleepers were analyzed after removing the effects of BMI and gender. Hochberg’s sharpened Bonferroni correction was applied by adjusting the overall type I error rate of 0.05 (two-tailed) for multiple comparisons [15]. All analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
A total of 83 subjects, 33 healthy males and 50 healthy females, were recruited. The mean age of the 83 participants was 34.62 (±SD = 12.94) years. According to the results of the PSQI, 39 good sleepers and 44 poor sleepers were included in this study.
Statistical analyses showed that the poor sleepers had significantly higher PSQI scores compared to the good sleepers. Except for differences in gender, there were no significant differences in age, DAT availability (including the caudate, putamen, and striatum), and educational level between the two groups (Table 1).
After partialling out BMI [14] and gender effects, no significant correlation was found between PSQI score (including global and individual-component scores) and DAT availability in the good sleeper group. However, in the poor sleeper group, there was a significant negative correlation between DAT availability in the caudate and sleep duration score (ρ = −0.31, P = 0.049; Table 2c). This indicates that healthy poor sleepers with a lower striatal DAT availability in the caudate sleep for a shorter length of time.
Discussion
DA modulates many vital physiological functions such as movement, motivation, cognition, reward, emotional behavior, and neuroendocrine regulation [16]. DA has not been traditionally considered a modulator of the sleep–wake cycle because midbrain DA neurons do not display robust alterations in the firing rate between sleeping and waking [17]. However, there exists compelling evidence of DA’s modulation of the arousal state. This evidence arises primarily from animal studies and from patient data. Our previous study investigated the relationship between striatal postsynaptic dopamine D2/D3 receptor availability and sleep quality in healthy adult humans. Our previous results showed that healthy good sleepers, with a lower D2/D3 receptor availability in the caudate, slept for a shorter length of time [7]. Because high doses of dopamine agonists cause arousal via the postsynaptic receptors, we interpreted that the shorter sleep duration in healthy good sleepers was the result of a higher synaptic level of dopamine. A later study by Volkow et al. in healthy volunteers demonstrated decreased D2/D3 receptor radioligand binding in the striatum after one night of sleep deprivation. They concluded that an increase of DA in the synaptic cleft was needed to maintain wakefulness after sleep deprivation [18]. Therefore, their findings were consistent with our previous work.
The current study also demonstrates a role of DA in modulating the sleep–waking state; i.e., healthy poor sleepers with a lower presynaptic DAT availability in the caudate sleep for a shorter length of time. DAT is the target for most wake-promoting medications [6]. Nishino et al., using canines and DA uptake inhibitors, showed that presynaptic activation of DA transmission was a key pharmacological property mediating the wake-promoting effects of currently available CNS stimulants. In addition, presynaptic modulation of DA is critical in the control of wakefulness [19]. Dopamine is cleared from the synaptic cleft via presynaptic DAT, and DAT plays a crucial role in the regulation of extracellular DA [20, 21]. A 300-times slower clearance of released DA was observed in DAT-knockout homozygous mice as compared with control mice, and elevated extracellular levels of DA were observed in the former [21]. These mice demonstrated a significant increase in wake time and a reduction in non-REM sleep [3]. Mice lacking the DAT gene are also easily aroused [22]. Even fruit flies with a genetic lesion in the DAT gene exhibited reduced sleep and a decreased arousal threshold [23]. Based on these animal studies, we considered that a lower DAT availability indicates a lower DA reuptake by DAT; therefore, these poor sleepers have shorter sleep duration. An interesting recent study, using TRODAT-1 SPECT to explore influence of sleep deprivation in healthy volunteers, showed a negative correlation between DAT availability and slow-wave sleep (SWS) latency in the recovery nights after total sleep deprivation. It was speculated that reduced SWS latency might be related to augmentation of SWS activity in the recovery nights [24]. All of the above findings indicate a role for DAT in maintaining wakefulness.
Both our previous postsynaptic D2/D3 receptor study and the current presynaptic DAT study reveal that the DA level in the caudate is related to sleep in healthy adults. Additional support for our findings, regarding the importance of the caudate in modulating sleep, can be found in the literature. In the 1950s, low-frequency stimulation studies demonstrated that the caudate nucleus regulated the waking–sleep cycle [25, 26]. In cats, removal of the caudate led to a month-long reduction in SWS [27]. An additional human imaging study demonstrated hypoperfusion of the caudate in narcoleptics with cataplexy [28].
Neither our previous work nor our current study showed a difference in either postsynaptic D2/D3 receptor availability or presynaptic DAT availability between good and poor sleepers. Furthermore, postsynaptic D2/D3 receptor availability correlates with sleep duration in good sleepers while presynaptic DAT availability correlates with sleep duration in poor sleepers. Because only D2/D3 receptor availability or only DAT availability was assessed, our results may have been caused by changes in D2/D3 receptor density, DAT density, or synaptic DA level, and further studies are needed to evaluate the level of DA at the synapse (for example, through the use of the occupancy model [29]). Nevertheless, our studies may indicate different roles of the D2/D3 receptor and DAT in regulating sleep duration in good and poor sleepers, respectively, and these results warrant further DA imaging studies to investigate sleep disorders.
Conclusion
This in vivo study reveals that healthy poor sleepers, with a lower striatal presynaptic DAT availability, sleep for a shorter length of time. In addition, we have demonstrated that DAT is involved in modulating the sleep–wake cycle and our results warrant further use of DA imaging studies to investigate sleep disorders.
References
Mignot E, Taheri S, Nishino S (2002) Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci 5(Suppl):1071–1075
Isaac SO, Berridge CW (2003) Wake-promoting actions of dopamine d1 and d2 receptor stimulation. J Pharmacol Exp Ther 307:386–394
Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM (2001) Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21:1787–1794
Rye DB, Jankovic J (2002) Emerging views of dopamine in modulating sleep/wake state from an unlikely source: Pd. Neurology 58:341–346
Gottesmann C (1999) Neurophysiological support of consciousness during waking and sleep. Prog Neurobiol 59:469–508
Boutrel B, Koob GF (2004) What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep 27:1181–1194
Lee BF, Chiu NT, Yang YK, Chu CL (2007) The relation between striatal dopamine d2/d3 receptor availability and sleep quality in healthy adults. Nucl Med Commun 28:401–406
quiz 34–57
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Chen ML, Lin LC, Wu SC, Lin JG (1999) The effectiveness of acupressure in improving the quality of sleep of institutionalized residents. J Gerontol A Biol Sci Med Sci 54:M389–M394
Chang L (1978) A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci 25:643–683
Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F (2002) Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 53:737–740
Fictenberg NL, Putnam SH, Mann NR, Zafonte RD, Millard AE (2001) Insomnia screening in postacute traumatic brain injury: utility and validity of the Pittsburgh Sleep Quality Index. Am J Phys Med Rehabil 80:339–345
Chen PS, Yang YK, Yeh TL et al (2008) Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers—a SPECT study. Neuroimage 40:275–279
Hochberg Y, Benjamini Y (1990) More powerful procedures for multiple significance testing. Stat Med 9:811–818
Roth RH, Elsworth JD (1995) Psychopharmacology: the fourth generation of process. Bull Cancer. Raven, New York
Miller JD, Farber J, Gatz P, Roffwarg H, German DC (1983) Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res 273:133–141
Volkow ND, Wang GJ, Telang F et al (2008) Sleep deprivation decreases binding of [11c]raclopride to dopamine d2/d3 receptors in the human brain. J Neurosci 28:8454–8461
Nishino S, Mao J, Sampathkumaran R, Shelton J (1998) Increased dopaminergic transmission mediates the wake-promoting effects of CNS stimulants. Sleep Res Online 1:49–61
Gainetdinov RR, Jones SR, Fumagalli F, Wightman RM, Caron MG (1998) Re-evaluation of the role of the dopamine transporter in dopamine system homeostasis. Brain Res Brain Res Rev 26:148–153
Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 95:4029–4034
Spielewoy C, Roubert C, Hamon M, Nosten-Bertrand M, Betancur C, Giros B (2000) Behavioural disturbances associated with hyperdopaminergia in dopamine-transporter knockout mice. Behav Pharmacol 11:279–290
Kume K, Kume S, Park SK, Hirsh J, Jackson FR (2005) Dopamine is a regulator of arousal in the fruit fly. J Neurosci 25:7377–7384
Martins RC, Andersen ML, Garbuio SA et al (2010) Dopamine transporter regulation during four nights of REM sleep deprivation followed by recovery—an in vivo molecular imaging study in humans. Sleep 33:243–251
Akert K, Andersson B (1951) An experimental contribution to the physiology of the caudate nucleus. Acta Physiol Scand 22:281–298
Hess R Jr, Koella WP, Akert K (1953) Cortical and subcortical recordings in natural and artificially induced sleep in cats. Electroencephalogr Clin Neurophysiol 5:75–90
Villablanca JR, Marcus RJ, Olmstead CE (1976) Effects of caudate nuclei or frontal cortex ablations in cats. II. Sleep–wakefulness, EEG, and motor activity. Exp Neurol 53:31–50
Joo EY, Hong SB, Tae WS et al (2005) Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage 28:410–416
Laruelle M (2000) Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20:423–451
Acknowledgments
This study was partly supported by grants from the National Science Council of Taiwan (NSC 93-2314-B-006-017, NSC-95-2314-B-006-115-MY2, and NSC-97-2314-B-06-006-MY3) and the Atomic Energy Council of Taiwan (NSC-93-NU-7-006-004 and NSC 97-NU-7-006-001). The authors also wish to thank Ms. Ching Lin Chu, Ms. Tsai Hua Chang, Ms. Yun-Hsuan Chang, and Mr. Chien Ting Lin.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiu, NT., Lee, BF., Yeh, T.L. et al. Relationship Between Striatal Dopamine Transporter Availability and Sleep Quality in Healthy Adults. Mol Imaging Biol 13, 1267–1271 (2011). https://doi.org/10.1007/s11307-010-0442-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-010-0442-6