Abstract
Purpose
The aim of this study was to demonstrate the ability to use human clinical positron emission tomography/computed tomography (PET/CT) to detect and investigate head and neck cancers chemically induced by 4-nitroquinoline-1-oxide (4-NQO) in a rat model.
Study design
The study design was prospective animal research.
Procedures
A head and neck squamous cell carcinoma was established in 20 immunocompetent rats, who drank a 4-NQO solution during 16 weeks. 2-Deoxy-2-[F-18]fluoro-d-glucose (FDG)-PET/CT was performed for five of them, 34 weeks after the start of the experiment to characterize the tumors. A day following the FDG-PET/CT, rats were euthanized and pathological features were evaluated by hematoxylin–eosin staining.
Results
All rats had head and neck tumor at various locations at 34 weeks. Among the five rats selected for having FDG-PET/CT, the clinical examination detected exophytic tumors grown in the oral cavity for three of them (one on the inferior lip, one on the hard palate, and one on the internal side of the cheek). FDG-PET/CT confirmed the presence of those tumors and detected ones located on the base of tongue for three of them. Tumor extensions were characterized and tumor metabolic volumes were measured. The smallest lesion detected measured 3 × 3 × 4 mm. Pathologic examination using hematoxylin–eosin staining confirmed squamous cell carcinoma.
Conclusions
This study demonstrated that FDG-PET/CT is a feasible examination to detect occult primary tumors in rat models. It is useful to follow tumor progression and evaluate therapeutics efficacy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most common human neoplasms. The main etiological factors are tobacco and alcohol. In order to study the behavior of head and neck tumors and to test different chemotherapy agents, we have reproduced an immunocompetent rat model using the carcinogen 4-nitroquinoline-1-oxide (4-NQO; Sigma) in the drinking water to induce tumorigenesis. This model is well known to induce HNSCC and has been described in various studies [1–6]. Imaging of living animals was performed using 2-deoxy-2-[F-18]fluoro-d-glucose-positron emission tomography (FDG-PET) coupled with computed tomography (CT). After FDG studies, pathological analysis was performed to characterize the tumor model. The goal of this study was to demonstrate the ability to use a human clinical PET/CT to detect and investigate head and neck cancers chemically induced by 4-NQO in a rat model.
Materials and Methods
Animal Models and Experimental Protocol
Twenty-five immunocompetent male rats (Sprague Dawley), 21 days old and weighing 30 g, were used for the study. All procedures and care given to the rats were performed according to the Institutional Animal Use and Guidelines. They were kept in plastic cages, with two animals in each cage and fed with standard rat diet. A day to night cycle of 12:12 hours was maintained at 68°F. The 4-NQO stock solution was prepared weekly and diluted with tap water at a concentration of 0.001% for the experiment. Water containing 4-NQO was administrated orally as drinking water ad libitum for 20 of them during 16 weeks. Consumption of 4-NQO solution was checked once a week and bottles were refilled with fresh 4-NQO solution. The control group (five rats) drank normal water. Oral cavities were examined once a week for 34 weeks.
FDG-PET/CT Imaging
FDG-PET/CT was performed for five 4-NQO rats (three of them had an oral tumor on clinical examination) and five control rats (without HNSCC) in order to determine FDG physiological uptake by the head and neck area. During each procedure, including radiotracer injection and animal imaging, animals were anesthetized with 1% to 3% isoflurane (Baxter, Belgium) in 100% oxygen (4l/min), using an animal anesthetizing evaporator. PET/CT imaging was performed 34th weeks after the start of the experiment. Rats were 37 weeks old and weighed 220 g. Food was withheld for 12 h. FDG (18.5 mBq/100 g; Cyclopharma, Laboratoire Saint Beauzire, France) was injected intravenously 2 h before imaging. Small animal imaging studies were performed with a combined PET/CT (Biograph 6) approved for human clinical use (Siemens Medical Solutions; Knoxville, TE, USA). This CT performed simultaneous acquisition of 325 transaxial slices of 0.6 mm, for each bed position of PET acquisition. Transaxial PET resolution was 4.5-mm full width at half maximum. The technical parameters used for CT portion of PET/CT were a detector row configuration of 6 × 0.5 mm, 1.8 mm pitch, 80 kVp, and 130 mAs. This acquisition mode was used for attenuation correction and automatic image fusion. Field of view selected for CT reconstruction was 25 cm. CT data were reconstructed by classical filtered back projection to obtain 1-mm-thick transversal slices. A 10-min three-dimensional acquisition of one bed centered from the nose to the abdomen was performed after the CT. PET data were reconstructed after an iterative process (eight iterations, 16 subsets) with postfiltration by using a low pass 1.0 threshold to obtain 337 × 337 pixels images, zoomed by a factor 2. Contiguous 2.0 mm transaxial, 2.7 mm sagittal, and coronal slices were obtained. The tumor uptake of FDG was quantified in standardized uptake value (SUV). SUV is the tumor activity concentration (MBq/ml) normalized by the injected dose (MBq) per body weight (ml). Both SUV max and SUV mean were determined. Tumor metabolic volume was calculated using 40% max threshold volume of interest.
Pathology
One day following the FDG-PET/CT, all rats were euthanized with potassium chloride (KCl) injection under isoflurane sedation. Oral cavity, pharynx, trachea, esophagus, cervical lymph nodes, lungs, and liver were dissected and fixed with 10% buffered formalin. Each sample was sectioned and embedded in paraffin. Five-micrometer (μm) sections of each specimen were prepared and stained with hematoxylin–eosin (HE).
Results
Clinical Examination
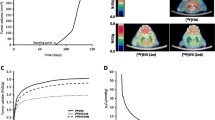
After 16 weeks 4-NQO treatment (N = 20), we did not observe any lesions. We observed leukoplasia that had developed on mobile tongue of ten rats, 20 weeks after the start of the experiment. Oral tumors appeared between weeks28 and 34 in 14 rats (70% of the cohort; Fig. 1). At week34, among the five rats selected for FDG-PET/CT, clinical examination detected exophytic tumors that had grown in the oral cavity for three rats (one on the inferior lip, one on the hard palate, and one on the internal side of the cheek) and no tumors for two rats. By clinical examination, no double localization of primary tumors was identified in the cohort. In the control group (N = 5), we did not observe any tumors at anytime.
FDG-PET/CT
In the 4-NQO group, FDG-PET/CT confirmed the presence of tumors diagnosed in the oral cavity by clinical examination (N = 3; Fig. 2) and discovered pharyngeal tumors located at the base of tongue (N = 3; Fig. 3). Imaging showed the retro-orbital extension of a tumor expanded on the hard palate (Fig. 4) and showed a double tumor localization for one (rat 1—inferior lip and base of tongue). SUV max, SUV mean, and measurement of tumor metabolic volumes were determined (Table 1). Volumes (V) were calculated using the formula: V = 1/6π a × b × c (a, b, and c were the tumor diameters in three dimensions). The tumor metabolic volume of the smallest lesion, located on the inferior lip, was 3 × 3 × 4 mm (19 mm3; Fig. 5). The tumor metabolic volume of the largest one, located at the base of tongue, was 7 × 9 × 24 mm (792 mm3). FDG intratumoral distribution was always uniform and no central hypofixation was noted. For rat 5, with a tumor at the base of tongue, FDG uptake was observed in one cervical lymph node. No pathological uptake was found in liver and lungs. In the control group, mean FDG physiological tongue uptake was measured at 2.0 ± 0.7 for SUV max and at 1.8 ± 0.7 for SUV mean.
Rat 2. a Histopathology (hematoxylin–eosin, magnification ×200): well-differentiated keratinizing squamous cell carcinoma showing trabecular and nodular architecture and numerous horn pearls (black arrows). CT–PET/CT–PET. b Transaxial slices, c coronal slices, d sagittal slices: retro-orbital extension of a hard palate tumor (arrows).
Pathology
Autopsy revealed a macroscopic tumor for all 20 4-NQO rats and discovered a double tumor localization for six of them (30% of the cohort; Table 2). HE-stained tumor sections showed lobulated nodular sheets and islands of SCC. The tumors displayed a circumscribed broad pushing growth front with a fibrovascular capsule and intervening fibrous septae. Tumor cells consisted of pleomorphic squamous epithelial cells exhibiting round to oval vesicular nuclei with prominent nucleoli, pink cytoplasm, scant keratinization, and atypical mitotic figures. The histopathological grade of the squamous cell carcinoma was squamous cell carcinoma of a well-differentiated type (Fig. 3). There was no central necrosis in the tumors. Microscopic examination of HE-stained sections of lungs, liver, and lymph nodes did not reveal metastases. The cervical lymph node that had an FDG uptake was normal (Fig. 6). In the control group, no tumors were found.
Discussion
4-NQO is a water-soluble carcinogen that produces HNSCC in rodents [6]. This chemical model was originally described by Wallenius and Lekholm in 1973 [7]. It is an easy to handle immunocompetent model, unlike the HNSCC xenograft model in nude rats [8]. Furthermore, 4-NQO-induced rat HNSCC have histological and biochemical characteristics close to human tumors [5, 9, 10]. Our current results and other studies clearly demonstrated that 4-NQO in drinking water induced histopathological changes overtime in the oral mucosa proceeding from normal epithelium to leukoplasia, hyperkeratosis, premalignant dysplasia, carcinoma in situ, and finally, invasive SCC [6]. The major advantage of chemically induced tumors, compared to grafted tumors, is that both tumors and their premalignant stages can be studied [2]. Various tumor localizations and sometimes double ones can be obtained. The main disadvantage is the time needed before tumor growth: 34 weeks in our studies, similar to others [4]. Six double localizations of primary tumor were observed in our experiment but none of the rats developed esophageal ones as described by others [4]. Imaging can be proposed in order to detect earlier tumoral localization. In fact, PET imaging with FDG is used routinely in clinical oncology. FDG is a glucose analog that accumulates in metabolically active tumors. Our experiment confirms that FDG-PET/CT can be used for HNSCC detection and measurement of metabolic tumor volume. Metabolic imaging not only confirmed the presence of tumors diagnosed by clinical examination in oral cavity but also detected occult primary tumors like those located at the base of tongue. It is a useful examination for showing the tumor extension. In our experiments, we observed that all rats, even in the control group, had a baseline uptake of FDG by the entire tongue, with a large standard deviation of SUV values. This is probably due to normal tongue muscle movements in rodents, which is of variable intensity. Therefore, the presence of tumor development on the tongue is not indicated by a significant increase in uptake but by a focused one. We think that a focused FDG uptake is more predictable of tumor location than SUV values which are not specific parameters. By using an FDG-PET/CT approved for human clinical use, the smallest tumor volume detected was 3 × 3 × 4 mm. This confirms that it is a leading instrument for tumor detection. Furthermore, we think that a dedicated small animal PET/CT scanner would be more sensitive and would probably enhance imaging of smaller tumors in rats. FDG-PET/CT showed a uniform intratumoral distribution of FDG in all tumors, correlated with an absence of necrosis by pathological examination unlike in xenograft rat model which showed a heterogeneous diffusion and central necrosis [8, 11]. In our experiments, we only observed one false positive FDG uptake located in a cervical lymph node that was considered normal on histopathology. FDG-PET/CT can be used for tumor staging and detection of metastasis [8, 12]. This imaging is also interesting for following tumor progression and for evaluating therapy efficacy [13, 14]. By using FDG to monitor treatment, accurate intratumoral distribution information would lead to a better understanding of therapy efficacy which may prove to be crucial to a better evaluate therapy.
Conclusions
This experiment confirms that using 4-NQO in drinking water results in a reproducible immunocompetent model of HNSCC which is very similar with human tumors. Human clinical PET/CT can detect and stage tumors. It is a valuable tool to screen occult primary tumors that develop in a location that is inaccessible by clinical examination in small animals such as rats. Using dedicated microimaging should certainly allow detection and follow-up of smaller tumors.
References
Hawkins BL, Heniford BW, Ackermann DM et al (1994) 4NQO carcinogenesis: a mouse model of oral cavity squamous cell carcinoma. Head Neck 16:424–432
Nauta JM, Roodenburg JL, Nikkels PG et al (1996) Epithelial dysplasia and squamous cell carcinoma of the Wistar rat palatal mucosa: 4NQO model. Head Neck 18:441–449
Kanojia D, Vaidya MM (2006) 4-Nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol 42:655–667
Tang XH, Knudsen B, Bemis D et al (2004) Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res 10:301–313
Ohne M, Satoh T, Yamada S et al (1985) Experimental tongue carcinoma of rats induced by oral administration of 4-nitroquinoline 1-oxide (4NQO) in drinking water. Oral Surg Oral Med Oral Pathol 59:600–607
Ribeiro DA, Fávero Salvadori DM, da Silva RN et al (2004) Genomic instability in non-neoplastic oral mucosa cells can predict risk during 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Oral Oncol 40:910–915
Wallenius K, Lekholm U (1973) Oral cancer in rats induced by the water-soluble carcinogen 4-nitrochinoline N-oxide. Odontol Revy 24:39–48
Bao A, Phillips WT, Goins B et al (2006) Setup and characterization of a human head and neck squamous cell carcinoma xenograft model in nude rats. Otolaryngol Head Neck Surg 135:853–857
Niwa S, Ueno S, Shirasu R (2001) Alteration of pRb expression in the development of rat tongue carcinoma induced by 4-nitroquinoline 1-oxide. Oral Oncol 37:579–585
Okazaki Y, Tanaka Y, Tonogi M et al (2002) Investigation of environmental factors for diagnosing malignant potential in oral epithelial dysplasia. Oral Oncol 38:562–573
Tatsumi M, Nakamoto Y, Traughber B et al (2003) Initial experience in small animal tumor imaging with a clinical positron emission tomography/computed tomography scanner using 2-[F-18]fluoro-2-deoxy-d-glucose. Cancer Res 63:6252–6257
Monteil J, Dutour A, Akla B et al (2005) In vivo follow-up of rat tumor models with 2-deoxy-2-[F-18]fluoro-d-glucose/dual-head coincidence gamma camera imaging. Mol Imaging Biol 7:220–228
Dutour A, Monteil J, Paraf F et al (2005) Endostatin cDNA/cationic liposome complexes as a promising therapy to prevent lung metastases in osteosarcoma: study in a human-like rat orthotopic tumor. Mol Ther 11:311–319
Kostakoglu L, Goldsmith SJ (2004) PET in the assessment of therapy response in patients with carcinoma of the head and neck and of the esophagus. J Nucl Med 45:56–68
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aubry, K., Shao, Z., Monteil, J. et al. FDG-PET/CT of Head and Neck Squamous Cell Carcinoma in a Rat Model. Mol Imaging Biol 11, 88–93 (2009). https://doi.org/10.1007/s11307-008-0183-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0183-y