Abstract
Purpose
This study was undertaken to determine if artifacts from misalignment of cardiac emission to transmission data is present in adenosine stress studies and if the artifact could be reproduced by intentional misalignment in normal exams.
Procedures
Seventy consecutive 82Rb myocardial perfusion studies were reviewed. Utilizing a quality control program, misalignment was assessed. The study was reprocessed after manual realignment to determine if the defect extent changed. Emission and transmission acquisitions in six normal studies also were intentionally misaligned.
Results
Twenty of 69 rest studies (29.0%) and 17 of 69 (24.6%) stress studies demonstrated misalignment. In four patients with stress misalignment, there was a significant change in clinical interpretation. Upon intentionally misaligning six normal studies, a lateral wall defect was reproduced.
Conclusions
Emission–transmission misalignment occurs in 29.0% and 24.6% of 82Rb rest and adenosine stress studies, respectively. While there is a positive correlation of artifactual defects with misalignment, the presence and size of artifacts is variable and unpredictable at seemingly lesser degrees of misalignment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac positron emission tomography (PET) at rest and stress with Rubidium-82 has proven useful in the evaluation of known or suspected coronary disease [1, 2]. The extent of perfusion defects has been demonstrated to have prognostic significance [3]. Thus, the reliability of artifact-free perfusion mapping is important. A variety of artifacts have been well described with single photon emission computed tomography (SPECT) [4]. Because of the availability of attenuation correction on most PET systems, PET is considered less prone to artifact from soft tissue attenuation, offering greater specificity and spatial resolution than SPECT [4–6]. Yet, attenuation correction itself may introduce artifacts into PET images [7–9].
Loghin and coworkers [7] have investigated the cause of a potential artifact in 82Rb and 13N-ammonia myocardial PET perfusion imaging performed with dipyridamole pharmacologic stress. Overall frequency of artifactual defects due to misalignment was 21.4%. The artifact was corrected when the emission and transmission images were properly aligned and post-processed using a manual correction technique. These potential misregistration errors are reported to be more frequent, severe, and complex with positron emission tomography/computerized tomography (PET/CT) devices [9].
The use of adenosine for pharmacologic stress is preferable to that of dipyridamole for a number of reasons including a more reliable vasodilatory response with adenosine, as well as fewer serious side effects resulting in a lower adjusted cost than with dipyridamole [6, 10–12]. Yet, because of the shorter duration of action of adenosine, it may not be suitable for certain myocardial PET perfusion protocols [6]. It could be postulated that if the adenosine effect on the emission images had abated by the time of the transmission scan, the heart would shift back into baseline position, causing an even greater emission–transmission mismatch than with dipyridamole studies in which the action of dipyridamole would last through the emission and transmission scans.
The purpose of this retrospective study is to determine if emission–transmission misalignment is present not only with dipyridamole stress as has been reported [7] but also in adenosine stress 82Rb myocardial PET studies. In addition, images were purposely misaligned to investigate the nature of such induced artifacts.
Materials and Methods
Patient Selection
We retrospectively studied 70 consecutive patients who underwent adenosine stress 82Rb perfusion studies completed at the Emory Cardiac Imaging Center between 8/19/04 and 12/7/04. The perfusion studies were performed for standard clinical indications such as chest pain, assessment of functional significance of coronary stenosis, and equivocal exercise or myocardial SPECT stress exams. The inclusion criteria for this study were as follows: any 82Rb PET cardiac adenosine rest and stress exam with segmented attenuation correction in which the data could be restored and reanalyzed. This study was approved by the Emory University Institutional Review Board.
PET Imaging and Stress Protocol
Patients were kept nil per os for 4–6 h before the exam and told to refrain from caffeine or caffeinated beverages for 24 h before the study. All scans were obtained on an ECAT EXACT 921 PET scanner (CTI/Siemens) in 2-D mode with the following protocol: A 2-min positioning scan was acquired with 82Rb 370 MBq intravenously (IV), and the patient’s chest was marked, or the bed coordinates were noted for subsequent positioning. Once optimal positioning was achieved with the positioning scan, the patient was kept in this position for the resting exam and left in the gantry for adenosine stress after the resting study was acquired. MBq 82Rb (1,036–2,368; mean dose 1,794.5 ± 336.7) from a generator was injected IV, and a 5-min resting emission image was obtained at 90 s post injection. A 5-min transmission scan was then acquired using the three rotating (nominally 25 MBq each) Germanium-68 rod sources built into the scanner.
Adenosine (0.14 mg kg−1 min−1) was then infused intravenously while pulse and blood pressure were monitored with continuous electrocardiogram under the supervision of a board-certified cardiologist. The total infusion time was 6 min. At 3 min into the adenosine infusion, the same dose of 82Rb was then injected. A 5-min stress emission scan was acquired at 90 s post injection. Approximately 1 min after the emission scan, a 5-min transmission scan was obtained.
Processing and Data Presentation
Images were processed using filtered back projection using a Hann filter cutoff at one cycle per centimeter. Attenuation correction was derived by segmenting the transmission image using the method of Xu et al. [13] as implemented by the scanner manufacturer. The transaxial images for all cardiac scans were resliced, and the short axis images saved for processing in our processing and quantization software (Emory Cardiac Toolbox, Atlanta, GA, USA) including quantitative comparison to a normal file described elsewhere [14–17].
Quality Control Program
After reconstruction, the transmission and emission images were entered into a quality control program to assess the amount of misregistration [See below for screen captures of the quality control (QC) program]. A modification of the method of Loghin et al. [7] was used to quantitate the amount of misregistration. Briefly, a threshold of 0.05/cm was used to convert the raw transmission image to a binary image. This threshold effectively differentiates between lung and tissue regions in the attenuation map. A seed was automatically placed inside the left lung, and a region-growing routine was used to segment the left lung from the image. Then, the emission image was converted to a binary image using a threshold of 0.3 times the maximum pixel value in the emission image. Finally, the overlap between the segmented left lung and emission binary images was determined. The overlap was quantitated both in terms of the number of pixels and volume (ml).
If the amount of overlap was non-zero, the transmission image was manually reoriented. The user has the ability to rigidly translate and rotate the transmission volume in all three (transaxial, coronal, and sagittal) planes. Once corrected for motion, the transmission image was foreprojected to make a new set of attenuation correction factors, and the emission data was reconstructed again. If this was done, the entire quality control procedure was repeated to verify there was no remaining myocardial tissue in the emission image overlapping lung on the transmission scan.
Misalignment Evaluation
One board-certified nuclear medicine physician (RH) and one board-certified nuclear radiologist (DMS) used the QC program to determine if the lateral wall of the myocardium on the emission map overlapped with that of lung on the transmission images. Misalignment of left ventricular emission activity to lung attenuation on the stress and rest exams was assessed and recorded in terms of pixels and size in cubic centimeters. Pixel size is 0.088 cc (0.51 × 0.51 × 0.34 cm).
In a subset of 17 patients with stress misalignment, the entire study was reprocessed after manual realignment of the cardiac emission data to the expected location of the heart on the transmission scan. Pre- to post-realignment slices and bull’s-eye images were compared to determine if the defect extent changed quantitatively and qualitatively and if this change resulted in a significant difference in clinical interpretation. Patient height, weight, peak maximal heart rate, and change of heart rate with adenosine infusion were recorded. Body mass index was calculated using the equation: body mass index (BMI) = weight in kilograms/(height in meters)2. The standard definition of obesity employs a BMI of 30 kg/m2 or greater.
Reproduction of Artifacts
A series of six normal stress studies were randomly selected to determine the sensitivity of creating artifacts with misregistration. The attenuation image was intentionally shifted to varying degrees left and right (5–20 mm) resulting in overlap of cardiac emission data to the lung on the transmission image. These images were then reprocessed, and the QC program was employed to determine overlap in pixel number and area. The reprocessed images were compared to a normal quantitative map to determine the presence and size of the resultant artifactual defect.
Statistical Considerations
Mean, standard deviation, as well as correlation coefficients, odds ratios, and logistical regressions and their significance were interpreted using Excel 2003 (Microsoft), SPSS 13.0 (SPSS) software, and SAS System software version 9.1. Significance was determined at p < 0.05 and marginal significance at p < 0.1.
Results
A total of 140 studies were retrospectively reviewed from 70 patients. One patient did not undergo stress and was excluded. Patient demographics are as outlined in Table 1. There were 25 normal and 44 abnormal exams as originally reported. The adequacy of the heart rate response to adenosine was also examined. Heart rate data was not available for four patients. Of the patients, 58.5% (38 of 65) had an optimal [≥20 increase in beats per minute (BPM)] heart rate response to adenosine. The mean heart rate response was 23.2 ± 13.9. Of the patients, 38.5% (25 of 65) had a tachycardic (>100 BPM) response to adenosine.
Analyses of Clinical Images
Of 69 rest studies, 49 (71.0%) had no misalignment; 10 of 69 (14.5%) had 0.1–0.9 cc misalignment; 10 of 69 (14.5%) had 1.0–6.0 cc misalignment. Mean rest study misalignment was 5.8 ± 14.2 pixels (0.5 ± 1.3 cc). The range of misalignment on the rest studies was 0–67 pixels (0–6 cc).
Of 69 stress exams, 52 (75.4%) had no misalignment; 16 of 69 (23.2%) had 0.1–0.9 cc misalignment; 1 of 69 (1.4%) had 1.0 cc misalignment. Mean stress study misalignment was 1.2 ± 2.6 pixels (0.10 ± 0.23 cc). The range of misalignment on the stress studies was 0–11 pixels (0–1 cc). Of 69 studies, 8 (11.6%) had both rest and stress misalignment.
Obesity was associated with a statistically significant increased risk of misalignment on stress studies (odds ratio 4.1; Table 2) and a nonsignificant trend to increased risk of misalignment on resting studies (Table 3).
A suboptimal heart rate response to adenosine (<20 BPM) was a marginally significant risk factor for misalignment during the stress exam (Table 4). Additionally, the presence of tachycardia alone at peak stress did not result in increased risk for stress misalignment (Table 5); in fact, there was a nonsignificant trend to decreased risk for misalignment with tachycardia.
Seventeen studies which demonstrated stress misalignment were realigned and evaluated for perfusion defects post-realignment. These are presented in Table 6. Of the 17 studies in which there was stress misalignment, pre- to post-realignment comparison revealed that in four, there was a significant change in clinical interpretation with correction.
Reproduction of Artifact on Intentionally Misaligned Studies
Analysis of the data for the six normal studies that were intentionally misaligned at stress demonstrated that with each degree of intentional shift (5 mm, 7.5 mm, etc) there was a significant increased risk of misalignment (Table 7) and that there was a significant correlation (Fig. 1) of the size of the artifactual defect (as compared to a normal file quantitative algorithm) with misalignment. Yet, this effect was variable. While misalignment of 10 cc or greater always resulted in some artifactual defect, misalignment of less than this amount in some cases resulted in no significant artifactual defect or relatively large defects. Artifactual defects of 5% or greater occurred with apparent misalignment of less than 1 cc in some instances.
Scatter plot correlating the size of the artifactual defect (% left ventricular mass as defined by the quantitative algorithm) to misalignment/misregistration of the emission/transmission images in six shifted normal studies. On average, the artifactual defect increases by 1.14% per cubic centimeter increase in misalignment. The rate of increase is statistically significantly (p < 0.0001). Note though, that no defect may occur with a misalignment of up to 9.2 cc, and defects 5% or greater may occur with misalignment of less than 1 cc in an individual patient.
Discussion
Several investigators have reported emission–transmission misalignment as a potential source of error for myocardial PET perfusion with dipyridamole stress and for viability studies [7, 8, 18]. This potential cause of artifact has also been described in the American Society of Nuclear Cardiology PET myocardial imaging guidelines [19]. Techniques to detect and compensate for this problem have also been reported in the fluorodeoxyglucose PET [20, 21] and the myocardial SPECT [22] literature.
The purpose of our study was not to correlate our results with that of catheterization data or clinical outcome but simply to determine if a misalignment artifact, caused primarily by cardiac emission data mapped onto lung transmission data, was present in our institutional protocol with adenosine. In our series, 29% of rest and 24.6% of adenosine stress studies demonstrated some degree of misalignment, a similar frequency to that of Loghin and coworkers in which dipyridamole stress was employed [7]. Of the 17 studies in which there was stress misalignment, pre- to post-realignment comparison revealed that in four there was a significant change in clinical interpretation with manual realignment. Figures 2 and 3 are examples from a clinical case subsequent to collection of the above data in which an artifactual defect was recognized and corrected, which changed the reading from “ischemia” to “no significant abnormality.”
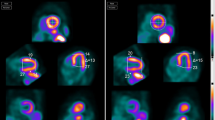
QC program (a), multiplanar slices (b) and polar images (c) from a patient with misalignment of cardiac activity (straight arrow in a). The QC display shows the binarized emission data (red) overlaying the transmission data (gray). The algorithm has determined that 125 pixels occupying 11.2 ml from the emission image lie within the left lung of the transmission image. The user can shift or rotate the transmission image in any of the planes to correct this problem (see Fig. 3). Note large apparent reversibility in lateral wall (angled arrows in b). This degree of artifact is unusual in our experience.
Same patient as in Fig. 2 after correction of misalignment. Note corresponding QC program (a), multiplanar slices (b) and polar images (c) in which the artifactual lateral wall reversibility has normalized (angled arrows in b). A small partially reversible inferior wall defect is not clinically significant in this patient with cardiomyopathy.
Loghin and coworkers [7] suggest that an underlying cause of the misalignment artifact at rest is due to an upward repositioning of the diaphragm upon assuming the supine position due to slow moving abdominal contents and is accentuated in a patient with a larger body mass index.
Similarly, in the current study, the presence of obesity was a significant risk factor for stress misalignment and marginally significant for rest misalignment. It is likely that a patient with a greater BMI, especially with an enlarged abdominal girth, may undergo accentuated shifting of abdominal contents with the increased respiratory and heart rate changes induced by adenosine and then a settling as the adenosine effects resolve, leading to misalignment between the sequentially acquired emission and transmission acquisitions.
In addition, Loghin and coworkers [7] suggest that stress misalignment is due in part to pharmacologic stress causing the heart to descend during systolic contraction and the apex to recoil forward, and that with tachycardia, the heart is in recoil position for a longer time during the cardiac cycle. Also dipyridamole stress results in a “feeling of chest fullness and labored breathing associated with tachypnea” and that this shifts the diaphragm inferiorly and narrows the mediastinum.
It is interesting to note that in our study, there was a marginally significant decreased risk of misalignment with an optimal response to adenosine. The presence of tachycardia at peak stress also decreased the risk of misalignment, though this was not statistically significant. This seems counterintuitive and should be further investigated.
After examining the subset of 17 stress exams with misalignment and noting that even a small misalignment error could produce a large and even clinically significant artifactual defect, we attempted to reproduce the characteristic lateral wall defect by purposely misaligning the emission and transmission images in six normal exams. Yet while we were able to duplicate this artifact and noted there was a significant correlation of the size of the artifactual defect to the volume of misalignment, the effect could be quite variable in this small patient sample. In fact, the most impressive examples of correctable misalignment resulting in a lateral wall defect in the clinical stress cohort occurred with only 1 pixel (0.1 cc) of misaligned volume (patients 14 and 15 in Table 6). This suggests that misalignment artifacts are multifactorial and complex and that a QC tool which only examines cardiac emission overlap with that of segmented lung transmission data is not adequate to precisely account for all causes of artifactual defects.
We believe that robust tools must be made available to correctly discern proper registration of cardiac emission data to the true myocardial muscle. In this work, the entire transmission image was shifted and rotated to eliminate any overlap. In truth, the heart and abdominal contents are shifting within the body contours. A potentially better method would be to warp the position of the heart and abdominal contents within the confines of the body. In addition, segmented transmission images are not of sufficient quality to discern mediastinal and cardiac soft tissue from that of fat. Realignment may therefore be imperfect in terms of accurately registering cardiac emission activity to anatomic cardiac parenchyma. So-called realignment may actually be using fat lateral to the heart for attenuation correction rather than heart muscle itself. While in most patients, misalignment of cardiac emission activity with lung seems to be the most critical factor in the production of artifactual defects, it is possible that some patients may demonstrate defects from alignment of cardiac emission activity to that of mediastinal fat itself.
We did not directly compare heart and diaphragmatic displacement between rest and stress, nor did we measure heart size. Loghin and coworkers [7] reported an artifact rate due to misalignment of 21% and comprehensively analyzed varying causal factors. Similarly in a subsequent investigation with cardiac PET/CT [23], we found a misregistration rate of 28% due to patient motion and cardiac drift which could not be corrected by varying the CT acquisition protocol. We concentrated on the end phenomenon of misalignment to lung because this has been determined to be the most significant factor and one in which a semi-automated or automated QC program could be more easily developed. Other authors also note that lung overlap is the most significant factor [8]. In our analysis of the six normal studies which were purposely shifted, none of the patients were misaligned in the z-direction, yet variable effects were still observed. Our primary aim was to determine overall artifact rate, which was similar between rest (29%) and stress (24.6%) studies. Once we realigned the misregistered stress studies and found that 23.5% (4 of 17) in which there was misregistration resulted in a clinically significant difference in interpretation, we determined that this artifact was of sufficient importance to be addressed by some type of comprehensive re-registration method. Misregistered rest studies were not realigned if the stress studies had no misalignment. Thus, we cannot determine the overall rate of clinical significance of this artifact across all studies, only those with stress misalignment.
Ideally, a direct comparison of dipyridamole to adenosine stress in the same patient on the same equipment would yield definitive differences between the two pharmacologic agents, but this would have to be done in a prospective comparative study in which a patient would be stressed twice.
We agree with other authors that cardiac PET/CT scanning will result in more potential for misalignment [7, 9, 24]. Yet, the ability to precisely overlay cardiac parenchyma to emission data will be possible for cardiac PET/CT. Such a preprocessing QC tool in which nonsegmented (true CT) data is used for registration should be made available and possibly automated.
Conclusions
Emission–transmission misalignment occurs in 29.0% of 82Rb rest studies and 24.6% of adenosine stress studies acquired with our specific adenosine protocol, which is similar to that of Loghin and coworkers with a dipyridamole protocol [7]. This misalignment caused a clinically significant correctable artifactual defect in 4 of 17 stress studies in which there was such misalignment. We found both in the clinical cases and deliberately shifted images, instances of 5% or greater defects with very small cardiac emission misalignment with lung, which suggest that the interaction between degree of misalignment and severity of perfusion defects is complex and multifactorial. Because of the potential of such artifacts, the inclusion of a quality control tool with cardiac PET software is warranted, but a program which relies only on rigid registration of cardiac emission data to lung is not sufficient at small degrees of misalignment. Visualizing the heart parenchyma and adjacent structures with PET/CT (and not simply segmenting the attenuation maps) and using this QC tool to precisely overlay emission and transmission data may be a potential solution to this problem.
References
Gould KL (1991) Clinical cardiac positron emission tomography: state of the art. Circulation 84:I22–I36
Gropler RJ, Soto P (2004) Recent advances in cardiac positron emission tomography in the clinical management of the cardiac patient. Curr Cardiol Rep 6:20–26
Marwick TH, Shan K, Patel S, Go RT, Lauer MS (1997) Incremental value of rubidium-82 positron emission tomography for prognostic assessment of known or suspected coronary artery disease. Am J Cardiol 80:865–870
DePuey EG, Garcia EV (1989) Optimal specificity of thallium-201 SPECT through recognition of imaging artifacts. J Nucl Med 30:441–449
Freedman N, Schechter D, Klein M, Marciano R, Rozenman Y, Chisin R (2000) SPECT attenuation artifacts in normal and overweight persons: insights from a retrospective comparison of Rb-82 positron emission tomography and TI-201 SPECT myocardial perfusion imaging. Clin Nucl Med 25:1019–1023
Bateman TM (2004) Cardiac positron emission tomography and the role of adenosine pharmacologic stress. Am J Cardiol 94:19D–24D, (discussion 24D–25D)
Loghin C, Sdringola S, Gould KL (2004) Common artifacts in PET myocardial perfusion images due to attenuation-emission misregistration: clinical significance, causes, and solutions. J Nucl Med 45:1029–1039
McCord ME, Bacharach SL, Bonow RO, Dilsizian V, Cuocolo A, Freedman N (1992) Misalignment between PET transmission and emission scans: its effect on myocardial imaging. J Nucl Med 33:1209–1214, (discussion 1214–1205)
Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S (2007) Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med 48:1112–1121
Holmberg MJ, Mohiuddin SM, Hilleman DE, Lucas BD Jr, Wadibia EC (1997) Outcomes and costs of positron emission tomography: comparison of intravenous adenosine and intravenous dipyridamole. Clin Ther 19:570–581, (discussion 538–579)
Gupta NC, Esterbrooks D, Mohiuddin S et al (1991) Adenosine in myocardial perfusion imaging using positron emission tomography. Am Heart J 122:293–301, (discussion 302–296)
Siffring PA, Gupta NC, Mohiuddin SM et al (1989) Myocardial uptake and clearance of T1-201 in healthy subjects: comparison of adenosine-induced hyperemia and exercise stress. Radiology 173:769–774
Xu EZ, Mullani NA, Gould KL, Anderson WL (1991) A segmented attenuation correction for PET. J Nucl Med 32:161–165
Garcia EV, Cooke CD, Van Train KF et al (1990) Technical aspects of myocardial SPECT imaging with technetium-99 m sestamibi. Am J Cardiol 66:23E–31E
Faber TL, Cooke CD, Folks RD et al (1999) Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med 40:650–659
Santana CA, Folks RD, Cooke CD, Faber TL, Garcia EV (2001) Rubidium-82 myocardial perfusion PET: development and validation of combined normal limits. J Nucl Cardiol 8(4):S134, (abstract)
Van Train KF, Areeda J, Garcia EV et al (1993) Quantitative same-day rest-stress technetium-99m-sestamibi SPECT: definition and validation of stress normal limits and criteria for abnormality. J Nucl Med 34:1494–1502
Chin BB, Nakamoto Y, Kraitchman DL, Marshall L, Wahl R (2003) PET-CT evaluation of 2-deoxy-2-[18F]fluoro-d-glucose myocardial uptake: effect of respiratory motion. Mol Imaging Biol 5:57–64
Schelbert HR, Beanlands R, Bengel F et al (2003) PET myocardial perfusion and glucose metabolism imaging: Part 2-Guidelines for interpretation and reporting. J Nucl Cardiol 10:557–571
Bettinardi V, Gilardi MC, Lucignani G et al (1993) A procedure for patient repositioning and compensation for misalignment between transmission and emission data in PET heart studies. J Nucl Med 34:137–142
Bacharach SL, Douglas MA, Carson RE et al (1993) Three-dimensional registration of cardiac positron emission tomography attenuation scans. J Nucl Med 34:311–321
Fricke H, Fricke E, Weise R, Kammeier A, Lindner O, Burchert W (2004) A method to remove artifacts in attenuation-corrected myocardial perfusion SPECT Introduced by misalignment between emission scan and CT-derived attenuation maps. J Nucl Med 45:1619–1625
Nye JA, Esteves F, Votaw JR (2007) Minimizing artifacts resulting from respiratory and cardiac motion by optimization of the transmission scan in cardiac PET/CT. Med Phys 34:1901–1906
Fitzpatrick GM, Wells RG (2006) Simulation study of respiratory-induced errors in cardiac positron emission tomography/computed tomography. Med Phys 33:2888–2895
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schuster, D.M., Halkar, R.K., Esteves, F.P. et al. Investigation of Emission–Transmission Misalignment Artifacts on Rubidium-82 Cardiac PET with Adenosine Pharmacologic Stress. Mol Imaging Biol 10, 201–208 (2008). https://doi.org/10.1007/s11307-008-0139-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0139-2