Abstract
Purpose
We sought to identify an anesthetic regime that, unlike isoflurane in air, would maintain glucose homeostasis in mice undergoing Positron emission tomography (PET) imaging with 2-deoxy-2-[18F]fluoro-d-glucose (FDG).
Materials and Methods
FDG uptake was also measured in normal and tumor tissues. Athymic and Balb/c nude mice were studied. Blood glucose levels were measured before and after 30 min of FDG PET imaging under isoflurane or sevoflurane carried in air or oxygen. FDG uptake was quantified as a percentage of the injected dose and using Patlak analysis yielding K i values.
Results
Blood glucose levels were more stable under sevoflurane than under isoflurane, especially in the athymic nude mice. Under isoflurane, FDG uptake into myocardium was higher than under sevoflurane and was strongly correlated with the intrascan change in blood glucose.
Conclusion
Sevoflurane should be preferred for physiologic imaging in mice, minimizing changes in glucose and, for FDG PET, reducing signal spillover from the myocardium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Measurements of the metabolic rate of glucose uptake (MRGlu) have been made in many tissues since the introduction of positron emission tomography (PET) imaging with 2-deoxy-2-[F-18]fluoro-d-glucose (FDG). This is typically done by combining dynamic imaging of FDG uptake kinetics with measurements of plasma glucose levels and a lumped constant [1–3]. Our early experiences with FDG PET imaging in athymic nude mice suggested that the plasma glucose levels were the weak link in this measurement chain since our standard light isoflurane anesthesia regime (~2% in air, adjusted to effect) brought about a substantial and statistically significant reduction in blood glucose levels during a typical 30-min scan, even though the body temperature was well-controlled and the induction period kept to a minimum. Blood glucose levels in humans under anesthesia have been reported to rise during surgery, explained by anesthetic-induced endocrine changes [4]. In mice, similar increases in blood glucose were reported by Toyama in Balb/c mice and by Fueger in severe combined immunodeficiency mice [5, 6], but Loepke reported hypoglycemic effects of isoflurane similar to our own observations albeit in C57Bl6-crossed mouse pups [7]. More recently, observations similar to our own and in the same mouse strain have been published by Dandekar et al., confirming the hypoglycemic effect of isoflurane in adult mice [8]. Corrections for blood glucose levels can be made in quantitative studies of FDG uptake [9, 10], but these methods are based on the assumption that the plasma glucose level is constant rather than drifting during the scan. In this study, we sought to identify an anesthetic regime that would maintain glucose homeostasis and to assess how different anesthetic regimes affected tissue uptake of FDG. We studied both athymic nude mice, which are commonly employed for tumor xenograft models in our institution, as well as Balb/c mice, which have been reported to have chronic hyperglycemia [11].
Materials and Methods
Mice and Tumor Cell Lines
Female athymic nude and Balb/c nude mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and maintained in Association for Assessment of Laboratory Animal Care-accredited animal facilities until 6 to 8 weeks old. Animal protocols were approved by our Institutional Animal Care and Use Committee. Two hundred mice were studied, 120 with xenograft tumors and 80 without. The tumor cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA). The 200 mice were divided into five cohorts of 40: (1) naïve athymic nudes, (2) athymic nude mice bearing HCT116 colon carcinoma xenografts, (3) athymic nude mice bearing NCI-PC3 prostate cancer xenografts, (4) naïve Balb/c nudes, and (5) Balb/c nude mice bearing IGROV-1 ovarian cancer xenografts. The tumor volumes ranged from approximately 100 to 500 mm3. IGROV-1 tumor growth was supported by the implantation of 17-beta-estradiol pellets (Innovative Research Of America, Sarasota, FL, USA). Each of the five cohorts was subdivided into four groups of ten mice, one group for each of the four type of anesthetic/carrier combinations being (1) isoflurane in air, (2) isoflurane in oxygen, (3) sevoflurane in air, and (4) sevoflurane in oxygen.
Animal Handling and Blood Glucose Measurements
All mice were fasted overnight with access to water. Animals were anesthetized singly in a prewarmed induction box prior to being promptly transferred to the bed of the PET scanner. Light anesthesia was maintained for restraint only at the minimum concentration required to ensure the mice did not wake up during the scan, about 2% v/v for isoflurane (Baxter Healthcare, Deerfield, IL, USA) or about 3.5% v/v for sevoflurane (Abbott Labs, North Chicago, IL, USA) consistent with previous reports [12].. The eyes were lubricated with ophthalmic ointment. Body temperature was regulated at 37°C in a feedback system based on warm air flows. Blood glucose levels were measured in triplicate readings of blood from the saphenous vein (~1 μl per mouse) at approximately 5 min postinduction (prescan) and again at 35 min (postscan) using the OneTouch Ultra meter by LifeScan (Milpitas, CA, USA).
Small Animal PET Imaging
Mice were imaged using Focus 120 MicroPET systems (Siemens Preclinical Solutions, Knoxville, TN, USA), which have been previously characterized [13]. Promptly after measuring the prescan blood glucose on the scanner bed, animals were injected via the lateral tail vein with approximately 9.25 MBq (250 μCi) of 18F-FDG (PETNET, Palo Alto, CA, USA) in 100 μl of saline to begin a 30-min dynamic scan. All 32 image frames (6 × 10, 6 × 20, 6 × 30, 6 × 60, and 8 × 135 s) were reconstructed using vendor-supplied software implementing the ordinary Poisson maximum a posteriori reconstruction method [14] with a zoom factor of 2.14 and the hyperparameter beta set to 0.05 yielding a uniform image resolution of approximately 1.3 mm across the field of view. The reconstructed voxel size was 0.4 × 0.4 mm in-plane × 0.8 mm through-plane.
Image Analysis
Region of interest (ROI) measurements were made on multiple axial slices of the brain, myocardium, liver, tumor, Harderian gland, and muscle tissues using ASIPro™ software version 6.6.2 (Siemens Preclinical Solutions, Knoxville, TN, USA). ROI quantitation was in percentage of the decay-corrected injected dose per milliliter of ROI, used interchangeably with percent injected dose (%ID) per gram in soft tissue. The standardized uptake value (SUV) is commonly employed in PET, but for mice, it is unclear how to appropriately normalize for physiological variables; some publications quote SUV values based on multiplying [6] and some based on dividing [8] by body weight. We have chosen to normalize SUV only by injected dose (SUV=%ID/g) and not to correct for body weight. The SUV values are determined from the last 6 min of the scan giving an effective uptake time of 24 min. Uptake rates are reported as the rate constant K i, in units of reciprocal seconds; K i corresponds to the slope of the linear phase of the Patlak plot. Converting from FDG uptake rates into glucose uptake rates requires multiplication by the appropriate glucose concentration and by a lumped constant (LC) accounting for the differential transport and trapping kinetics of glucose and FDG. Reports of the LC in humans and rats vary from approximately 0.4 to 1.4 [15, 16], but the true LC can vary even within a tissue as various glucose transport and hexokinase isoforms come into play [17]. Given these uncertainties and the lack of published data for the LC measured in mice, we chose to use a LC of unity in our calculations of MRGlu. For the glucose concentration, we employed the mean of pre- and postscan blood glucose measurements, although there is the possibility, even likelihood, that the poorly perfused tumor glucose concentration is depleted compared to plasma levels. There is some value in considering these subtly different metrics, and so FDG uptake was quantified using all three (SUV, K i, and MRGlu).
Statistical Analysis
Statistical calculations were performed using R version 2.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Student’s t test and two-way analysis of variance (ANOVA) were used for group comparisons of tissue FDG uptake and comparisons of percent change in postscan from prescan blood glucose measurements under the four different anesthetic regimes. p values less than 0.01 were considered statistically significant. The Pearson’s correlation coefficient test was applied to measure the relationships between blood glucose and tissue uptake. p values are represented on the face of the figures using asterisks as follows: *: p < 0.01, **: p < 0.001, and ***: p < 0.0001. We did not observe any statistically significant differences due to interactions.
Results
Blood Glucose Levels
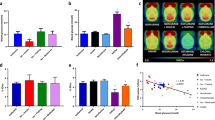
Blood glucose levels measured pre- and postscan for all 200 mice were grouped according to their cohort and anesthetic/carrier gas group for comparison (Fig. 1). The raw data blood glucose levels are shown (Fig. 1a) along with the normalized data showing percentage change (Fig. 1b).
a Prescan and postscan glucose levels for the five cohorts of mice under each of the four different anesthesia regimes: IA (isoflurane in air), IO (isoflurane in oxygen), SA (sevoflurane in air), and SO (sevoflurane in oxygen). The triangles and squares represent the means of the prescan glucose and postscan glucose, respectively. The vertical bars are the 95% confidence intervals. b Percent changes in blood glucose levels for the five cohorts of mice under each of the four different anesthesia regimes. The horizontal bars represent the mean of the group and the vertical bars the 95% confidence intervals. n = 10 per anesthesia regime, per animal group. In the panel of Balb/c nude with IGROV tumors, there is one outlier point (>100% increase) not plotted in the isoflurane in oxygen set. Asterisks represent statistical significance levels of a two-way ANOVA test to determine the effect of anesthetic agent and/or carrier on percent change in blood glucose as follows: asterisk, 0.001 < p < 0.01; double asterisk, 0.0001 < p < 0.001; triple asterisk, p < 0.0001.
Athymic Mice
For the athymic nude mice, we observe that glucose homeostasis is better preserved under sevoflurane than under isoflurane anesthesia as seen in the smaller percent changes in blood glucose. There is a statistically significant difference between the blood glucose stability under isoflurane versus sevoflurane, but the differences between air and oxygen are not statistically significant.
Balb/c Mice
We observe the baseline hyperglycemia reported in this strain [11]. Unlike the athymic nudes, the postscan glucose actually increased in the Balb/c mice except for the combination isoflurane in air, where the glucose level dropped. The percent change in blood glucose was significantly affected by both anesthetic and carrier gas. There is a statistically significant difference between the blood glucose stability under isoflurane/oxygen versus isoflurane/air (Student’s t test, p = 0.008), but the differences between air and oxygen conditions are not statistically significant under sevoflurane (p = 0.212).
FDG Tissue Uptake
Myocardium
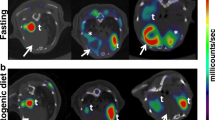
Unexpectedly, we observed that the heart was typically much more conspicuous in images from the isoflurane anesthetized mice, as can be seen in the long-axis views obtained from the hearts of the naïve athymic and Balb/c nude mice (Fig. 2a). Images are represented from hearts with the lowest, the highest, and the closest-to-median uptake values for all four anesthetic/carrier conditions. This was the result of greater uptake rates into the myocardium, as can be seen in the representative time activity curves (Fig. 2b), and was evident by any measure of uptake (Fig. 3, left column for myocardium). Only in the Balb/c nude mice carrying IGROV-1 tumors and estrogen pellets was the FDG uptake significantly affected by the carrier gas.
a PET images of FDG uptake in the heart. Mice were anesthetized with either isoflurane or sevoflurane in air or oxygen for the 30-min duration of image acquisition as indicated (IA, SA, IO, SO). Coronal images show images of the observed maximum, median, and minimum myocardial uptake (%ID/g) under each of the four different anesthesia regimes. b Group-averaged time–activity curves for FDG uptake in myocardium (top) and liver (bottom). The error bars represent the mean ± one standard error of the mean. n = 10.
K i (influx rate), SUV (%ID/g), and metabolic rates of glucose uptake in myocardial and brain tissues of the naïve mice groups under the different anesthetic regimes: IA (isoflurane in air), IO (isoflurane in oxygen), SA (sevoflurane in air), and SO (sevoflurane in oxygen) for each group of mice. The error bars represent the mean ± one standard deviation. n = 10 for every bar. Asterisks represent statistical significance levels of a two-way ANOVA test to determine the effect of anesthetic agent and/or carrier on FDG uptake as follows: asterisk, 0.001 < p < 0.01; double asterisk, 0.0001 < p < 0.001; triple asterisk, p < 0.0001.
Brain
FDG uptake in the brain was much less variable than that of the myocardium, and statistically significant differences were observed (Fig. 3, right column for brain). As with myocardium, the uptake rate constant K i was affected only by the choice of the anesthetic agent in athymic nude mice, but in Balb/c nude mice, both the anesthetic and carrier had a statistically significant effect.
Tumor
FDG uptake as %ID/g, K i, and the MRGlu was calculated for each of the three tumor types studied, HCT116, PC3, and IGROV-1 (Fig. 4). Asterisks on the figure denote statistical significance for an effect of anesthetic agent or carrier gas.
K i (influx rate), SUV (% ID/g), and metabolic rates of glucose uptake in tumor tissue of the mice groups implanted with tumors under the different anesthetic regimes: IA (isoflurane in air), IO (isoflurane in oxygen), SA (sevoflurane in air), and SO (sevoflurane in oxygen) for each group of mice. The error bars represent the mean ± one standard deviation. n = 10 for every bar, except for the mice implanted with PC3 cells and underwent isoflurane in air or isoflurane in oxygen, where n = 9. Asterisks represent statistical significance levels of a two-way ANOVA test to determine the effect of anesthetic, and carrier gas as follows: asterisk, 0.001 < p < 0.01; double asterisk, 0.0001 < p < 0.001; triple asterisk, p < 0.0001.
Liver
No systematic differences were observed in the time–activity curves from liver tissue under any of the anesthetic conditions studied, consistent with its use a blood reference tissue [18]. Small differences in amplitude and half-life were seen between the athymic and Balb/c nude mouse strains (Fig. 2b).
Other Tissues
Uptake in the Harderian glands was highly variable with very low near-background uptake observed several times, but no statistically significant pattern emerged.
Blood Glucose Levels and their Correlations with FDG Uptake
FDG uptake rates into normal brain tissue showed a statistically significant inverse relationship with mean glucose levels, and this is illustrated for naïve athymic nude mice (Fig. 5a). Similar relationships were evident for all four anesthetic conditions. In the myocardium, a significant correlation was observed between the percentage change in the intrascan blood glucose and the FDG uptake; it is important to note that this phenomenon was only evident in animals anesthetized with isoflurane, so the data are presented separately for isoflurane and sevoflurane conditions (Fig. 5b).
a Scatter plot of FDG uptake in brain vs mean glucose in athymic nude mice. n = 40. Mean glucose is the average of pre- and postscan glucose. b Scatter plot of FDG uptake in myocardium vs percent change in glucose in Athymic nude mice. n = 40. Each panel includes the Pearson’s correlation coefficients (r), their corresponding p value (p) and slope (s). A low p value means there is a statistically significant relationship between percent change in glucose and K i. The dashed line corresponds to fitted line for the groups that underwent sevoflurane. The solid line corresponds to the fitted line of the groups under isoflurane.
Discussion
Blood Glucose Levels
Our data demonstrate that during a typical 30-min imaging procedure with athymic nude mice, light isoflurane anesthesia in air can decrease blood glucose levels significantly. This observation concurs with recent data from Dandekar et al. [8] who reported a progressive drop in blood glucose in mice anesthetized with 3% isoflurane in oxygen; after 30 min, the blood glucose had dropped by approximately 50% compared to the 14% reported here. The difference may be attributed to our use of isoflurane at a relatively low concentration (~2% vs 3%). The largest decrease in blood glucose occurred in mice carrying PC3 tumors anesthetized with isoflurane/air, a mean drop of more than 50% from baseline over the course of 30 min. We note that PC3 tumors reportedly secrete cachectic factors, and these might affect glucose metabolism systemically [19]. The cohort implanted with estradiol pellets and IGROV-1 tumors showed much greater variability in blood glucose levels compared to any other group, and this was consistent across the different anesthetic regimes. Estrogen is known to affect glucose through its effects on insulin metabolism, and this may directly account for this exaggerated variability or set up an increased sensitivity to other factors [20].
We have shown that the use of sevoflurane greatly attenuates the change in blood glucose, which accompanied the use of isoflurane. The decrease in blood glucose under isoflurane/air could be moderated in Balb/c mice by using oxygen as an alternative carrier gas, but oxygen is not always available or desirable from a safety or experimental design standpoint. It is also desirable to have independent control of the oxygen tension for studies such as the elegant imaging of Chan et al., who used different inhaled gases and FDG-PET to determine the effects of blood oxygen levels on tumor metabolism [21]. Such studies may benefit from anesthesia with sevoflurane because the oxygen tension of the inhaled gas could be varied independently with fewer metabolic complications arising from isoflurane-induced changes in blood glucose levels.
Published data have shown that blood glucose levels are higher in fed than in fasted mice [6, 22]. We considered that these higher blood glucose levels might be experimentally acceptable, even desirable, if they were more stable under anesthesia. However, preliminary experiments suggested that there was no such compensatory benefit; blood glucose drops under isoflurane even in unfasted animals (data not shown).
FDG Tissue Uptake
The combined effect of the anesthetic agent and carrier gas revealed some potentially useful differences in FDG uptake in the myocardium, which has been associated with spillover effects interfering with detection and complicating quantitation of adjacent structures such as lung tumors or the blood pool of the left ventricle [23, 24]. Isoflurane tends to elevate myocardial FDG uptake above the levels seen in conscious animals [25], so the lower uptake seen under sevoflurane is perhaps consistent with the notion that sevoflurane causes less metabolic perturbance than isoflurane and the knowledge that the myocardium can switch rapidly between glucose and other energy sources under hormonal influences with insulin levels being the key [26]. There are reports of cardioprotective effects of volatile anesthetics, possibly mediated by interactions with adenosine triphosphate-dependent potassium channels [27], and one might speculate that this kind of direct cardiac action could add to the effects of hormonal changes in causing the different FDG uptake patterns observed under the different anesthetic regimes.
The inverse relationship between tumor FDG uptake and blood glucose levels in the extreme case of clamped hyperglycemia (500 mg/dL) has been previously demonstrated in rat models [28]. Our results from mice in the normo- to hypoglycemic range show some statistically significant impacts of the anesthetic regime on tumor FDG uptake and uptake rates (Fig. 4). Carrier gas had a statistically significant effect on the glucose uptake rate in the HCT116 tumors, being lower under oxygen, possibly a Pasteur effect, while the anesthetic agent seemed to have the greater effect upon PC3 tumors, the uptake rate being lower under isoflurane. No discernible relationship was observed for the IGROV-1 tumors, possibly because the measurement variability was much greater in these tumors. Given the highly variable nature of xenograft tumors, it is difficult to draw general conclusions other than that tumor FDG uptake can indeed be significantly influenced by the anesthetic regime and blood glucose levels, so appropriate care should be taken in designing quantitative studies of tumor metabolism, e.g., for measuring response to therapy.
Early observations led us to consider the effect of anesthesia on the Harderian glands, which are also responsible for spillover effects, in this case confounding attempts to quantify FDG uptake in adjacent areas of the brain [29]. Although the lowest uptake was observed under sevoflurane/oxygen, the variability was relatively high, and none of the trends observed turned out to have statistical significance. Further work is required to identify an anesthetic regime that reliably minimizes FDG uptake into the Harderian glands.
Blood Glucose Levels and their Correlations with FDG Uptake
In scatter plots of tissue uptake versus plasma glucose levels, we observed in normal brain the expected strong negative correlation between FDG uptake rates (K i) and blood glucose levels in this tightly regulated obligate glucose-consuming tissue. The relationship between glucose concentration and FDG uptake is based on the competitive access to glucose transport. In the myocardium, the situation was more complex. Myocardial FDG uptake trended toward an inverse correlation with the plasma glucose levels, but this effect did not reach statistical significance (data not shown). However, unlike the brain, there was a very strong correlation between FDG uptake and the percentage change in glucose levels but only under isoflurane. This would be explained if the transport competition was occurring against a background of endocrine (presumably insulin) changes increasing the tissue capacity for glucose (and FDG) transport. This phenomenon has been recognized in clinical applications of FDG PET imaging where oral glucose loading is often used to give an (seemingly paradoxical) increase in myocardial FDG uptake [30, 31].
Adoption of Sevoflurane in the Small Animal Imaging Setting
The clinical use of sevoflurane has expanded at the expense of isoflurane in recent years because it has a number of advantages. Sevoflurane generates fewer hepatotoxic metabolites and has a more rapid induction and recovery profile [12], and it is less stressful because sevoflurane has a relatively pleasant aroma, which does not elicit a cough reflex or irritate the mucous membranes [32]. These benefits may also be relevant to small animal imaging, particularly in fragile models of human disease such as stroke, chronic heart failure, myocardial infarction, or lung tumor burden where our experience has been that even light isoflurane anesthesia can be accompanied by serious adverse events.
One present disadvantage of sevoflurane is its higher cost. This may not be an obstacle for much longer, since price decreases might be expected now that generic versions may be available. This has been the experience with isoflurane, which 10 years ago cost about the same as sevoflurane does today. If sevoflurane/air is used in place of isoflurane/oxygen, then there is some compensatory cost saving to be made in the use of air and perhaps some logistical benefit if portable oxygen tanks are replaced by breathing air “on tap” from a compressor. Neither does vaporizer replacement have to be an obstacle to the adoption of sevoflurane. Existing isoflurane vaporizers can often be inexpensively modified and calibrated for use with sevoflurane, in contrast to the special equipment needed for some other modern agents such as desflurane.
We have not explored a wide range of anesthetic depths or durations nor all the carrier gas possibilities such as the nitrous oxide and oxygen mixtures, which have been reported to improve pulmonary gas exchange [33]. Further studies may explore the biochemical and hormonal basis for these empirical observations.
Conclusion
Sevoflurane may be preferred to isoflurane for routine anesthesia in mice undergoing small animal imaging, especially where blood glucose levels can affect the results such as in FDG-PET imaging. In athymic nude mice, sevoflurane improved the homeostasis of blood glucose; in Balb/c nude mice, it made the blood glucose effects independent of carrier gas. In both strains, sevoflurane reduced uptake in the myocardium. This could be especially beneficial in studies where the oxygen tension is varied or in PET studies where there are concerns about cardiac spillover into adjacent structures such as the cavity of the left ventricle or tumors in the lungs.
References
Patlak CS, Blasberg RG, Fenstermacher JD (1983) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 3(1):1–7
Schiffer WK, Mirrione MM, Dewey SL (2007) Optimizing experimental protocols for quantitative behavioral imaging with 18F-FDG in rodents. J Nucl Med 48(2):277–287
Bacharach SL, Sundaram SK (2002) 18F-FDG in cardiology and oncology: the bitter with the sweet. J Nucl Med 43(11):1542–1544
Lattermann R, Schricker T, Wachter U, Georgieff M, Goertz A (2001) Understanding the mechanisms by which isoflurane modifies the hyperglycemic response to surgery. Anesth Analg 93(1):121–127
Toyama H et al (2004) Absolute quantification of regional cerebral glucose utilization in mice by 18F-FDG small animal PET scanning and 2–14C-DG autoradiography. J Nucl Med 45(8):1398–405
Fueger BJ et al (2006) Impact of animal handling on the results of 18F-FDG PET studies in mice. J Nucl Med 47(6):999–1006
Loepke AW, McCann JC, Kurth CD, McAuliffe JJ (2006) The physiologic effects of isoflurane anesthesia in neonatal mice. Anesth Analg 102(1):75–80
Dandekar M, Tseng JR, Gambhir SS (2007) Reproducibility of 18F-FDG microPET studies in mouse tumor xenografts. J Nucl Med 48(4):602–607
Lindholm P et al (1993) Influence of the blood glucose concentration on FDG uptake in cancer—a PET study. J Nucl Med 34(1):1–6
Langen KJ et al (1993) The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med 34(3):355–359
Zeidler A, Tosco C, Kumar D, Slavin B, Parker J (1982) Spontaneous hyperglycemia and impaired glucose tolerance in athymic nude BALB/c mice. Diabetes 31(9):821–825
Sun Y et al (2006) High throughput modular chambers for rapid evaluation of anesthetic sensitivity. BMC Anesthesiol 6:13
Kim JS et al (2007) Performance measurement of the microPET focus 120 scanner. J Nucl Med 48(9):1527–1535
Li Q, Leahy RM (2006) Statistical modeling and reconstruction of randoms precorrected PET data. IEEE Trans Med Imaging 25(12):1565–1572
Botker HE et al (1999) Myocardial glucose uptake measured with fluorodeoxyglucose: a proposed method to account for variable lumped constants. J Nucl Med 40(7):1186–1196
Crane PD, Pardridge WM, Braun LD, Oldendorf WH (1983) Kinetics of transport and phosphorylation of 2-fluoro-2-deoxy-D-glucose in rat brain. J Neurochem 40(1):160–167
Muzi M et al (2001) Kinetic characterization of hexokinase isoenzymes from glioma cells: implications for FDG imaging of human brain tumors. Nucl Med Biol 28(2):107–116
Green LA et al (1998) Noninvasive methods for quantitating blood time-activity curves from mouse PET images obtained with fluorine-18-fluorodeoxyglucose. J Nucl Med 39(4):729–734
Wang Z et al (2003) Expression of the human cachexia-associated protein (HCAP) in prostate cancer and in a prostate cancer animal model of cachexia. Int J Cancer 105(1):123–129
Muraki K, Okuya S, Tanizawa Y (2006) Estrogen receptor alpha regulates insulin sensitivity through IRS-1 tyrosine phosphorylation in mature 3T3-L1 adipocytes. Endocr J 53(6):841–851
Chan LW et al (2006) The influence of tumor oxygenation on 18F-FDG (fluorine-18 deoxyglucose) uptake: a mouse study using positron emission tomography (PET). Radiat Oncol 1:3
Lee KH et al (2005) Effects of anesthetic agents and fasting duration on 18F-FDG biodistribution and insulin levels in tumor-bearing mice. J Nucl Med 46(9):1531–1536
Wu HM et al (1996) Derivation of input function from FDG-PET studies in small hearts. J Nucl Med 37(10):1717–1722
Ferl GZ, Zhang X, Wu HM, Huang SC (2007) Estimation of the 18F-FDG input function in mice by use of dynamic small-animal PET and minimal blood sample data. J Nucl Med 48(12):2037–2045
Meyer PT et al (2006) Simplified quantification of small animal [18F]FDG PET studies using a standard arterial input function. Eur J Nucl Med Mol Imaging 33(8):948–954
Calvani M, Reda E, Arrigoni-Martelli E (2000) Regulation by carnitine of myocardial fatty acid and carbohydrate metabolism under normal and pathological conditions. Basic Res Cardiol 95(2):75–83
Kohro S, Hogan QH, Nakae Y, Yamakage M, Bosnjak ZJ (2001) Anesthetic effects on mitochondrial ATP-sensitive K channel. Anesthesiology 95(6):1435–340
Wahl RL, Henry CA, Ethier SP (1992) Serum glucose: effects on tumor and normal tissue accumulation of 2-[F-18]-fluoro-2-deoxy-D-glucose in rodents with mammary carcinoma. Radiology 183(3):643–647
Kuge Y et al (1997) Positron emission tomography for quantitative determination of glucose metabolism in normal and ischemic brains in rats: an insoluble problem by the Harderian glands. J Cereb Blood Flow Metab 17(1):116–120
Berry JJ et al (1991) The effect of metabolic milieu on cardiac PET imaging using fluorine-18-deoxyglucose and nitrogen-13-ammonia in normal volunteers. J Nucl Med 32(8):1518–1525
Knuuti MJ et al (1992) Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med 33(7):1255–1262
Doi M, Ikeda K (1993) Airway irritation produced by volatile anaesthetics during brief inhalation: comparison of halothane, enflurane, isoflurane and sevoflurane. Can J Anaesth 40(2):122–126
Agarwal A, Singh PK, Dhiraj S, Pandey CM, Singh U (2002) Oxygen in air (FiO2 0.4) improves gas exchange in young healthy patients during general anesthesia. Can J Anaesth 49(10):1040–1043
Acknowledgements
We would like to thank Michel Friesenhahn, Jed Ross and Jeff Tinianow for their help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flores, J.E., McFarland, L.M., Vanderbilt, A. et al. The Effects of Anesthetic Agent and Carrier Gas on Blood Glucose and Tissue Uptake in Mice Undergoing Dynamic FDG-PET Imaging: Sevoflurane and Isoflurane Compared in Air and in Oxygen. Mol Imaging Biol 10, 192–200 (2008). https://doi.org/10.1007/s11307-008-0137-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-008-0137-4