Abstract
Neuroimaging studies have provided a major contribution to our understanding of the mechanisms of the placebo effect in neurological and psychiatric disorders. Expectation of symptom improvement has long been believed to play a critical role in the placebo effect, and is associated with increased endogenous striatal dopamine release in Parkinson’s disease and increased endogenous opioid transmission in placebo analgesia. Evidence from positron emission tomography and functional magnetic resonance imaging studies suggests that expectations of symptom improvement are driven by frontal cortical areas, particularly the dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortices. The ventral striatum is involved in the expectation of rewarding stimuli and, together with the prefrontal cortex, has also been shown to play an important role in the placebo-induced expectation of therapeutic benefit. Understanding the mechanisms of the placebo effect has important implications for treatment of several medical conditions, including depression, pain, and Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroimaging has made invaluable contributions to our understanding of neurological disorders, particularly in the field of Parkinson’s disease (PD). Through its ability to specifically tag the dopaminergic system, positron emission tomography (PET) has been used extensively to track the progression of the disease, detect presymptomatic individuals, and to increase our general understanding of the function of the monoaminergic systems underlying voluntary movement, cognition, and mood. The ability to quantitatively image dopamine function in the human brain has driven scientific research forward into areas that had been previously untapped. One such example is the placebo effect, which remained a largely clinical phenomenon until the neurochemical mechanisms could begin to be elucidated through the use of PET and functional magnetic resonance imaging (fMRI). Such techniques have significantly advanced our understanding of the placebo effect in the fields of PD, pain, and depression, and have provided important insights into the brain processes involved in the way we integrate environmental contexts, cognition, and affective self-regulation. The present article reviews the contributions of neuroimaging to our understanding the placebo effect in neurological and psychiatric conditions, with emphasis on PD and the role of expectation and the dopamine system.
Dopamine and the Neuropathology of PD
Dopamine neurons projecting to the forebrain are classified into three different systems according to their terminal projections [1–3]: from the ventral tegmentum to the frontal cortex (mesocortical system); to limbic areas including the amygdala, nucleus accumbens, and hypothalamus, among others (mesolimbic system); and to the striatum, comprising the putamen and caudate nucleus (nigrostriatal system). Dopamine released in these different terminal regions may be associated with functions as diverse as working memory, learning, motivation, addictive behaviors, reward processing, and voluntary movement. Disrupted dopamine function can result in parkinsonism, loss of incentive salience, addictive behavior, and psychosis. In PD, the primary deficit involves the selective degeneration of nigrostriatal dopamine-producing cells (although at the later stages of disease, other dopamine projections, as well as other neurotransmitter systems, may also be affected), and is therefore mainly characterized by alterations in the control and execution of voluntary salient movement. Given the critical role of dopamine in modulating the corticostriatothalamocortical circuits of the basal ganglia [4, 5], the dopamine depletion that occurs in PD results in aberrant processing in these circuits, the overall impact of which is a reduced thalamic drive to the motor cortex, which results in difficulties initiating movement (akinesia), bradykinesia (slowness of movement), rigidity, tremor at rest, and postural instability. PD pharmacotherapy aims at replacing the lost dopamine by either direct means using dopamine precursors, which are enzymatically converted into dopamine (levodopa), or synthetic agonists acting at dopamine receptors.
The Placebo Effect In PD: Clinical Results
The placebo effect can be defined as any “genuine psychological or physiological effect which is attributable to receiving a substance or undergoing a procedure, but is not due to the inherent powers of that substance or procedure” (adapted from Stewart-Williams and Podd [6]). Any sort of treatment can act as a placebo, but it is the response of the patient (either positive or negative) to that treatment that determines whether or not a placebo effect has occurred. The magnitude of the placebo effect itself is related to the type of placebo administered; the greater the potency of treatment, the greater the placebo effect [7]. For example, placebo surgery seems to be more effective than a placebo pill [8–10] and, as a recent study for arthroscopic knee surgery suggested, may produce the same outcome as the actual surgical procedure [11]. Placebo effects have been documented throughout the course of history in a wide variety of medical disorders, including depression, pain, and many others [10], and given the lack of specificity of the different (and frequently bizarre) remedies, it could be said that the history of medicine is, in fact, the history of the placebo effect [12].
Substantial placebo effects occur in PD that are, for the most part, detected in placebo-controlled trials aimed at testing new pharmacological, surgical, or physical therapies. For example, in a double-blind trial of pergolide, significant improvement with respect to baseline was seen in both the pergolide-treated group (30% after 24 weeks) and the placebo group (23% after 24 weeks) [13]. In the large clinical trial of deprenyl and tocopherol antioxidative therapy of parkinsonism, 21% of patients demonstrated a blinded, investigator-determined, “objective” improvement in motor function during placebo therapy over six months [14]. Goetz and colleagues reported that 14% of the patients enrolled in a six-month, randomized, placebo-controlled clinical trial of ropinirole monotherapy achieved a 50% improvement in motor function while on placebo treatment [15]. In this particular study, all domains of parkinsonism were subject to the placebo effect, but bradykinesia and rigidity—those features of PD which are best correlated to dopamine function—tended to be more susceptible than tremor, gait, or balance. Finally, in a metareview, Shetty and colleagues [16] demonstrated that 12 of 36 articles reported a 9–59% improvement in PD patient motor symptoms following placebo treatment.
The importance of including a placebo group when investigating the efficacy of surgical procedures for treating PD has been emphasized [17] but remains a source of controversy [18–20]. In a recent study on the effect of intrastriatal implantation of fetal porcine ventral mesencephalic tissue to treat PD [21], the degree of motor performance improvement at 18 months was substantial, but was the same in the sham group. In one multicenter, randomized, double-blind, sham surgery-controlled study of human fetal transplantation for Parkinson’s [22], there was no significant clinical benefit of the transplant compared to sham surgery, although pilot studies performed using an identical technique had demonstrated substantial benefit [23]. Indeed, in another study of human fetal transplantation, both subjective and objective (blinded examiner) outcomes were better predicted by which treatment the patient thought s/he was assigned to rather than the actual treatment assignment [24, 25].
Clinical results such as these have provided the impetus for experiments that aim to study the placebo effect itself. These studies face significant ethical and logistical challenges as, to study the placebo effect (as opposed to detecting it in a standard clinical trial), deliberate deception must often be used to maintain the expectation of improvement in the subject. PD is an excellent model in which to study the placebo effect due to the ability to objectively measure the motor responses in these patients using standard clinical neurological tests by a blinded examiner [15, 26]. For example, Mercado and colleagues (2006) demonstrated that patients with subthalamic nucleus deep-brain stimulation (STN-DBS) as treatment for PD had a greater degree of improvement in their motor performance, as measured by the Unified PD Rating Scale, when they thought that their stimulators were turned on, and performed even worse when they thought that their stimulators were off, compared to the conditions in which they were blind to stimulator function [27]. Benedetti and colleagues used standard clinical measures to demonstrate that sham STN-DBS can improve bradykinesia [28–30], and also that saline given in the guise of apomorphine can reduce rigidity [31] in patients conditioned to the effects of the active medication. However, it is equally important to emphasize that the clinical scales used for measuring motor function are subjective themselves, and patients may also be less prone to report clinical changes than the clinicians are to observe them [24], which adds another dimension of subjectivity.
The Placebo Effect in PD: Imaging Results
Neuroimaging has been critical in establishing a clear physiological basis for the placebo effect in PD, and placebo studies represent an example of how imaging can enable significant strides to be made into areas where most previous investigation had depended on conjecture based on clinical observation. Presynaptic extracellular dopamine release can be indirectly estimated using [11C]-raclopride PET [32–34], and several studies have used this technique to measure endogenous dopamine release in response to pharmacological challenges [35–40], drugs of abuse [41–43], drug-related cues [44], metabolic stress [45], and monetary reward [46, 47] in both healthy and disease states. Most relevant to PD, Piccini and colleagues (2003) demonstrated endogenous striatal dopamine in response to methamphetamine in advanced PD patients, indicating that it is possible for these patients to release endogenous dopamine, albeit to a lesser degree than healthy controls [48].
Using [11C]-raclopride PET, de la Fuente-Fernandez et al. (2001) demonstrated that a placebo could induce the release of endogenous dopamine in the striatum of PD patients [49]. In this study, patients underwent four PET scans and were aware that they would be receiving an injection of active drug (apomorphine, a dopamine receptor agonist) for three of the scans and a placebo for another scan, but they were not told the scan order. The investigators found a substantial dopamine release in response to placebo (approximately 17% decrease in [11C]-raclopride binding, corresponding to a change of 200% or more in extracellular dopamine concentration and comparable to the response to amphetamine in subjects with an intact dopamine system) (Fig. 1). Furthermore, the dopamine release in the motor areas of the striatum (putamen and dorsal caudate) was greater in those patients who reported clinical improvement (i.e., who perceived a placebo effect). In a recent related study, Strafella and colleagues (2006) also used [11C]-raclopride PET to demonstrate striatal dopamine release in response to sham repetitive transcranial magnetic stimulation (rTMS) in PD patients. In this study, patients underwent two PET scans, one baseline scan in which no rTMS was used, and a placebo scan, where they were told that they had a 50/50 chance of receiving either real rTMS or sham rTMS, but in all cases received the sham treatment. Interestingly, those authors found that the changes in [11C]- raclopride binding were greater in the hemisphere contralateral to the more affected side, particularly in the putamen [50]. Although the patients who perceived clinical benefit had a slightly higher amount of dopamine release in the dorsal and ventral striatum, this difference failed to reach statistical significance. Taken together, these results indicate that the biochemical basis for the placebo response in PD is to replace the depleted dopamine in those striatal areas that are responsible for motor symptoms, leading to clinical improvement. These results are corroborated by an electrophysiology study performed in PD patients undergoing STN-DBS surgery, in which it was shown that a placebo evoked a decrease in mean neuronal firing frequency and a shift to nonbursting activity in the STN, which was highly correlated with a reduction in upper-limb rigidity [31]. It is likely that the change in STN neuron firing is a downstream effect of placebo-induced dopamine release in the caudate and putamen.
[11C]raclopride PET scans of a patient with PD at baseline and following injection of saline (placebo). Decreased [11C]raclopride binding to D2 receptors in the striatum after placebo in the After placebo image indicates tracer displacement by placebo-induced endogenous dopamine release. Reprinted from Mercado et al. (2006) [27] with permission.
Expectation and the Placebo Effect
What remains unclear is how this biochemical placebo response (i.e., dopamine release) is produced in the first place. The importance of expectation in placebo effects has long been recognized, and a prominent theory of the mechanism of the placebo effect is that it is driven by the expectation of therapeutic benefit. Through prior experience with a particular treatment, expectations are generated about the resulting physical response to that treatment, in what Kirsch has termed “response expectancy” [51], which is proposed to be central in producing the physiological placebo effect. In our previous work, we used a paradigm in which the patients expected apomorphine for three out of four scans; thus, they knew that their chance of receiving active drug was 75% for each scan. Although all patients in the study showed biochemical placebo responses, only half of the patients reported placebo-induced motor improvement. Those patients also released larger amounts of dopamine in the dorsal striatum, suggesting a relationship between the amount of dorsal striatal dopamine release and perceived clinical benefit. However, this relationship was not seen in the ventral striatum, where all patients displayed increased dopamine release regardless of whether they felt any improvement as a result of placebo administration (Fig. 2) [52]. Compared to the dorsal striatum, which is involved in voluntary movement, the ventral striatum is classically associated with motivation [53, 54], goal-directed behavior [55], and reward anticipation [56–60]. The investigators concluded that the dopamine released in the ventral striatum was associated with the patients’ expectation of improvement in their symptoms, which could in turn be considered a form of reward. Thus, dopamine release in the ventral striatum can be seen as necessary but not sufficient for the placebo effect to occur. This is in keeping with other studies, in which ventral striatal dopamine release is better correlated with “drug wanting” than the perceived subjective effects of the drug [38, 61]. In the Strafella (2006) study, patients had a 50% expectation of receiving real rTMS, and also demonstrated increased dopamine release in the ventral striatum. Thus, both studies used a significant component of expectation in the paradigms and a dopaminergic response was seen in the ventral striatum that occurred independently of the benefit felt by the subjects. Several other clinical studies have also demonstrated the importance of expectation to the placebo effect in PD [25, 28, 29, 49, 52, 62].
Placebo-induced changes in [11C]raclopride (RAC) binding potential (BP) in the ventral (nucleus accumbens, Acc) and dorsal (caudate nucleus, Caud; putamen, Put) striatum of six patients with PD. The changes represent the difference in RAC BP between baseline (B) and post-placebo (P) values (i.e., B–P). In the nucleus accumbens, there were no differences in placebo-induced RAC BP changes between patients who perceived a clinical benefit after placebo injection (solid bars, n = 3) and those who did not (open bars, n = 3) (p = 0.23). In contrast, both in the caudate nucleus and in the putamen, this biochemical placebo effect was greater in patients who reported placebo-induced clinical benefit than in those without (p < 0.05 for the caudate and p < 0.01 for the putamen). Reprinted from Berridge and Robinson (1998) [53] with permission from Elsevier.
The Mesolimbic Dopamine System and Reward Expectation
Among its diverse roles in the brain, dopamine is strongly associated with reward processing. Dopamine neurons that project to the ventral striatum increase phasic, burst firing in response to natural (i.e., food, liquid, and sex) and drug rewards; intense, novel stimuli; and stimuli with rewarding or attentional properties [63, 64]. They are also able to track reward learning, such that once an animal has learned the association between a reward-predicting stimulus and reward delivery, the dopamine cell firing shifts to the stimulus (i.e., in anticipation of reward delivery), and if the reward is then omitted, the dopamine neurons reduce firing at the time when the reward should have occurred [64, 65]. Thus, dopamine neurons fire in response to unexpected rewards but they do not respond physically to predictable rewards, providing the basis for the hypothesis that phasic dopamine signals occur not in response to the reward itself, but instead to the reward prediction error [58, 66, 67], which is the discrepancy between the predicted reward and the actual occurrence of the reward. Dopamine neurons also demonstrate a more sustained, tonic firing pattern during the reward expectation period [57], the magnitude of which varies with the probability that the reward will occur: neuron activity is maximal at p = 0.5 (when the uncertainty is the greatest), declines at p = 0.25 and p = 0.75, and is virtually zero at both extremes (i.e., p = 0 and 1) when the chance of reward is known with certainty [57]. These results were mirrored in a recent study in humans using fMRI; although dopamine neuron activity could not be assessed directly, midbrain blood-oxygen-level-dependent (BOLD) signals tracked the error prediction signal transiently and demonstrated more sustained activity that correlated with uncertainty [68]. Thus, through different profiles of neural activity, dopamine neurons have the capacity to code the occurrence, prediction, expectation, and uncertainty of reward-related stimuli as the organism learns to optimize its goal-directed behavior.
Dopamine release in the ventral striatum is particularly associated with reward prediction, and has been extensively demonstrated in animals [69–71]. The phasic firing of dopamine neurons occurs on the millisecond time scale, whereas the tonic firing occurs over seconds, or perhaps minutes, and so these properties of dopamine neurons are difficult to demonstrate in humans using the slower time resolution of most neuroimaging techniques. Despite these limitations, the expectation of rewarding stimuli has been associated with an increased BOLD signal in the ventral striatum as measured by fMRI in several studies [68, 72–76]. An fMRI study in human cocaine addicts showed increased BOLD signals in the nucleus accumbens during the preinfusion period for both saline and cocaine, indicating that the nucleus accumbens activity may reflect a computation of expectancy [72, 77]. A recent 2-deoxy-2-[F-18]fluoro-d-glucose (FDG)-PET study measured brain glucose metabolism during the expectation of receiving methylphenidate in drug-naive subjects, and reported that when subjects expected to receive methylphenidate but received placebo, significant metabolic increases were seen in the ventral cingulate gyrus and nucleus accumbens, and the effect was largest in subjects who had not yet experienced the active drug, suggesting the involvement of these areas in processing expectation for what the authors termed “uncertain drug effects” [78]. This possibility is supported by a wealth of neuroimaging studies indicating increased activity of the ventral striatum during the expectation of other types of rewards, including monetary [68, 73, 74, 76, 79–81], primary [82, 83], and drug rewards [77, 84]. Whether an increase in BOLD signal or glucose metabolism in the ventral striatum indicates an increase in dopamine release per se, or reflects an increase in glutamatergic transmission from cortical or limbic structures that may be modulated by dopamine, is unknown.
However, there are PET studies that have shed some light on the role of dopamine in reward processing. Reward-related increases in dopamine release have been shown in both the dorsal [35, 41, 46, 85, 86] and ventral [36, 38–40, 42, 46, 47, 86, 87] striatum, although it remains unclear as to what extent the functional roles within these structures can be dissociated, particularly because the neural response to reward varies with the experimental paradigm, type of rewarding stimulus used, and personal experience of the subject. That being said, ventral striatal dopamine release has been demonstrated using [11C]-raclopride PET in response to monetary rewards [46, 47], drugs of abuse [42, 86, 87], and psychostimulants [36, 38–40], although the slow time resolution makes it difficult to tease apart the dopamine release related to the expectation of reward and to the reward itself. Despite this, the expectation of caffeine in habitual coffee drinkers was shown to increase dopamine release in the thalamus as estimated by a change in [11C]-raclopride binding [88], although the responses in the dorsal or ventral striatum did not reach significance.
Imaging the Placebo Effect in Pain
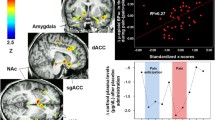
Prominent placebo effects occur in many disorders other than PD. Much of this research has occurred in the field of pain, where the investigator can recruit healthy subjects and induce various types of experimental pain. In fact, the first neurochemical evidence for the mechanism of the placebo effect was published in 1978, when it was shown that placebo analgesia could be blocked by naloxone, indicating that it was mediated by endogenous opioids [89]. Since then, several studies have further implicated endogenous opioids in the mechanism of placebo analgesia [90–93]. Zubieta and colleagues (2001) used displacement of the μ-opioid receptor antagonist PET tracer [11C] carfentanil to indirectly demonstrate endogenous opioid release during a sustained pain challenge in the anterior cingulate cortex (ACC), prefrontal cortex (PFC), insula, amygdala, thalamus, and nucleus accumbens [94]. In a later study [95], the same investigators examined the neural networks underlying placebo analgesia by administering placebo with the expectation of analgesia during the same pain challenge, and found endogenous opioid release in the rostral ACC, dorsolateral prefrontal cortex (DLPFC), anterior insula, and the nucleus accumbens (Fig. 3). In the high placebo responders, increased opioid transmission in the nucleus accumbens was positively correlated with the subjective change in pain intensity ratings and reductions in the negative affective ratings experienced during the pain challenge. In the DLPFC, μ-opioid system activation was negatively correlated with the magnitude of the expected analgesic effect of the placebo rated before placebo administration, suggesting that a reduction in opioid inhibitory control in this region has a permissive effect on the engagement of other pain control regions, such as the insula, ACC, thalamus, and/or midbrain [95]. These results substantiate those of an fMRI study that separated the neural activations underlying pain anticipation and experience [96]. The investigators used a well-established expectancy-manipulation paradigm [97–99] to enhance belief in the placebo by surreptitiously decreasing the level of thermal pain when a topical placebo cream was applied on the forearm. Placebo treatment substantially decreased the subjects’ reported pain and also the pain-related activity in the insula, contralateral thalamus, and ACC. During the expectation of analgesia, increased BOLD signal was observed in the DLPFC, orbitofrontal cortex (OFC), and ACC, as well as in the periaqueductal gray area (PAG) of the brainstem, and the PAG increases were positively correlated with DLPFC and OFC activation. Given that the PAG is an area strongly linked to the descending control of pain and the endogenous opioid system, these results suggest that opioid systems are engaged by prefrontal cortically driven expectations of analgesia [100]. These data echo results of a PET study measuring regional cerebral blood flow (rCBF) during thermal pain in which remifentanyl or placebo was given, and it was shown that both interventions increased rCBF in the OFC and ACC and that these increases covaried with rCBF increases in the brainstem (PAG, pons, and medulla) [101]. Finally, a recent study used an expectancy manipulation paradigm similar to that reported by Wager et al. (2004) to investigate the BOLD signal changes during heat pain before and after placebo acupuncture [102], and observed significant differences in the anterior insula, lateral PFC, rostral ACC, and the inferior parietal lobule. They also found a negative correlation between the activity in the lateral/orbital PFC, rostral ACC, cerebellum, pons, and right fusiform and parahippocampal gyri, and the corresponding difference in subjective pain ratings, indicating that the stronger the placebo analgesia (i.e., lower pain ratings), the greater the activity in these brain areas [102]. That these results contrast those of Wager et al. (2004), who found placebo-induced BOLD signal reductions in the thalamus, insula, and ACC during pain, highlights the important issue of the intrinsic variability of the placebo response; although both studies used similar expectation-enhancement procedures, they used different placebos (cream vs. acupuncture), different methodologies, and the subjects were given vastly differing instructions in different environments and thus had different expectations. In light of these findings, it reasonable to postulate that there is a spectrum of placebo analgesic effects, the underlying mechanisms of which are dictated by the environmental context and the experience of the individual during the experiment. In the case of placebo analgesia, these mechanisms can engage endogenous opioid [29, 92, 95, 103] and nonopioid [90, 93, 104] systems in varying degrees, depending on the experimental circumstances. However, as described, certain prefrontal cortical structures are involved consistently across placebo analgesia studies, including the superior medial PFC; midrostral dorsal anterior cingulate; and the dorsolateral, ventrolateral, and orbitofrontal cortices. Interestingly, these areas are frequently implicated in studies examining the voluntary regulation of affective responses [100].
Effects of placebo (saline injection) on the activation of μ-opioid receptor-mediated neurotransmission as measured by [11C]carfentanil-PET in 20–30-year-old, right-handed males (n = 14). Significant (p < 0.0001) effects of placebo on μ-opioid system activity were detected in the left dorsolateral prefrontal cortex (DLPFC), rostral ACC (RACing), left nucleus accumbens (Nacc), and right anterior insula (Ins) (p < 0.05) after correction for multiple comparisons. The posterior right insula achieved subthreshold levels of significance (p < 0.0001 uncorrected for multiple comparisons). Z scores of statistical significance are represented by the pseudocolor scale on the right and are superimposed over an anatomically standardized MRI image in coronal views. The left side of the axial and coronal images corresponds to the right side of the body (contralateral to pain). Reprinted from Zubieta et al. (2005) [95] with permission.
Imaging the Placebo Effect in Depression
Clinical trials of antidepressants have shown particularly strong placebo effects [105], which can in some cases be indistinguishable from those of the active drug [106]. Indeed, Kirsch and Sapierstein concluded from their meta-analysis of 19 trials of antidepressants that about 75% of the effectiveness of these drugs results from the placebo effect [107]. Detecting true placebo responses in depression is complicated by the natural waxing and waning of symptoms in some patients, the difficulties in measuring improvement using rating scales, and the unavoidable confound of selecting patients who have had multiple different treatments and thus bring expectations and learning with them into the study (e.g., they know that antidepressant medications require more than three weeks to take therapeutic effect) [100]. Despite these and other variables, some studies have successfully mapped out the placebo response in depressed patients. Mayberg and colleagues (2002) conducted an FDG-PET study that examined the brain regional glucose metabolism in response to fluoxetine or placebo treatment in a group of depressed men, where scans were acquired at baseline, one and six weeks following treatment. The PET data showed an overlap between the areas of metabolic change in the fluoxetine and placebo groups at six weeks, although the fluoxetine group had additional areas not seen in the placebo group (Fig. 4) [106, 108]. This metabolic pattern was completely different in patients who received cognitive behavioral therapy, indicating that the physiological placebo response closely matches the active drug response that it is designed to simulate [100] (and also that cognitive behavioral therapy is not simply a placebo). As discussed, this may also be the case in placebo analgesia [101] and in PD [49]. However, Leuchter and colleagues (2002) used quantitative electroencephalography (EEG) to demonstrate that, although depressed medication responders and placebo responders were virtually indistinguishable clinically, the placebo responders had changes in prefrontal cordance that were not seen in medication responders or in nonresponders (to either medication or placebo). This suggests that the placebo response may depend on altered prefrontal activity early in therapy and that the placebo response was not functionally equivalent to the drug response [109]. Thus, it remains unclear if placebo-derived improvements in depression share a common mechanism with the therapeutic effect of active treatment.
Changes in regional glucose metabolism as measured by FDG-PET in fluoxetine, placebo, and cognitive behavioral therapy (CBT) responders measured before and after a standard course of each respective treatment in depressed patients. Increases are in red and decreases are in blue. The fluoxetine and placebo groups were studied as part of the same double-blind controlled experiment [104, 106]. A pattern of cortical increases (prefrontal, parietal, and posterior cingulate cortices) and limbic-paralimbic decreases (subgenual cingulate) is shared by both groups, with the fluoxetine group showing additional changes in the brainstem, hippocampus, insula, and caudate. In contrast, the CBT response is associated with dorsolateral and medial frontal decreases and hippocampal increases. Subgenual cingulate BA 25 (ACing), posterior cingulate (PCing), pons (P), hippocampus (Hc), PFC BA 9 (PFC), anterior insula (Ins), caudate (Cau), orbital frontal cortex BA 11 (OFC), medial frontal cortex BA 9 (MFC). Reprinted from Benedetti et al. (2005) [100] with permission.
The effects of expectation of clinical improvement in depression have also been examined. In the study by Mayberg and colleagues (2002), at one week, before any clinical antidepressant effect was seen, both the fluoxetine and placebo groups demonstrated ventral striatal and OFC glucose metabolic changes (Fig. 5), which were not seen in those patients who were ultimately drug nonresponders. Because none of the patients in either group demonstrated any signs of clinical improvement at this time, the investigators interpreted these results as the expectation component of the subsequent antidepressant response [100]. These data are supported by a recent EEG study conducted in depressed subjects which demonstrated that a positive clinical outcome appeared to be predicted in part by decreases in prefrontal EEG cordance that occurred during the first week of the clinical trial during the placebo lead-in phase, in the absence of drug (venlafaxine or fluoxetine) treatment [110]. Although it is not possible to identify the specific brain areas involved, the authors suggested that early neurophysiological changes in prefrontal brain areas represent nonspecific changes that occur in response to the treatment environment, such as interactions with study personnel, pill administration, and structured assessments, shaping the expectations of the patient that have the capacity to influence the treatment outcome [110]. Thus, in depression, as well as in PD, expectation plays an important role in the placebo response, and likely plays an important role in active treatment as well.
Time course of regional metabolic changes in fluoxetine nonresponders (left), fluoxetine responders (middle), and placebo responders (right). Ventral striatal and orbital frontal increases are seen uniquely at one week (top panel, middle and right images) of both active and sham treatment in those patients that go on to show clinical response at six weeks. Such changes are not seen in patients who failed to respond (left image) and are no longer present in either group of responders once clinical remission has been achieved (six-week time point; bottom). In contrast, response-specific changes in PFC and subgenual cingulate are seen only at six weeks (bottom) and not at the one-week time point. Ventral striatum (VST), orbital frontal cortex (OFC), medial frontal cortex (MFC), subgenual cingulate BA 25 (ACing25), prefrontal cortex (PFC), anterior insula (Ins), anterior cingulate BA 24 (ACing24). Reprinted from Benedetti et al. (2005) [100] with permission.
Conclusion
It is clear that the mechanisms underlying placebo effects are beginning to be unraveled owing to our ability to image the human brain and quantify neurotransmission. Without these techniques, the placebo effect would likely continue to be considered a nuisance, obscuring the results of clinical trials. The placebo effect should be seen within the context of brain circuitry that enables semivoluntary control over affective and physiological responses [100]. Based on the evidence derived from PD, pain, and depression, it is clear that there is not one placebo effect but many, with different underlying mechanisms. However, they all have in common a component of expectation, which may well involve the DLPFC and dopaminergic activity in the ventral striatum. This could be considered a “permissive” component, integrating motivational and reward-expectation circuitry enabling the belief that there will be improvement in one’s symptoms [111]. This state of expectation, driven by prefrontal cortical and limbic areas, may in turn trigger a downstream biochemical response specific to the condition in question; in the case of PD, dopamine release in the dorsal striatum, and in placebo analgesia, endogenous opioid release. The degree of overlap between the mechanisms of placebo responses in different conditions is unknown, and it is likely that the placebo responses in most conditions involve the combined effects of many neurochemicals, including monoamines, opioids, serotonin, and hormones; however, it is not yet known with any certainty if this is the case. It is interesting to note that in two of the studies mentioned here, the magnitude and location of the biochemical placebo effect correlated with symptomatic improvement: (1) the dopamine release in the putamen of the dorsal striatum in the PD patients who perceived the most improvement in their motor functions [49] and (2) the increased endogenous opioid release in the ACC, insula, and nucleus accumbens in subjects who experienced relatively less pain by measures of intensity, unpleasantness, and affect [95]. This suggests that the placebo effect goes where it is most needed, or serves a protective or adaptive function based on the environmental context in which the subjects find themselves. This could explain the high variability of the placebo response in that it is tailored to reflect the perceived needs of the individual, which differ greatly among subjects. Despite this heterogeneity, which can only be controlled to some extent in clinical trials, it is remarkable that, based on the evidence to date, the neurochemical placebo effect appears to mirror the pharmacological effect which it is designed to mimic [100], as seen in Mayberg et al. (2002), Petrovic et al. (2002), and de la Fuente-Fernandez et al. (2001). This indicates a crucial role for the context, particularly the expectations generated by the environment, in the manifestation of placebo responses, whether it is a clinical trial or an experiment designed to study the placebo effect itself. As neuroimaging techniques continue to be refined and improved, researchers will continue to gain further insights into not only the mechanisms underlying the placebo effect, but also the larger fundamental processes of how we integrate environmental cues into the way we regulate our thoughts, emotions, and physiological state for our behavior and survival.
References
Lindvall O, Bjorklund A (1978) Anatomy of the dopaminergic neuron systems in the rat brain. Adv Biochem Psychopharmacol 19:1–23
Haber SN, Fudge JL (1997) The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol 11(4):323–342
Joel D, Weiner I (2000) The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 96(3):451–474
Haber SN (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26(4):317–330
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Stewart-Williams S, Podd J (2004) The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull 130(2):324–340
de Craen AJ, Tijssen JG, de Gans J, Kleijnen J (2000) Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol 247(3):183–188
Brody H (1980) Placebos and the philosophy of medicine: clinical, conceptual, and ethical issues. Chicago: The University of Chicago Press
Kaptchuk TJ, Goldman P, Stone DA, Stason WB (2000) Do medical devices have enhanced placebo effects? J Clin Epidemiol 53(8):786–792
Shapiro AK, Shapiro E (1997) The placebo: is it much ado about nothing? In: Harrington A (ed) The placebo effect: an interdisciplinary exploration. Cambridge: Harvard University Press, pp 12–36
Moseley JB, O’Malley K, Petersen NJ, et al. (2002) A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 347(2):81–88
de la Fuente-Fernandez R, Stoessl AJ (2002) The biochemical bases for reward. Implications for the placebo effect. Eval Health Prof 25(4):387–398
Diamond SG, Markham CH, Treciokas LJ (1985) Double-blind trial of pergolide for Parkinson’s disease. Neurology 35(3):291–295
Goetz CG, Leurgans S, Raman R (2002) Placebo-associated improvements in motor function: comparison of subjective and objective sections of the UPDRS in early Parkinson’s disease. Mov Disord 17(2):283–288
Goetz CG, Leurgans S, Raman R, Stebbins GT (2000) Objective changes in motor function during placebo treatment in PD. Neurology 54(3):710–714
Shetty N, Friedman JH, Kieburtz K, Marshall FJ, Oakes D (1999) The placebo response in Parkinson’s disease. Parkinson Study Group. Clin Neuropharmacol 22(4):207–212
Freeman TB, Vawter DE, Leaverton PE, et al. (1999) Use of placebo surgery in controlled trials of a cellular-based therapy for Parkinson’s disease. N Engl J Med 341(13):988–992
Macklin R (1999) The ethical problems with sham surgery in clinical research. N Engl J Med 341(13):992–996
Weijer C (2002) I need a placebo like I need a hole in the head. J Law Med Ethics 30(1):69–72
London AJ, Kadane JB (2002) Placebos that harm: sham surgery controls in clinical trials. Stat Methods Med Res 11(5):413–427
Watts RL, Freeman TB, Hauser RA, et al. (2001) A double-blind, randomised, controlled, multicenter clinical trial of the safety and efficacy of stereotaxic intrastriatal implantation of fetal porcine ventral mesencephalic tissue (Neurocell™-PD) vs. imitation surgery in patients with Parkinson’s disease (PD). Parkinsonism Relat Disord 7:S87
Olanow CW, Goetz CG, Kordower JH, et al. (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol 54(3):403–414
Hauser RA, Freeman TB, Snow BJ, et al. (1999) Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 56(2):179–187
Freed CR, Greene PE, Breeze RE, et al. (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344(10):710–719
McRae C, Cherin E, Yamazaki TG, et al. (2004) Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry 61(4):412–420
Fuente-Fernandez R, Schulzer M, Stoessl AJ (2002) The placebo effect in neurological disorders. Lancet Neurol 1(2):85–91
Mercado R, Constantoyannis C, Mandat T, et al. (2006) Expectation and the placebo effect in Parkinson’s disease patients with subthalamic nucleus deep brain stimulation. Mov Disord 21(9):1457–1461
Pollo A, Torre E, Lopiano L, et al. (2002) Expectation modulates the response to subthalamic nucleus stimulation in Parkinsonian patients. Neuroreport 13(11):1383–1386
Benedetti F, Pollo A, Lopiano L, et al. (2003) Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci 23(10):4315–4323
Benedetti F, Colloca L, Lanotte M, et al. (2004) Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain Res Bull 63(3):203–211
Benedetti F, Colloca L, Torre E, et al. (2004) Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci 7(6):587–588
Dewey SL, Smith GS, Logan J, et al. (1993) Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography in normal human subjects. Proc Natl Acad Sci U S A 90(24):11816–11820
Endres CJ, Kolachana BS, Saunders RC, et al. (1997) Kinetic modeling of [11C]raclopride: combined PET-microdialysis studies. J Cereb Blood Flow Metab 17(9):932–942
Breier A, Su TP, Saunders R, et al. (1997) Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A 94(6):2569–2574
Volkow ND, Wang GJ, Fowler JS, et al. (1999) Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2 receptors. J Pharmacol Exp Ther 291(1):409–415
Drevets WC, Gautier C, Price JC, et al. (2001) Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49(2):81–96
Volkow ND, Wang G, Fowler JS, et al. (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21(2):RC121
Leyton M, Boileau I, Benkelfat C, et al. (2002) Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology 27(6):1027–1035
Martinez D, Slifstein M, Broft A, et al. (2003) Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23(3):285–300
Oswald LM, Wong DF, McCaul M, et al. (2005) Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology 30(4):821–832
Schlaepfer TE, Pearlson GD, Wong DF, Marenco S, Dannals RF (1997) PET study of competition between intravenous cocaine and [11C]raclopride at dopamine receptors in human subjects. Am J Psychiatry 154(9):1209–1213
Brody AL, Olmstead RE, London ED, et al. (2004) Smoking-induced ventral striatum dopamine release. Am J Psychiatry 161(7):1211–1218
Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A (2004) The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse 54(2):65–71
Volkow ND, Wang GJ, Telang F, et al. (2006) Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26(24):6583–6588
Adler CM, Elman I, Weisenfeld N, et al. (2000) Effects of acute metabolic stress on striatal dopamine release in healthy volunteers. Neuropsychopharmacology 22(5):545–550
Zald DH, Boileau I, El Dearedy W, et al. (2004) Dopamine transmission in the human striatum during monetary reward tasks. J Neurosci 24(17):4105–4112
Koepp MJ, Gunn RN, Lawrence AD, et al. (1998) Evidence for striatal dopamine release during a video game. Nature 393(6682):266–268
Piccini P, Pavese N, Brooks DJ (2003) Endogenous dopamine release after pharmacological challenges in Parkinson’s disease. Ann Neurol 53(5):647–653
de la Fuente-Fernandez R, Ruth TJ, Sossi V, et al. (2001) Expectation and dopamine release: mechanism of the placebo effect in parkinson’s disease. Science 293(5532):1164–1166
Strafella AP, Ko JH, Monchi O (2006) Therapeutic application of transcranial magnetic stimulation in Parkinson’s disease: the contribution of expectation. Neuroimage 31(4):1666–1672
Kirsch I (1997) Specifying nonspecifics: psychological mechanisms of placebo effects. In: Harrington A (ed) The placebo effect: an interdisciplinary exploration. Cambridge: Harvard University Press, pp 166–186
de la Fuente-Fernandez R, Phillips AG, Zamburlini M, et al. (2002) Dopamine release in human ventral striatum and expectation of reward. Behav Brain Res 136(2):359–363
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28(3):309–369
Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev 31(1):6–41
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14(2–3):69–97
Apicella P, Scarnati E, Ljungberg T, Schultz W (1992) Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 68(3):945–960
Fiorillo CD, Tobler PN, Schultz W (2003) Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299(5614):1898–1902
Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275(5306):1593–1599
Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80(1):1–27
Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12(12):4595–4610
Evans AH, Pavese N, Lawrence AD, et al. (2006) Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol 59(5):852–858
Colloca L, Lopiano L, Lanotte M, Benedetti F (2004) Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol 3(11):679–684
Horvitz JC (2000) Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 96(4):651–656
Ljungberg T, Apicella P, Schultz W (1992) Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol 67(1):145–163
Mirenowicz J, Schultz W (1994) Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol 72(2):1024–1027
Montague PR, Dayan P, Sejnowski TJ (1996) A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16(5):1936–1947
Montague PR, Hyman SE, Cohen JD (2004) Computational roles for dopamine in behavioural control. Nature 431(7010):760–767
Dreher JC, Kohn P, Berman KF (2006) Neural coding of distinct statistical properties of reward information in humans. Cereb Cortex 16(4):561–573
Phillips AG, Blaha CD, Fibiger HC (1989) Neurochemical correlates of brain-stimulation reward measured by ex vivo and in vivo analyses. Neurosci Biobehav Rev 13(2–3):99–104
Garris PA, Kilpatrick M, Bunin MA, et al. (1999) Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature 398(6722):67–69
Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422(6932):614–618
Breiter HC, Rosen BR (1999) Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci 877:523–547
Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA (2000) Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84(6):3072–3077
Elliott R, Friston KJ, Dolan RJ (2000) Dissociable neural responses in human reward systems. J Neurosci 20(16):6159–6165
Pagnoni G, Zink CF, Montague PR, Berns GS (2002) Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci 5(2):97–98
Knutson B, Adams CM, Fong GW, Hommer D (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21(16):RC159
Beiter HC, Gollub RL, Weisskoff RM, et al. (1997) Acute effects of cocaine on human brain activity and emotion. Neuron 19(3):591–611
Volkow ND, Wang GJ, Ma Y, et al. (2006) Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage 32(4):1782–1792
Knutson B, Westdorp A, Kaiser E, Hommer D (2000) FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12(1):20–27
Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P (2001) Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30(2):619–639
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001) Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12(17):3683–3687
Berns GS, McClure SM, Pagnoni G, Montague PR (2001) Predictability modulates human brain response to reward. J Neurosci 21(8): 2793–2798
O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002) Neural responses during anticipation of a primary taste reward. Neuron 33(5):815–826
Risinger RC, Salmeron BJ, Ross TJ, et al. (2005) Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage 26(4):1097–1108
Small DM, Jones-Gotman M, Dagher A (2003) Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 19(4):1709–1715
Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A (2004) The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse 54(2):65–71
Martinez D, Gil R, Slifstein M, et al. (2005) Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58(10):779–786
Kaasinen V, Aalto S, Nagren K, Rinne JO (2004) Expectation of caffeine induces dopaminergic responses in humans. Eur J Neurosci 19(8):2352–2356
Levine JD, Gordon NC, Fields HL (1978) The mechanism of placebo analgesia. Lancet ii:654
Gracely RH, Dubner R, Wolskee PJ, Deeter WR (1983) Placebo and naloxone can alter post-surgical pain by separate mechanisms. Nature 23(306):264–265
Levine JD, Gordon NC (1984) Influence of the method of drug administration on analgesic response. Nature 312(5996):755–756
Benedetti F (1996) The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain 64(3):535–543
Amanzio M, Benedetti F (1999) Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 19(1):484–494
Zubieta JK, Smith YR, Bueller JA, et al. (2001) Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293(5528):311–315
Zubieta JK, Bueller JA, Jackson LR, et al. (2005) Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci 25(34):7754–7762
Wager TD, Rilling JK, Smith EE, et al. (2004) Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303(5661):1162–1167
Voudouris NJ, Peck CL, Coleman G (1989) Conditioned response models of placebo phenomena: further support. Pain 38(1):109–116
Montgomery GH, Kirsch I (1997) Classical conditioning and the placebo effect. Pain 72(1–2):107–113
Price DD, Milling LS, Kirsch I, et al. (1999) An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain 83(2):147–156
Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK (2005) Neurobiological mechanisms of the placebo effect. J Neurosci 25(45):10390–10402
Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia—imaging a shared neuronal network. Science 295(5560):1737–1740
Kong J, Gollub RL, Rosman IS, et al. (2006) Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci 26(2):381–388
Benedetti F, Arduino C, Amanzio M (1999) Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci 19(9):3639–3648
Colloca L, Benedetti F (2005) Placebos and painkillers: is mind as real as matter? Nat Rev Neurosci 6(7):545–552
Walsh BT, Seidman SN, Sysko R, Gould M (2002) Placebo response in studies of major depression: variable, substantial, and growing. JAMA 287(14):1840–1847
Mayberg HS, Silva JA, Brannan SK, et al.(2002) The functional neuroanatomy of the placebo effect. Am J Psychiatry 159(5):728–737
Kirsch I, Sapierstein G (1998) Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medications. Prev Treat 1(6):2a
Mayberg HS, Brannan SK, Tekell JL, et al. (2000) Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48(8):830–843
Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M (2002) Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry 159(1):122–129
Hunter AM, Leuchter AF, Morgan ML, Cook IA (2006) Changes in brain function (quantitative EEG cordance) during placebo lead-in and treatment outcomes in clinical trials for major depression. Am J Psychiatry 163(8):1426–1432
Lidstone SC, de la Fuente-Fernandez R, Stoessl AJ (2005) The placebo response as a reward mechanism. Semin Pain Med 4(1):37–42
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11307-007-0098-z.
Rights and permissions
About this article
Cite this article
Lidstone, S.C.C., Stoessl, A.J. Understanding the Placebo Effect: Contributions from Neuroimaging. Mol Imaging Biol 9, 176–185 (2007). https://doi.org/10.1007/s11307-007-0086-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-007-0086-3