Abstract
Objectives
2-Deoxy-2-[F-18]fluoro-d-glucose (FDG)-positron emission tomography (PET)/computed tomography (CT) is widely available as a powerful imaging modality, combining the ability to detect active metabolic processes and their morphologic features in a single exam. The role of FDG-PET is proven in a variety of cancers, including melanoma, but the estimates of sensitivity and specificity are based in the majority of the published studies on dedicated PET, not PET/CT. Therefore, we were prompted to review our experience with FDG-PET/CT in the management of melanoma.
Methods
This is a retrospective study on 106 patients with melanoma (20–87 years old; average: 56.8 ± 15.9), who had whole-body FDG-PET/CT at our institution from January 2003 to June 2005. Thirty-eight patients (35.9%) were women and 68 patients (64.1%) were men. Reinterpretation of the imaging studies for accuracy and data analysis from medical records were performed.
Results
All patients had the study for disease restaging. The primary tumor depth (Breslow’s thickness) at initial diagnosis was available for 76 patients (71.7%) and ranged from 0.4 to 25 mm (average: 3.56 mm). The anatomic level of invasion in the skin (Clark’s level) was determined for 70 patients (66%): 3, level II; 13, level III; 43, level IV; 11, level V. The administered dose of 18F FDG ranged from 9.8 to 21.6 mCi (average: 15.4 ± 1.8 mCi). FDG-PET/CT had a sensitivity of 89.3% [95% confidence interval (CI): 78.5–95] and a specificity of 88% (95% CI: 76.2–94.4) for melanoma detection.
Conclusion
This study confirms the good results of FDG-PET/CT for residual/recurrent melanoma detection, as well as for distant metastases localization. PET/CT should be an integral part in evaluation of patients with high-risk melanoma, prior to selection of the most appropriate therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Melanoma is not a rare disease and the number of new melanomas diagnosed in the United States is increasing on a yearly basis: from 1973 to 1994, melanoma incidence and mortality increased 120% and 39%, respectively [1]. Approximately 59,580 new cases of invasive melanoma and 35,000 new cases of in situ melanoma are estimated to be diagnosed yearly. About 7,910 were expected to die in 2005 in the United States [2]. Long-term survival is excellent for patients diagnosed with in situ and early invasive disease, making an early diagnosis and accurate staging extremely important in the management of melanoma patients [3].
2-Deoxy-2-[F-18]fluoro-d-glucose (FDG)-positron emission tomography (PET)/computed tomography (CT) is becoming widely available as a powerful imaging modality, combining the ability to detect active metabolic processes and their morphologic features in a single exam. The role of FDG-PET is proven in a variety of cancers, including lymphoma, colorectal carcinoma, lung cancer, and melanoma, entities for which it changed the practice of oncology [4]. The current medical literature depicts excellent results for PET in melanoma, but the sensitivity and specificity calculations are based on dedicated PET acquisitions, not PET/CT in the majority of the published studies. However, there is evidence that PET/CT performs better than PET in various solid cancers [5]. Thus, we were prompted to review our experience with PET/CT in the management of patients with melanoma.
Materials and Methods
This is a retrospective study on 106 consecutive patients with melanoma, 20–87 years old (average: 56.8 ± 15.9), who had whole-body PET/CT at our institution from January 1, 2003 to June 30, 2005. Thirty-eight patients (35.9%) were women and 68 patients (64.1%) were men. The study was performed with the approval of the Institutional Review Board. The inclusion criteria were histopathologically proven diagnosis of melanoma and availability of PET/CT scans. Reports of PET/CT and pathology examinations were reviewed and their results were recorded. A joint Nuclear Medicine–Radiology readout assures the accuracy of the findings on the CT portion of the exams during routine interpretation of the PET/CT exams. Reinterpretation of the studies by board-certified Nuclear Medicine physicians was performed for consistency.
The FDG-PET/CT scans were acquired by using a Discovery LS PET/CT unit (GE Medical Systems, Milwaukee, WI). The patients fasted at least 6 hours before imaging and their blood glucose levels were less than 150 mg/dl at the time of the tracer injection. A standard dose of 15 mCi was prescribed for adult patients. Approximately 60 minutes after tracer administration, a CT scan (5 mm contiguous axial cuts) was obtained in four integrated multislice helical noncontrast CT, from top of the head to the ankles. The acquisition was obtained in helical mode, using 140 kV, 40 mA s and a 512 × 512 matrix size, acquiring a field of view (FOV) of 867 mm in 22.5 s. This CT-based scan was used for attenuation correction purposes and to help in anatomic localization of FDG. Immediately after the CT, an emission PET scan was acquired in 2D mode over the same anatomical regions starting at the level of the ankles for molecular/metabolic information. Acquisition time was 4 minutes per bed position (35 slices/bed) in eight beds, with a one-slice overlap at the borders of the FOV. PET emission scan was corrected by using segmented attenuation data of the CT scan. PET images were reconstructed with a standard iterative algorithm (OSEM, two iterative steps, 28 subsets) using GE software release 5.0. All images were reformatted into axial, coronal, and sagittal views and viewed with the software provided by the manufacturer (eNtegra, GE Medical Systems, Haifa, Israel).
Semiquantitative analysis of the FDG uptake in the suspected lesions was based on calculation of standard uptake value (SUV), defined as the ratio of activity per milliliter of tissue to the activity in the injected dose corrected by decay and per patient’s body weight. Precision is greater than three significant digits for maximum SUV (SUVmax) value [6]. Regions of interest were placed around the regions of increased FDG uptake for SUVmax determination. The cutoff SUVmax for malignant lesions was considered as 2.5.
Specificity and sensitivity for PET, CT, and PET/CT in detection of melanoma were calculated by using the pathology results (91.5% of the patients) or clinical follow-up (8.5% of the cases) as the gold standard, using a 2 × 2 contingency table. Confidence interval (CI) estimations were performed by using the Wilson score method [7].
Results
All patients had the study requested for disease restaging. The administered dose of FDG ranged from 9.8 to 21.6 mCi (average: 15.4 ± 1.8). The anatomic level of invasion in the skin (Clark’s level) was determined for 70 patients (66%): 3, level II; 13, level III; 43, level IV; 11, level V. The primary tumor depth (Breslow’s thickness) at initial diagnosis was available for 76 patients (71.7%) and ranged from 0.4 to 25 mm (average: 3.56 mm). Breslow’s depth was less than 1.0 mm in six patients: two patients had true negative PET/CT; one had false positive (reactive axillary lymphadenopathy) and false negative (the primary lesion was not seen) findings on PET/CT; and three patients had true positive lesions on PET/CT (sensitivity: 75%, specificity: 66.7%). Breslow’s thickness of 1.0–4.0 mm was present in 58 patients, resulting in 38 true positive, 28 true negative, four false positive, and three false negative lesions on PET/CT (sensitivity: 92.7%, specificity: 87.5%). A total of 12 patients had a Breslow’s depth of more than 4.0 mm and their PET/CT lesions were: 13 true positive; 3, true negative; 2, false positive; 3, false negative (sensitivity: 81.3%, specificity: 60%).
There were 30 patients with advanced malignant melanoma: four had stage IIIc and 26 had stage IV disease. All PET/CT scans were performed for restaging after therapy. In this subgroup, the disease was detected with PET/CT (four had widespread metastases) in 20 patients, whereas 10 patients had negative scans. PET/CT had a sensitivity of 100% (95% CI: 82.4–100) and a specificity of 83.3% (95% CI: 55.2–95.3).
Using pathology reports (91.5% of the patients) or clinical follow-up (8.5% of the cases) as gold standard, the sensitivity and specificity of PET, CT and PET/CT were calculated for all patients included in this patient population (106 patients). PET was 89.5% sensitive (95% CI: 78.9–95.1) and 81.6% specific (95% CI: 68.6–90.1), while CT was 68.5% sensitive (95% CI: 55.3–79.3) and 94.2% specific (95% CI: 84.4–98.1). The combined PET/CT had a sensitivity of 89.3% (95% CI: 78.5–95) and a specificity of 88% (95% CI: 76.2–94.4) for melanoma detection on a per-patient basis. FDG-PET/CT findings were true positive in 50 patients, false positive in six patients, false negative in six patients, and true negative in 44 patients. PET/CT detected residual melanoma in seven patients (6.6%), metastases in 34 patients (32%), and widespread metastatic disease in nine patients (8.5%).
In the per-lesion analysis, FDG-PET/CT had a sensitivity of 89.6% (95% CI: 81.5–94.5) and a specificity of 84.6% (95% CI: 72.5–91.9) for melanoma detection. There were 78 true positive lesions, eight false positive lesions, nine false negative lesions, and 44 true negative scans in the studied population. Of the eight false positive lesions, four were reactive lymph nodes, three were in areas of postsurgical changes and one was a granulomatous lung nodule. In the group of false negative results, six were recurrences at the resection site, two were subcentimeter soft tissue nodules and one was a brain lesion.
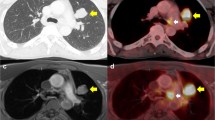
The estimated sensitivities and specificities of PET/CT in melanoma are presented in Fig. 1, based on Breslow’s depth, and per patient and per lesion analysis. Fig. 2 presents widespread metastases on the PET/CT scan of a 43-year-old woman with melanoma, Clark level IV, Breslow depth 2.6 mm at diagnosis. Pulmonary (arrowheads) and left adrenal (arrow) metastases are seen in Fig. 3 on the PET/CT scan of a 61-year-old woman with melanoma, Clark level IV, Breslow depth 2.2 mm at diagnosis. Another patient, a 25-year-old woman with melanoma, Clark level V, Breslow depth 5 mm at diagnosis, had liver metastases (arrowheads) noted on PET/CT and are presented in Fig. 4. The residual lesion shown in Fig. 5 on a PET/CT scan of a 51-year-old man with melanoma, Clark level IV, Breslow depth 2.7 mm at diagnosis, was negative on histopathological examination.
Discussion
Our study confirms FDG-PET/CT as an adequate imaging modality for melanoma patients, with high estimated values for sensitivity both on per patient and per lesion analysis. Patient selection in reference to PET/CT is important for the results of the scan. Thus, the best performance (100% sensitivity, 83.3% specificity) was achieved in patients with advanced disease (stages IIIc and IV), where four of the 30 patients had unexpected widespread metastases and disease management was changed from surgery to chemotherapy. PET/CT had excellent results (92.7% sensitive, 87.5% specific) in patients with Breslow’s depth of 1.0–4.0 mm, whereas the specificity decreased to 66.7% and 60% in patients with Breslow’s depth of less than 1.0 mm or more than 4.0 mm. Bias in patient selection is one of the limitations of our study, but this is difficult to overcome in retrospective reviews.
Various recommendations were proposed for baseline imaging studies in melanoma. The Swiss Society of Dermatology and Venereology recommended in 2005 different approaches for patients with a primary cutaneous malignant melanoma of Breslow’s tumor thickness more than 1.0 mm, but less than 4.0 mm (ultrasound examination of the regional lymph nodes and abdomen; chest radiograph at baseline and annually for 5 years) and those with Breslow’s thickness more than 4.0 mm and/or lymph node metastases (ultrasound examination of the regional lymph nodes, whole-body PET, CT of the chest and abdomen at baseline, and yearly for 5 years) [8]. These guidelines, as well as thorough physical examination and sentinel lymph node scintigraphy (SLNS), were used by Hafner et al. [9] in a prospective study on 100 patients. There were no patients with detectable and confirmed distant metastases at the time of enrollment in their patient population, suggesting that the value of whole-body staging at baseline remains limited. The authors conclude that baseline staging in primary cutaneous malignant melanoma should encompass a thorough physical examination and ultrasound of the regional lymph nodes [9].
The clinical impact of FDG-PET in various cancers, including melanoma, was predicted and described as early as 1991 [10]. Review articles propose the use of PET for detecting distant metastatic and recurrent melanoma, while recognizing its limited use in patients with early-stage disease [11]. In another review, Friedman and Wahl [12] conclude that FDG is the modality of choice for evaluating patients who fit into one of four categories: (1) individuals with a high risk for distant metastases based on extent of locoregional disease; (2) patients with findings that are suspicious for distant metastases; (3) individuals with known distant tumor deposits who still stand to benefit from customized therapies if new lesions are discovered or treated lesions regress; and (4) patients at high risk for systemic relapse who are considering aggressive medical therapy. Although FDG-PET is excellent in the detection of melanoma metastases, limitations exist with respect to detection of small lung nodules and brain metastases, which are better evaluated by CT and magnetic resonance imaging (MRI), respectively [12]. In our study, one of the false negative cases was a brain lesion missed by PET/CT, but identified on MRI.
In a prospective randomized trial of 144 patients (139 had primary cutaneous melanoma and five had local recurrent melanoma), Wagner et al. [13] noted that PET scanning did not impact the care of patients with early-stage melanoma already staged with standard techniques and thus is not recommended for the initial staging in this population. One of the major limitations of FDG-PET noted in the published studies is the detection of regional lymph node metastasis, probably as a result of the spatial resolution of this technology. Fink et al. [14] compared PET and SLNS in patients with primary malignant melanoma stages I and II. Of the 48 patients included in their study, eight (16.7%) had a positive biopsy of SLN. PET was positive in only one patient with a positive SNB, yielding a sensitivity of 13%. Their conclusion is that PET is not an adequate screening test for subclinical and sonographically inconspicuous lymph node metastases in patients with malignant melanoma stage I and II [14]. Similar results are reported by Havenga et al. [15]; melanoma metastases were found in the SLN of 13 of the 53 patients and PET detected the lymph node metastases in two of the 13 patients with SLN metastases. The prevalence of distant metastases is too small to justify routine use of PET [15]. In our experience (Quan V et al., Sentinel lymph node imaging and FDG-PET in the staging of melanoma, SNM Annual Meeting, Toronto, Canada, Jun 18–23, 2005), PET does not match SLNS for local lymph node metastases detection, but distant metastases were identified in three of the 21 patients included in the study.
A prospective trial on 18 patients with stage IV metastatic melanoma prior to attempted metastectomy compared PET and conventional imaging (CI: CT and MRI). Both had similar sensitivities (79% for PET, 76% for CI) and specificities (87% for both) on a lesion-by-lesion analysis. The combination of PET and CI resulted in an increased accuracy and PET has the benefit of being a real whole-body imaging study [16]. Gulec et al. [17] investigated PET prospectively in 49 patients with metastatic melanoma. The results of PET led to treatment changes in 49% of their patients. They conclude that PET provides more accurate assessment of extent of disease in patients with malignant melanoma, when compared to conventional imaging [17]. The impact of FDG-PET on surgical management of melanoma patients was investigated by Bastiaannet et al. [18]. In a retrospective review of 257 patients with stage III and IV melanoma and PET scans, 21.8% of the patients were upstaged as a result of PET findings and in 17.1% the treatment was changed from surgery to systemic therapy [18]. In an interesting approach, Wong et al. [19] tried to evaluate the impact of PET in the management of melanoma from the referring physician’s perspective; based on the responses received (51 questionnaires, 35% response rate), PET had a major impact and resulted in management changes in 53% of patients with melanoma [19].
Other retrospective trials report sensitivities of 74–92% and specificities of 86–97% for detection of metastasis in patients with stage III and IV malignant melanoma. According to these reports, PET shows greater ability to detect soft tissue, abdominal, and lymph node metastases than conventional imaging, but CT is better for pulmonary parenchymal melanoma detection, with PET appearing to be a useful adjunct in this instance [20–22]. Thus, it appears logical that a combination of PET and CT in a single exam should improve diagnostic accuracy, and this is the reason behind our retrospective review of melanoma patients studied with PET/CT. The sensitivities and specificities that we report for PET/CT (89.3% and 88% in the per-patient analysis, 89.6% and 84.6% in the per-lesion analysis) are similar to the published data. The addition of CT improved the specificity from 81.6% (95% CI: 68.6–90.1) for PET to 88% (95% CI: 76.2–94.4) for PET/CT. Colocalization of FDG uptake on PET to small lymph nodes or soft tissue nodules on CT brought added confidence to image interpretation in our study. However, small pulmonary nodules may be below the spatial resolution of PET and have no abnormal FDG uptake; they shall not be labeled benign in patients with melanoma.
The pattern of metastatic spread of disease is highly unpredictable, and PET/CT as a true whole-body examination (top of the skull to feet) appears as a very useful imaging modality for an accurate staging in patients with malignant melanoma. Further progress of the PET technology to improve spatial resolution and sensitivity and possibly new tracers may allow for better disease detection in less advanced stages of this disease.
Conclusions
This study confirms the good results of FDG-PET/CT for restaging melanoma patients, in particular patients with advanced disease (stages III and IV). The addition of CT increases specificity of PET/CT over PET alone, and adds diagnostic data for small lesions, such as subcentimeter pulmonary nodules, lymph nodes, and soft tissue nodules. Brain MRI remains important for detection of brain metastases. PET/CT should be an integral part in evaluation of patients with high-risk melanoma, prior to selection of the most appropriate therapy.
References
Hall HI, Miller DR, Rogers JD, Bewerse B (1999) Update on the incidence and mortality from melanoma in the United States. J Am Acad Dermatol 40(1):35–42, Jan
Jemal A, Murray T, Ward E, et al. (2005) Cancer statistics. CA Cancer J Clin 55(1):10–30, Jan–Feb
Lange JR, Balch CM (2005) Screening for cutaneous melanoma. Surg Oncol Clin N Am 14(4):799–811, Oct
Gambhir SS (2002) Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer 2(9):683–693, Sep
Antoch G, Saoudi N, Kuehl H, Dahmen G, Mueller SP, Beyer T, et al. (2004) Accuracy of whole-body dual-modality fluorine-18-2-fluoro-2-deoxy-d-glucose positron emission tomography and computed tomography (FDG-PET/CT) for tumor staging in solid tumors: comparison with CT and PET. J Clin Oncol 22(21):4357–4368, Nov 1
Sugawara Y, Zasadny KR, Neuhoff AW, Wahl RL (1999) Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology 213(2):521–525, Nov
Newcombe RG (1998) Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17(22):2635–2650, Nov 30
Dummer R, Panizzon R, Bloch PH, et al. (2005) Updated Swiss guidelines for the treatment and follow-up of cutaneous melanoma. Dermatology 210(1):39–44
Hafner J, Schmid MH, Kempf W, et al. (2004) Baseline staging in cutaneous malignant melanoma. Br J Dermatol 150(4):677–686, Apr
Strauss LG, Conti PS (1991) The applications of PET in clinical oncology. J Nucl Med 32(4):623–648, Apr
Kumar R, Alavi A (2005) Clinical applications of fluorodeoxyglucose-positron emission tomography in the management of malignant melanoma. Curr Opin Oncol 17(2):154–159, Mar
Friedman KP, Wahl RL (2004) Clinical use of positron emission tomography in the management of cutaneous melanoma. Semin Nucl Med 34(4):242–253, Oct
Wagner JD, Schauwecker D, Davidson D, et al. (2005) Inefficacy of F-18 fluorodeoxy-d-glucose-positron emission tomography scans for initial evaluation in early-stage cutaneous melanoma. Cancer 104(3):570–579, Aug 1
Fink AM, Holle-Robatsch S, Herzog N, et al. (2004) Positron emission tomography is not useful in detecting metastasis in the sentinel lymph node in patients with primary malignant melanoma stage I and II. Melanoma Res 14(2):141–145, Apr
Havenga K, Cobben DC, Oyen WJ, et al. (2003) Fluorodeoxyglucose-positron emission tomography and sentinel lymph node biopsy in staging primary cutaneous melanoma. Eur J Surg Oncol 29(8):662–664, Oct
Finkelstein SE, Carrasquillo JA, Hoffman JM, et al. (2004) A prospective analysis of positron emission tomography and conventional imaging for detection of stage IV metastatic melanoma in patients undergoing metastasectomy. Ann Surg Oncol 11(8):731–738, Aug
Gulec SA, Faries MB, Lee CC, et al. (2003) The role of fluorine-18 deoxyglucose positron emission tomography in the management of patients with metastatic melanoma: impact on surgical decision making. Clin Nucl Med 28(12):961–965, Dec
Bastiaannet E, Oyen WJ, Meijer S, et al. (2006) Impact of [(18)F]fluorodeoxyglucose positron emission tomography on surgical management of melanoma patients. Br J Surg 93(2):243–249, Feb
Wong C, Silverman DH, Seltzer M, et al. (2002) The impact of 2-deoxy18Ffluoro-d-glucose whole body positron emission tomography for managing patients with melanoma: the referring physician’s perspective. Mol Imaging Biol 4(2):185–190, Mar
Swetter SM, Carroll LA, Johnson DL, Segall GM (2002) Positron emission tomography is superior to computed tomography for metastatic detection in melanoma patients. Ann Surg Oncol 9(7):646–653, Aug
Fuster D, Chiang S, Johnson G, Schuchter LM, Zhuang H, Alavi A (2004) Is 18F-FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? J Nucl Med 45(8):1323–1327, Aug
Harris MT, Berlangieri SU, Cebon JS, Davis ID, Scott AM (2005) Impact of 2-deoxy-2[F-18]fluoro-d-glucose positron emission tomography on the management of patients with advanced melanoma. Mol Imaging Biol 23:1–5, Jul
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iagaru, A., Quon, A., Johnson, D. et al. 2-Deoxy-2-[F-18]fluoro-d-glucose Positron Emission Tomography/Computed Tomography in the Management of Melanoma. Mol Imaging Biol 9, 50–57 (2007). https://doi.org/10.1007/s11307-006-0065-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-006-0065-0