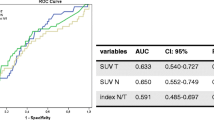
Abstract
Objective
This study was conducted to assess the effect of breast density, age, and menopausal status on the 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) uptake in normal breast tissue by quantitative standardized uptake values (SUV).
Methods
A total of 96 patients (premenopausal 54; postmenopausal 42) with histologically proven unilateral breast cancer who underwent FDG-positron emission tomography (PET) scans for staging were included in this study. The median age was 52±11 years (range 32–79 years). Fifty-nine patients had grade III or IV mammographic density (dense breast), whereas 37 patients had grade I or II breast density (nondense) according to the ACR Lexicon criteria. In the present study, we analyzed maximum and average SUVs for contralateral normal breast.
Results
Maximum and average SUVs for normal dense breasts were 1.02±0.30 and 0.84±0.27, respectively. Similar values for the nondense breasts were 0.66±0.24 and 0.53±0.23, respectively. Both maximum and average SUVs of dense breasts were significantly higher than those of nondense breasts (p<0.001). There was no significant difference in SUVs of nipple in patients with dense and nondense breasts. There was no significant effect of age and menopausal status on SUVs of normal breast. However, there were trends of negative relationship, i.e., decreasing SUVs with increasing age.
Conclusion
There was a significant difference in SUVs between the dense and nondense normal breast. However, the maximum SUVs in the dense breasts were well below the threshold of 2.5, a widely used cutoff value for malignancy. Menopausal status and age do not significantly affect the uptake of FDG.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most commonly diagnosed form of cancer among women in the United States. In 2005, it is estimated that 211,240 new cases of breast cancer will be diagnosed among women and 40,410 patients will die from breast cancer in the United States [1]. Although its incidence has increased over the past decades, the overall mortality from this disease has declined in recent years [2]. Screening with conventional mammography along with physical examination is a sensitive method for the early detection of breast cancer and has also been shown to decrease associated mortality [3–7]. However, mammography does have several limitations in clinical practice. Mammography is moderately sensitive for detecting breast lesions but has a low positive predictive value at approximately 36% [8]. In addition, diagnosis can be difficult in young women with dense breasts, in those with implants, and after surgery or irradiation to the breast tissue [9]. Moreover, the sensitivity of mammography for detecting breast cancer declines significantly with increasing breast density [8, 10]. Finally, increasing mammographic density is associated with a higher false-positive rate [11]. 2-Deoxy-2-[F-18]fluoro-d-glucose (FDG)-positron emission tomography (PET) imaging has been proposed as a diagnostic modality for improved detection of breast cancer and is shown to be highly accurate in characterizing palpable breast lesions [12–14]. One of the important advantages of FDG-PET over other imaging techniques is that it can provide a very high measure of contrast between normal and malignant tissues. FDG-PET has been compared with scintimammography and it was determined that FDG-PET is superior in identifying involved axillary lymph nodes and equivalent in identifying primary breast lesions [15]. Additionally, FDG-PET has been well established in differentiating between benign and malignant tumors including breast cancer and is also of value in evaluating loco-regional spread and distant metastasis [16, 17].
Similar to mammography, breast density may also have an effect on the interpretation of FDG-PET. This was confirmed for the first time in a retrospective study by Vranjesevic et al. [18]. Because dense breasts contain more cells per volume, one may expect an overall higher uptake of FDG in these tissues, which could make the diagnosis of breast malignancies more difficult. In our own experience, we have noticed that patients with clinically dense breasts tend to have increased FDG uptake on PET [19]. However, the authors in both studies enrolled a small number of patients. Any study with larger sample will be helpful in improved understanding of confounding factors, and investigating the discrimination value of increased FDG uptake would have implications on the accurate diagnosis of breast cancer in PET imaging. Therefore, the present study was aimed at assessing the relationship of standardized uptake value (SUV) in normal breast with age, breast density, and hormonal status.
Materials and Methods
Patient Population
A total of 96 patients (premenopausal 54; postmenopausal 42) with histologically proven unilateral breast cancer underwent multimodality imaging techniques such as utrasonography, digital mammography, computed tomography (CT), magnetic resonance imaging (MRI), and FDG-PET as a component of an NIH-funded project for characterizing the primary breast lesion and locoregional staging. In the present study, we retrospectively analyzed maximum and average SUVs for contralateral normal breast (48 left and 48 right breast). Informed consent was obtained from all patients. None of the patients had received chemotherapy or radiation therapy before undergoing a PET scan. The median age was 52±11 years (range 32–79 years). Fifty-nine patients had grade III or IV mammographic density (dense breast) whereas 37 patients had grade I or II breast density (nondense) according to the ACR Lexicon criteria. Among the 37 patients with nondense breasts, 35 had grade II and two had grade I breast density. All the mammographic studies were obtained within 4 weeks before to the FDG-PET scan. To analyze the relationship between age and SUV, patients were divided into four groups (I: ≤40 years, II: 41–50 years, III: 51–60 years and IV: ≥61 years).
FDG-PET Imaging
PET imaging was performed using a dedicated whole body PET scanner (Allegro, Philips Medical System, Philadelphia, PA, USA; C-PET, ADAC UGM, Philadelphia, PA). The patients fasted for at least 4 hours and serum glucose levels were <140 mg/dl in all patients. Immediately before the PET scan acquisition, all patients were asked to empty their bladder as they were imaged without an indwelling urinary catheter. PET scanning was initiated 60 minutes after intravenous administration of 2.5–5.2 MBq (0.068–0.14 mCi)/kg of FDG. Sequential overlapping scans were acquired to cover the neck, chest, abdomen, and pelvis. Transmission scans using a Cs137 point source were interleaved between the multiple emissions scans to correct for nonuniform attenuation. The images were reconstructed by using an iterative reconstruction algorithm, and both attenuation-corrected and nonattenuation-corrected images were interpreted.
Image Analysis
Two nuclear medicine physicians independently analyzed the data for this purpose. The SUVs were identical in 96% of cases with both observers. When there was difference, a mean was taken to determine the final SUV. The SUVs for normal breast tissue were calculated from the contralateral normal breast in all 96 patients with unilateral lesions. After image reconstruction, a region of interest (ROI) consisting of 12×12 mm (9 pixels) was carefully drawn on the consequent four to six PET scan slices in the normal breast at the same level as that of the lesion in contralateral abnormal breast. Average and maximum SUVs were calculated for each ROI. From these ROIs, the SUV was calculated according to the formula described below.
where ‘MBq’ = a Mega-Becquerel, and ‘g’ = grams.
Maximum and average SUVs for the normal breast were calculated from the mean values of 4–6 slices.
Statistical Analysis
Descriptive statistics were used to summarize the baseline clinical profile/data. Paired t test was used to obtain the mean significant difference among values of uptake between the left and right breasts, whereas unpaired t test was applied to assess the mean significant difference among the uptake between premenopausal and postmenopausal, and dense and nondense breasts. One-way analysis of variance (ANOVA) with post-hoc analysis was used to determine the mean significant difference among the values of uptake in various categories of age (≤40, 41–50, 51–60, and ≥61 years). Bivariate Pearson correlation was used to examine the linear relationship between various quantitative variables. In addition, stepwise multiple regression analysis was carried out to assess the effect of several independent covariates such as age, breast density, menopausal status, etc., on dependent (outcome) variable uptake. A p value of less than 0.05 was considered as a significant level. All statistical analyses were carried out by using statistical software SAS 8.2.
Results
The mean values of maximum and average SUVs for normal breast parenchyma were 0.88±0.32 and 0.72±0.29, respectively. Similar values for the normal tissue in the nipple region were 0.82±0.24 and 0.70±0.22, respectively. The highest maximum SUV for normal breast parenchyma and nipple was 1.8 and 1.4, respectively. There was no significant difference in SUVs between breast parenchyma and nipple.
Relationship of SUV with Breast Density
Maximum and average SUVs for normal tissue of dense breasts were 1.02±0.30 and 0.84±0.27, respectively. Similar values for the nondense breasts were 0.66±0.24 and 0.53±0.23, respectively. There was significant difference in maximum and average SUVs of breast parenchyma in patients with dense and nondense breasts (p≤0.001; Figs. 1 and 2). Maximum and average SUVs for nipple of dense breasts were 0.85±0.20 and 0.77±0.24, respectively. Similarly, the values were 0.76±0.29 and 0.57±0.21, respectively, for nondense breasts. There was no significant difference in SUVs of nipple in patients with dense and nondense breasts. The relationship of SUVs with breast density is shown in graphs (Figs. 1 and 2). No significant difference was noted in maximum and average SUVs among right and left breasts as well as right and left nipple.
Relationship of SUV with Age
The mean values of maximum and average SUVs for breast parenchyma and nipple in four different age groups are given in Table 1. There was no significant difference in maximum and average SUVs of breast parenchyma and nipple in patients of different age groups. However, there were trends of negative relationship, i.e., decreasing SUVs with increasing age (Figs. 3, 4, 5, and 6).
Relationship of SUV with Menopausal Status
The mean values of maximum and the average SUVs for right breast, left breast, right nipple, and left nipple in patients with premenopausal, perimenopausal, and postmenopausal status are given in Table 2. There was no significant difference in maximum and average SUVs of breast parenchyma and nipple in patients with different menopausal status. Stepwise multiple regressions revealed that breast density was the only significant predictor for FDG uptake in the normal breasts. Age and menopausal status did not emerge as significant predictors for FDG uptake in these patients.
Typical breast mammograms and corresponding transverse PET slices of four density categories are given in Fig. 7.
Discussion
Conventional screen-film mammography has limited sensitivity for detection of breast cancer especially in breasts with dense fibroglandular tissue [8]. Digital mammography was developed to address some of the limitations of screen-film mammography. However, accuracy of digital mammography is not substantially different from that of screen-film mammography [20]. FDG-PET has been shown to be highly useful for management of breast cancer [21]. Increased breast density is considered an independent risk factor for developing breast cancer. Recent studies reported the impact of breast density on FDG uptake in women with normal breast tissue [18, 19]. Vranjesevic et al. [18] retrospectively studied 45 women who had undergone whole-body FDG-PET for indications other than breast cancer. The study concluded that breast density and menopausal status affect the uptake of FDG, with average and peak SUVs significantly higher in dense versus fatty breasts (p<0.01). Our preliminary results also revealed that the maximum and the average SUVs of normal dense breasts were significantly higher than those of normal nondense breasts (p=0.003 for both) [19].
The present study evaluated the relationship of SUVs in normal breast with age, breast density, and menopausal status. For this purpose, analysis of data of 96 patients showed that normal tissue of dense breasts had significantly higher maximum and average uptake of FDG compared to nondense breasts (p<0.001 for both). Compared to the results gathered by Vranjesevic et al. [18], we report slightly higher peak and average SUVs in both dense and nondense breasts. The higher SUVs in the present study are most likely attributable to the difference in defining the ROI between the two studies. The mean of maximum SUVs for both dense and nondense breast was 1.02 and 0.66 for breast parenchyma, and 0.85 and 0.76 for the nipple, respectively. These higher SUVs in dense breasts are more attributable to fibrogladular tissue in dense breasts as compared to nondense breasts. The highest maximum SUVs for all normal breast tissues were below 1.8 for breast parenchyma and below 1.4 for nipple. These maximum SUVs of breast parenchyma and nipple are considerable lower than the threshold of 2.5, a widely used cutoff value for malignancy. Thus, although our results show that dense breasts had a significantly higher uptake of FDG than nondense breasts, a lesion with a SUV of 2.5 or higher should still be easily delineated from the surrounding tissue, indicating that the accuracy of FDG-PET in the diagnosis of breast malignancies should not be significantly affected in patients with dense breasts. However, with the significantly higher SUV in normal dense breast parenchyma, it remains to be seen if higher background FDG uptake in patients with dense breast has lower sensitivity in day-to-day reporting of FDG-PET studies in breast cancer. This increased background activity in dense breast may affect visual interpretation of the reader; however, it will not effect the quantitative (SUV) interpretation. Therefore, we feel that SUV threshold used for calling malignancy need not be adjusted according to the patient’s breast density.
The association between age and mammographic breast density has been well documented in the literature, with younger women tending to have denser breasts than older women [22–25]. Based on this, one may expect to see lower SUVs in normal breast tissue with increasing age. Our results did not show any significant difference in maximum and average SUVs of breast parenchyma and nipple in patients of different age groups, which is similar to the results reported by Vranjesevic et al. [18]. However, there were trends of decreasing SUVs with increasing age, which might become significant with larger sample size of patients with different age groups. We also did not find any significant impact of menopausal status on SUVs, as no significant difference was noted among premenopausal and postmenopausal patients. This finding is contrary to the results of Vranjesevic et al. [18], which showed significant effect of hormonal status on SUV uptake. They demonstrated that postmenopausal women receiving hormonal replacement therapy (HRT) had peak and average SUVs similar to those of premenopausal women, whereas the SUVs of postmenopausal women not receiving HRT were significantly lower. This can be explained by the fact that in the present study we have not classified postmenopausal patients based on the HRT as we have not had data of HRT status in many patients. However, we feel that further studies with large sample sizes may be helpful in settling this issue.
In our results, the SUVs were unadjusted for lean body mass or total body surface area, given that corrected and uncorrected SUVs were shown to be highly correlated by a regression analysis in the prior study [18]. Moreover, although correction for lean body mass or body surface area is generally important for proper standardization of FDG uptake, it does not correlate well in tissues with lower uptake values, where mean SUV is ca. 1 [26]. Thus, there is likely no need to correct for lean body mass when analyzing normal breast tissue, because the standardized FDG uptake values in these tissues are generally below 1.
Conclusions
There was a significant difference in SUVs between the dense and nondense normal breast. However, the maximum SUVs in dense breasts were well below the threshold of 2.5, a widely used cut-off value for malignancy. Menopausal status and age do not significantly affect the uptake of FDG.
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Surveillance, Epidemiology, and End Results (SEER) Program www.seer.cancer.gov) SEER Statistics Database: Incidence—SEE (1973–2000), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch
Rosenberg RD, Hunt WC, Williamson MR, Gilliland FD, Wiest PW, Kelsey CA, et al. (1998) Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology 209:511–518
Baines CJ, Miller AB, Wall C, McFarlane DV, Simor IS, Jong R, et al. (1986) Sensitivity and specificity of first screen mammography in the Canadian National Breast Screening Study: a preliminary report from five centers. Radiology 160:295–298
Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S (1993) Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst 85:1644–1656
Tabar L, Fagerberg G, Chen HH, Duffy SW, Smart CR, Gad A, et al. (1995) Efficacy of breast cancer screening by age. New results from the Swedish Two-County Trial. Cancer 75:2507–2517
Frisell J, Klund G, Hellstrom L (1991) Randomized study of mammography screening: preliminary report on mortality in the Stockholm trial. Breast Cancer Res Treat 18:49–56
Kolb TM, Lichy J, Newhouse JH (2002) Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 225:165–175
Salvatore M, Del Vecchio S (1998) Dynamic imaging: scintimammography. Eur J Radiol 27 Suppl 2:S259–264
Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. (2000) Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 92:1081–1087
Lehman CD, White E, Peacock S, Drucker MJ, Urban N (1999) Effect of age and breast density on screening mammograms with false-positive findings. AJR 173:1651–1655
Nieweg OE, Kim EE, Wong WH (1993) Positron emission tomography with fluorine-18-deoxyglucose in the detection and staging of breast cancer. Cancer 71:3920–3925
Hoh CK, Schiepers C (1999) 18-FDG imaging in breast cancer. Semin Nucl Med 29:49–56
Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL (1993) Evaluation of breast masses and axillary lymph nodes with [F-18]2-deoxy-2-fluoro- d-glucose PET. Radiology 187:743–750
Palmedo H, Bender H, Grunwald F, Mallmann P, Zamora P, Krebs D, et al. (1997) Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and technetium-99m methoxyisobutylisonitrile scintimammography in the detection of breast tumours. Eur J Nucl Med 24:1138–1145
Avril N, Dose J, Janicke F, Bense S, Ziegler S, Laubenbacher C, et al. (1996) Metabolic characterization of breast tumors with positron emission tomography using F-18 fluorodeoxyglucose. J Clin Oncol 14:1848–1857
Alavi A, Kung JW, Zhuang H (2004) Implications of PET based molecular imaging on the current and future practice of medicine. Semin Nucl Med 34:56–69
Vranjesevic D, Schiepers C, Silverman DH, Quon A, Villalpando J, Dahlbom M, et al. (2003) Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med 44:1238–1242
Kumar R, Schnall MD, Alavi A (2004) 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med 45:1423–1424
Pisano ED, Yaffe MJ (2005) Digital mammography. Radiology 234:353–362
Kumar R, Alavi A (2004) Fluorodeoxyglucose-PET in the management of breast cancer. Radiol Clin North Am 42:1113–1122
Ciatto S, Zappa MA (1993) A prospective study of the value of mammographic patterns as indicators of breast cancer risk in a screening experience. Eur J Radiol 17:122–125
Flook D, Gilhome RW, Harman J, Gravelle IH, Webster DJ (1987) Changes in Wolfe mammographic patterns with aging. Br J Radiol 60:455–456
Wolfe JN (1976) Breast parenchymal patterns and their changes with age. Radiology 121:545–552
Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V (1996) Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA 276:33–38
Zasadny KR, Wahl RL (1993) Standardised uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction. Radiology 189:847–850
Acknowledgments
This work was supported by Public Health Services Research Grant M01-RR00040 from NIH. Rakesh Kumar, M.D., was financially supported by UICC (International Union Against Cancer) Geneva, Switzerland under ACSBI fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, R., Chauhan, A., Zhuang, H. et al. Standardized Uptake Values of Normal Breast Tissue with 2-Deoxy-2-[F-18]Fluoro-d-glucose Positron Emission Tomography: Variations with Age, Breast Density, and Menopausal Status. Mol Imaging Biol 8, 355–362 (2006). https://doi.org/10.1007/s11307-006-0060-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-006-0060-5