Abstract
Introduction
Chronic kidney disease (CKD) is an important cause of disability and death, but its pathogenesis is poorly understood. Plasma metabolites can provide insights into underlying processes associated with CKD.
Objectives
To clarify the relationship of plasma metabolites with CKD and renal function in human.
Methods
We used a targeted metabolomics approach to characterize the relationship of 450 plasma metabolites with CKD and estimated glomerular filtration rate (eGFR) in 616 adults, aged 38–94 years, who participated in the Baltimore Longitudinal Study of Aging.
Results
There were 74 (12.0%) adults with CKD. Carnitine, acetylcarnitine, propionylcarnitine, butyrylcarnitine, trigonelline, trimethylamine N-oxide (TMAO), 1-methylhistidine, citrulline, homoarginine, homocysteine, sarcosine, symmetric dimethylarginine, aspartate, phenylalanine, taurodeoxycholic acid, 3-indolepropionic acid, phosphatidylcholines (PC).aa.C40:2, PC.aa.C40:3, PC.ae.C40:6, triglycerides (TG) 20:4/36:3, TG 20:4/36:4, and choline were associated with higher odds of CKD in multivariable analyses adjusting for potential confounders and using a false discovery rate (FDR) to address multiple testing. Six acylcarnitines, trigonelline, TMAO, 18 amino acids and biogenic amines, taurodeoxycholic acid, hexoses, cholesteryl esters 22:6, dehydroepiandrosterone sulfate, 3-indolepropionic acid, 2 PCs, 17 TGs, and choline were negatively associated with eGFR, and hippuric acid was positively associated with eGFR in multivariable analyses adjusting for potential confounders and using a FDR approach.
Conclusion
The metabolites associated with CKD and reduced eGFR suggest that several pathways, such as the urea cycle, the arginine-nitric oxide pathway, the polyamine pathway, and short chain acylcarnitine metabolism are altered in adults with CKD and impaired renal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chronic kidney disease (CKD), which affects 8–16% of adults worldwide, is an important cause of disability and death (Jha et al. 2013). Complications of progressive CKD include increased all-cause and cardiovascular mortality, cardiovascular disease, cognitive decline, anemia, mineral and bone disorders, and fractures (Jha et al. 2013). In 2017, an estimated 1.2 million people worldwide died from CKD (GBD Chronic Kidney Disease Collaboration 2020). Diabetes, metabolic syndrome, and hypertension are leading causes of CKD (Jha et al. 2013). CKD can progress to end-stage renal disease and require costly renal replacement therapies.
Asymptomatic CKD accounts for 80–90% of all cases (Jha et al. 2013) and is often difficult to detect (Gagnebin et al. 2018). As a consequence, many adults have undiagnosed CKD (Centers for Disease Control and Prevention 2019). The identification of novel biomarkers can potentially improve the diagnosis of CKD (Ye and Mao 2016) and provide insights into the pathophysiology and biological pathways leading to decline of renal function and development of CKD (Ye and Mao 2016). Metabolomics, the systematic analysis of metabolites in a biologic specimen, is a promising approach to characterize specific metabolic profiles of CKD (Cañadas-Garre et al. 2019; Hocher and Adamski 2017). Metabolomic studies have shown that circulating amino acids are associated with CKD (Benito et al. 2018, 2019; Chen et al. 2017; Li et al. 2018; Qi et al. 2012; Silva et al. 2018), and certain circulating metabolites such as uric acid, sugars, amino acids, lipids, and organic acids predict incident CKD or progression of CKD (Goek et al. 2013; Rhee et al. 2016). The relationship of plasma metabolites with CKD and renal function remains incompletely characterized (Cañadas-Garre et al. 2019; Hocher and Adamski 2017; Nkuipou-Kenfack et al. 2014).
Our goal was to gain further insight into the relationship of plasma metabolites with CKD and renal function. To address this goal, we conducted a hypothesis-free study of the relationship of plasma metabolites with CKD and renal function, assessed by estimated glomerular filtration rate (eGFR), using a targeted metabolomics platform in adults who participated in a well-characterized cohort of human aging.
2 Materials and methods
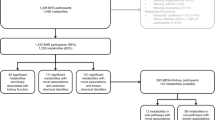
The study subjects consisted of 616 participants, aged 38 to 94 years, in the Baltimore Longitudinal Study of Aging (BLSA) who were seen between January 2006 and June 2016. The study characterized the cross-sectional association of plasma metabolites with CKD and eGFR at baseline. Participants were assessed at an in-patient study clinic for follow-up visits every one to four years, with more frequent follow-up for older participants. They underwent 2.5 days of medical, physiological, and psychological exams. The study protocol was approved by the institutional review boards of the National Institute of Environmental Health Science (NIH, North Carolina) and the Johns Hopkins School of Medicine and conducted in accordance with the 1964 Helsinki Declaration. At every visit, after the scope, procedures, and related risk were explained, participants signed an informed consent document.
Demographic and health characteristics of the participants with and without CKD were assessed by a self-administered questionnaire. Body mass index (BMI) was defined as kg/m2. Hypertension was defined as a systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive medications (Chobanian et al. 2003). Anemia was defined as circulating levels of hemoglobin < 13 g/dL in men and < 12 g/dL in women; depression was diagnosed with a Center for Epidemiologic Studies-Depression Scale (CES-D) score ≥ 16 (Radloff 1977). Glomerular filtration rate (GFR) was based on creatinine and estimated using the Modification of Diet in Renal Disease (MDRD) formula (Levey et al. 2006). CKD was defined as an eGFR of < 60 ml/min/1.73 m2 or an eGFR of < 30 ml/min/1.73 m2 if aged 80 and above (International Society of Nephrology and Kidney Disease Improving Global Outcomes (KDIGO) 2013; National Kidney Foundation 2012). The Mini-Mental State Examination (MMSE) (Folstein et al. 1975) and histories of myocardial infarction, cerebrovascular accident, peripheral artery disease, cancer, chronic obstructive pulmonary disease (COPD), angina, and stroke were ascertained through standardized questionnaires administered by trained personnel.
2.1 Measurement of serum metabolites
Blood was collected from participants who stayed overnight at the NIA Clinical Research Unit, Medstar Harbor Hospital in Baltimore, Maryland. Blood samples were drawn from the antecubital vein between 0700 and 0800 h after an overnight fast. Participants were not allowed to smoke, engage in physical activity, or take medications before the blood sample was collected. Blood samples were immediately stored at 4 °C, centrifuged within 4 h, then immediately aliquoted and frozen at − 80 °C. The collection of EDTA plasma in the BLSA is consistent with guidelines for biomarker studies (Tuck et al. 2009). Pre-analytical studies of the human plasma metabolome show that 98% of metabolites are stable during 7 years of storage and 74% of metabolites are stable during 16 years of storage at – 80° C (Wagner-Golbs et al. 2019). The metabolites most affected by long-term storage during 16 years were complex lipids (lysophosphatidylcholines, phosphatidylcholines) and certain amino acids (arginine, glycerate, aspartate, asparagine, pyruvate, cysteine, cystine) (Wagner-Golbs et al. 2019).
Plasma metabolites were measured using liquid chromatography tandem mass spectrometry (LC–MS/MS). Plasma samples were randomized across plates and run in a masked fashion. Metabolites were extracted and concentrations were measured at Biocrates Life Sciences AG (Innsbruck, Austria). Biocrates’ commercially available MxP® Quant 500 kit was used for the quantification of several endogenous metabolites of various biochemical classes. Lipids and hexoses were measured by flow injection analysis-tandem mass spectrometry (FIA-MS/MS) using a 5500 QTRAP® instrument (AB Sciex, Darmstadt, Germany) with an electrospray ionization source, and amino acids, related amino acids, carboxylic acids, fatty acids, indole derivatives, biogenic amines, bile acids, cresols, alkaloids, amine oxides, hormones, vitamins and cofactors and nucleobases related metabolites were measured by liquid chromatography-tandem mass spectrometry (LC–MS/MS) using the same 5500 QTRAP® instrument. The experimental metabolomics measurement technique is described in detail by patents EP1897014B1 and EP1875401B1 (accessible online at https://patents.google.com/patent/EP1897014B1 and https://patents.google.com/patent/EP1875401B1). Briefly, a 96-well based sample preparation device was used to quantitatively analyze the metabolite profile in the samples. The kit utilizes calibration standards in standard solution. For quantitation, calibration standards in seven concentration levels and stable isotope-labeled internal standards are used and the analytical performance is validated using quality controls at three concentration levels in matrix. The sample preparation device consists of inserts that have been spiked with internal standards, and a predefined sample amount was added to the inserts. Next, a phenyl isothiocyanate solution was added to derivatize some of the analytes (e.g. amino acids), and after the derivatization was completed, the target analytes were extracted with an organic solvent, followed by a dilution step. The obtained extracts were then analyzed by FIA-MS/MS and LC–MS/MS methods using multiple reaction monitoring to detect the analytes.
Two UHPLC methods were run using the MxP Quant 500 Column System for the LC–MS/MS methods. The mobile phase consisted of solvent A (water containing 0.2% formic acid) and solvent B (acetonitrile containing 0.2% formic acid), with the following gradient: LC1 Part: 0–0.25 min 0%B at 0.8 ml/min, 1.50 min 12%B at 0.8 ml/min, 2.70 min 17.5%B at 0.8 ml/min, 4.0 min 40%B at 0.8 ml/min, 4.50 min 100%B at 0.8 ml/min, 4.70 min 100%B at 1.0 ml/min, 5.00 min 100%B at 1.0 ml/min, 5.10 min 0%B at 1.0 ml/min, 5.80 min 0%B at 0.8 ml/min; LC2 Part: 0.25 min 0%B at 0.8 ml/min, 0.50 min 25%B at 0.8 ml/min, 2.00 min 50%B at 0.8 ml/min, 3.0 min 75%B at 0.8 ml/min, 3.50 min 100%B at 0.8 ml/min, 4.70 min 100%B at 1.0 ml/min, 5.00 min 100%B at 1.0 ml/min, 5.10 min 0%B at 1.0 ml/min, 5.80 min 0%B at 0.8 ml/min. For the FIA-MS/MS method, the FIA plate was run at a flow rate of 30 µL/min with FIA solvent as the mobile phase, with the following flow rate program: 0–1.6 min: 30 µL/min; 2.4 min: 200 µL/min; 2.80 min: 200 µL/min and 3.00 min: 30 µL/min.
Data were quantified using appropriate mass spectrometry software (Sciex Analyst®) and imported into Biocrates MetIDQ™ software for further analysis. The accuracy of the measurements as determined by internal calibrators was in the normal range of the method for all analytes. Quality control samples were within the predefined tolerances of the method. The data were not normalized across plates, and no imputation was performed.
The kit potentially measures 630 metabolites, but 180 metabolites were either completely below the limit of detection (most acylcarnitines and very long chain lysophosphatidylcholines) or were excluded from the analysis because they were below the limit of detection in more than 10% of subjects. After exclusions, 450 metabolites, including 7 acylcarnitines (AC), trigonelline, trimethylamine N-oxide (TMAO), 43 amino acids and biogenic amines, 10 bile acids, hexoses, 3 carboxylic acids, 21 ceramides, 15 cholesteryl esters (CE), p-cresol sulfate, 7 diglycerides, 7 dihexosylceramides, 2 dihydroceramides, 7 fatty acids, 14 hexosylceramides, cortisol, dehydroepiandrosterone (DHEA) sulfate, 3 indoles and derivatives, 11 lysophosphatidylcholines (LPC), hypoxanthine, 70 phosphatidylcholines (PC), 14 sphingomyelins (SM), 203 triglycerides (TG), 5 trihexosylceramides, and choline measurements were available for the data analysis. Based upon metabolite concentrations, additional ratios were calculated to aid the interpretation of pathways: the global arginine bioavailability ratio (GABR: ratio of arginine to [ornithine + citrulline]), putrescine/ornithine, ornithine/citrulline, proline/citrulline, serine/glycine, arginine/ornithine, arginine/symmetric dimethylarginine (SDMA), and arginine/asymmetric dimethylarginine (ADMA).
2.2 Statistical analysis
Differences in baseline characteristics between individuals with CKD and without CKD were tested using an unpaired Student’s t-test for continuous variables and chi-square and Fisher’s exact tests for categorical variables. All metabolites were natural log-transformed. The distributions of plasma metabolite concentrations were analyzed using the Kolmogorov–Smirnov test for normality. Depending on the distribution of the variables, either Student’s t-test or Mann Whitney U test was applied. Plasma metabolite concentrations were compared in adults < 65 years with those 65 years and older. Unadjusted bivariate and multivariate logistic regression models were used to examine the relationship between plasma metabolite concentrations and CKD at baseline. Unadjusted bivariate and multivariate linear regression models were used to examine the relationship between plasma metabolite concentrations and eGFR at baseline. In multivariable models, we adjusted for age, race, and sex in the first model, added smoking as a covariate in the second model, and storage time in the third model. Using the logistic and linear regression models, we estimated odds ratios or betas, respectively, per 1 standard deviation of each metabolite and 95% confidence intervals for metabolites independently associated with CKD or eGFR. Covariates included age, race, and sex in model 1; age, race, sex, and smoking status in model 2; age, race, sex, smoking status, and storage time (time from blood drawing to laboratory analysis) in model 3. Data analyses were performed using SPSS version 25.0 for Windows (IBM Corp., Armonk, NY, USA) and RStudio version 1.2.1335 (2009–2019 RStudio, Inc.). A false discovery rate (FDR) approach was used to control for multiple testing (Boca and Leek 2018). We calculated the FDR by the method of Benjamini and Hochberg (1995). A q-value cut-off of < 0.05 was used to define statistically significant associations (Benjamini and Hochberg 1995).
3 Results
The demographic and health characteristics in 616 adults with and without CKD are shown in Table 1. The prevalence of CKD was 12.0%. Those with CKD were more likely to be smokers (P = 0.04). There were no significant differences in age, race, sex, MMSE score, BMI, hemoglobin A1c, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, hypertension, myocardial infarction, peripheral artery disease, cancer, anemia, depression, chronic obstructive pulmonary disease, angina, or stroke between those with and without CKD.
The unadjusted cross-sectional relationships between plasma metabolites and CKD are shown in Table S1 (Online Resource 1). Those with CKD had higher concentrations of 152 metabolites (6 ACs, trigonelline, TMAO, 13 amino acids and biogenic amines, taurodeoxycholic acid, hexoses, 4 ceramides, 7 cholesteryl esters, 4 diglycerides, dihexosylceramide (Hex2Cer) d18:1/16:0, hexosylceramide (HexCer) d18:1/18:0, dehydroepiandrosterone sulfate, 3-indolepropionic acid, lysophosphatidylcholines.a.C20:3, 30 PCs, 3 SMs, 74 TGs, trihexosylceramide (Hex3Cer) d18:1/26:1, and choline) and lower concentrations of TG 17:2/36:2, serine/glycine ratio, and ornithine/citrulline ratio than those without CKD at P < 0.05. A FDR was not applied for these unadjusted comparisons in Table S1 (Online Resource 1).
Multivariate logistic regression models were used to examine the relationship between plasma metabolites and CKD in Table S2 (Online Resource 2). After adjusting for age, race, and sex in model 1, there were 70 metabolites that were associated with increased odds of CKD at a q-value < 0.05. After adjusting for age, race, sex, and smoking status in model 2, 42 metabolites (4 ACs, trigonelline, TMAO, 8 amino acids and biogenic amines, taurodeoxycholic acid, CE 22:6, DG 16:1/18:2, hex2Cer d18:1/16:0, 3-indolepropionic acid, 11 PCs, 2 SMs, 9 TGs, and choline) were associated with increased odds of CKD at a q-value < 0.05. After adjusting form age, race, sex, smoking status, and storage time in model 3, 22 metabolites (carnitine, acetylcarnitine, propionylcarnitine, butyrylcarnitine, trigonelline, TMAO, 1-methylhistidine, citrulline, homoarginine, homocysteine, sarcosine, symmetric dimethylarginine, aspartate, phenylalanine, taurodeoxycholic acid, 3-indolepropionic acid, PC.aa.C40:2, PC.aa.C40:3, PC.ae.C40:6, TG20:4/36:3, TG20:4/36:4, and choline) were associated with increased odds of CKD at a q-value < 0.05. The serine/glycine ratio and ornithine/citrulline ratio were associated with decreased odds of CKD in model 2 and 3. A volcano plot showing the relationship of odds ratios for plasma metabolites with CKD is shown in Fig. 1.
Bivariate linear regression models of the relationship between plasma metabolites and eGFR are shown in Table S3 (Online Resource 3). There were 188 metabolites (6 ACs, trigonelline, TMAO, 29 amino acids and biogenic amines, taurodeoxycholic acid, hexoses, aconitic acid, 6 ceramides, 6 CEs, 6 diglycerides, hex2Cer d18:1/16:0, eicosatrienoic acid, 2 HexCer, 2 indoles and derivatives, 4 LPCs, 31 PCs, 2 SMs, 83 TGs, 4 Hex3Cer, and choline) and the putrescine/ornithine and arginine/ornithine ratios were negatively associated with eGFR (P < 0.05). Glutamine, proline, hippuric acid, cortisol, and the serine/glycine, ornithine/citrulline, and proline/citrulline ratios were positively associated with eGFR (P < 0.05). A FDR approach was not applied for the unadjusted models in Table S3 (Online Resource 3).
The relationship between plasma metabolites and eGFR was examined in multivariable linear regression models shown in Table S4 (Online Resource 4). One hundred and twenty metabolites and the GABR, putrescine/ornithine, arginine/ADMA, serine/glycine, arginine/ornithine, ornithine/citrulline, and proline/citrulline ratios were significantly associated with eGFR, after adjusting for age, race, and sex in model 1. After adjusting for age, race, sex, and smoking status in model 2 and using a FDR approach with q-value < 0.05, 110 plasma metabolites were associated with eGFR. Six ACs, trigonelline, TMAO, 20 amino acids and biogenic amines, taurodeoxycholic acid, hexoses, 3 CEs, 3 diglycerides, hex2Cer d18:1/16:0, DHEA sulfate, 3-indolepropionic acid, 10 PCs, 3 SMs, 56 TGs, and choline, and the putrescine/ornithine, arginine/ADMA, and arginine/ornithine ratios were negatively associated with eGFR. Hippuric acid and GABR, serine/glycine, ornithine/citrulline, and proline/citrulline ratios were positively associated with eGFR. After adjusting for age, race, sex, smoking status, and storage time in model 3, 51 metabolites (6 ACs, trigonelline, TMAO, 18 amino acids and biogenic amines, taurodeoxycholic acid, hexoses, CE (22:6), DHEA sulfate, 3-indolepropionic acid, 2 PCs, 17 TGs, choline) and the putrescine/ornithine ratio were negatively associated with eGFR using a FDR approach with q-value < 0.05. Hippuric acid, serine/glycine, ornithine/citrulline, and proline/citrulline ratios were positively associated with eGFR. A volcano plot showing the relationship the betas for plasma metabolites with eGFR is shown in Fig. 2.
The unadjusted cross-sectional relationships between plasma metabolites with age are shown in Table S5 (Online Resource 5). One hundred and one plasma metabolites and the GABR were negatively associated with age. Twelve plasma metabolites and the serine/glycine, arginine/SDMA, ornithine/citrulline, and proline/citrulline ratios were positively associated with age.
4 Discussion
The present study shows that 22 plasma metabolites were significantly associated with prevalent CKD in community-dwelling adults after adjusting for potential confounders and dealing with multiple comparisons. Fifty-one plasma metabolites were negatively associated with eGFR, and one metabolite, hippuric acid, was positively correlated with eGFR. The metabolites associated with CKD and eGFR suggest that several pathways, such as the urea cycle, the arginine-nitric oxide pathway, the polyamine pathway, and short chain acylcarnitine metabolism are altered in adults with CKD and impaired renal function, as discussed below. Metabolite biomarkers of CKD may be also markers of glomerular filtration. In addition, some metabolites suggest that there may be differences in dietary intake and in the gut microbiome between adults with and without CKD.
In the present study, plasma citrulline was elevated in adults with CKD. Citrulline is an intermediates in the urea cycle in which ammonia is converted to urea for excretion. Elevated citrulline is a marker for several urea cycle disorders and one of several diagnostic amino acid biomarkers for hyperammonemia which also include ornithine, arginine, and lysine (Garg and Smith 2017). The present study found that citrulline and lysine were negatively associated with eGFR. Citrulline is an intermediates in the urea cycle in which ammonia is converted to urea for excretion. Elevated plasma citrulline has been associated with CKD (Benito et al. 2018; Li et al. 2018; Nkuipou-Kenfack et al. 2014). A negative association of citrulline with eGFR has been described previously (Goek et al. 2013).
Citrulline and arginine are precursors in the biological synthesis pathway of nitric oxide (NO) in which NO synthase catalyzes the conversion of arginine to citrulline and NO. Citrulline is converted to arginine in the kidneys and is involved in arginine/NO balance. CKD has been associated with decreased NO production (Baylis 2008; Reddy et al. 2015). The decrease could result from a decrease in NO synthesis or reduced bioavailability of NO (Reddy et al. 2015). ADMA and SDMA are endogenous amino acids that can inhibit NO synthase (Reddy et al. 2015). Plasma SDMA was higher in adults with CKD, and both SDMA and ADMA were inversely correlated with eGFR in the present study. These findings are consistent with previous studies showing elevated SDMA in CKD (Schepers et al. 2011) and higher ADMA in adults with lower eGFR (Nkuipou-Kenfack et al. 2014). These data suggest that there is lower NO synthesis in adults with CKD, consistent with previous studies. However, the GABR, which has been proposed to approximate NO synthetic capacity in vivo (Moaddel et al. 2018), was not associated with either CKD or eGFR in the present study.
Arginine is also required for polyamine synthesis, where it is converted to ornithine by arginase, the initial enzyme in the polyamine biosynthetic pathway (Morris 2016). Ornithine is subsequently converted to putrescine via ornithine decarboxylase (ODC). Previous studies have shown that putrescine was elevated in patients with CKD (Hocher and Adamski 2017). While in our study, putrescine was not significantly different in adults with CKD. The putrescine/ornithine ratio, which reflects ODC activity, was negatively associated with eGFR. Previous studies have implicated the arginine-ornithine-polyamine pathway in enlargement of the diabetic kidney (Thomson et al. 2001), and shown increased ornithine/citrulline and proline/citrulline ratios from increased arginase activity in patients with type 2 diabetes (Kövamees et al. 2016). In the present study, the ornithine/citrulline ratio was lower in CKD compared with controls, and the ornithine/citrulline and proline/citrulline ratios were positively associated with eGFR, suggesting decreased arginase activity and impaired polyamine synthesis.
The serine/glycine ratio was lower in adults with CKD compared to those without CKD, and the serine/glycine ratio was positively associated with eGFR. A previous study also reported a positive association of serine with eGFR (Laidlaw et al. 1994). Impaired conversion of glycine to serine via serine hydroxymethyltransferase SHMT, a key enzyme in the folate mediated one-carbon metabolism, in the kidney could account for lower plasma serine in adults with low eGFR (Laidlaw et al. 1994; Nonaka et al. 2019). Glycine may also derive from the choline which can be generated from the metabolism of phosphatidylcholine and is converted to betaine and then to glycine (Wu 2013). Sarcosine is an intermediate in the generation of glycine from choline. Higher plasma choline and sarcosine concentrations were found in adults with CKD, and plasma choline and sarcosine were negatively associated with eGFR. While betaine was not significantly associated with CKD or eGFR, the concurrent increase of phosphatidylcholines and choline with decreasing eGFR could potentially contribute to, in part, the increased circulating concentrations of glycine. Betaine can be converted to dimethylglycine via betaine-homocysteine methyltransferase, which transfers a methyl group from betaine to produce methionine and dimethylglycine from homocysteine through the methionine cycle (Long and Nie 2016).
Homocysteine was higher in adults with CKD, and both plasma homocysteine and methionine were negatively associated with eGFR. Higher plasma homocysteine was described in a cross-sectional study of over 17,000 adults in Israel (Cohen et al. 2019). In addition, a population-based cohort study of 1477 Japanese community-dwelling individuals suggested that moderately elevated serum homocysteine concentrations are a significant risk factor for the development of CKD in the general population (Ninomiya et al. 2004).
Plasma TMAO was elevated in adults with CKD in the present study, consistent with a previous study (Kim et al. 2016). Both choline and carnitine, precursors for the formation of trimethylamine (TMA), were higher in adults with CKD. TMAO is produced when dietary choline is converted to TMA by the gut microbiome and then oxidized to TMAO in the liver via flavin-containing monooxygenase isoform 3 (Kim et al. 2016). The higher levels of TMAO may reflect the gut microbiome-mediated synthesis of TMA or the clearance of TMAO by transporters expressed in the intestine, liver and kidney (Kim et al. 2016).
Three short-chain acylcarnitines (acetylcarnitine, C2; proprionylcarnitine, C3; butyrylcarnitine, C4) were significantly higher in adults with CKD compared to those without CKD. These findings are consistent with a relationship of serum acylcarnitines with eGFR described in the general population described by Goek et al. (2013). Short-chain acylcarnitines, such as proprionylcarnitine, can be generated from catabolism of branched chain amino acids (BCAA) but not fatty acid β-oxidation (Aguer et al. 2015). Acylcarnitines are proinflammatory (Rutkowsky et al. 2014) and have been shown to increase reactive oxygen species and induce insulin resistance in skeletal muscle (Aguer et al. 2015). Acylcarnitines are freely filtered by the glomerulus and only 25% are reabsorbed (Fouque et al 2006); a lower eGFR could potentially contribute to increased circulating acylcarnitines.
Trigonelline was elevated in adults with CKD. Trigonelline, a pyridine alkaloid, is found in high concentrations in coffee, legumes, and some vegetables, and has been used as an epidemiological marker of coffee intake (Midttun et al. 2018). In contrast, a recent meta-analysis of 25,849 subjects showed that higher coffee intake was associated with a reduced risk of incident CKD (Srithongkul and Ungprasert 2020).
Plasma 1-methylhistidine was elevated in adults with CKD compared to those without CKD. Anserine, a dipeptide of 1-methylhistidine and β-alanine, is found in muscles of many vertebrates. Plasma 1-methylhistidine is a marker of meat consumption and results from the metabolism of the anserine (Dragsted 2010; Hagen et al. 2020; Zhou et al. 2010). Circulating 1-methylhistidine has been correlated with dietary meat intake (Mitry et al. 2019). The finding of elevated 1-methylhistidine in adults with CKD is consistent with epidemiological studies showing that a dietary pattern characterized by a high consumption of red and processed meats is associated with greater risk of CKD (Ajjarapu et al. 2019). Plasma 3-methylhistidine, a modified amino acid that is formed by post-translational methylation of histidine residues of actin and myosin, was elevated in adults with low eGFR in the present study (Long et al. 1975; Sheffield-Moore et al. 2014; Wang et al. 2012). Urinary excretion of 3-methylhistidine has been used to determine the rate of skeletal muscle protein degradation (Long et al. 1975; Sheffield-Moore et al. 2014). CKD has a negative effect on muscle mass and endurance (Watanabe et al. 2019). The finding from the present study is consistent with a previous report of elevated serum 3-methylhistidine in patients with CKD (Zhang et al. 2016). The present study showed that plasma 3-indolepropionic acid, an aromatic amino acid which is produced by the gut microbiota, was elevated in CKD. These findings are contrary to a previous study in which lower serum 3-indolepropionic acid concentrations were described in adults with CKD compared with normal controls (Sun et al. 2019). Circulating 3-indolepropionic acid has been associated with dietary fiber intake (Tuomainen et al. 2018).
The present study identified only one plasma metabolite, hippuric acid, had a significant positive association with eGFR. Hippuric acid, the glycine conjugate of benzoic acid, is generated from dietary polyphenols and the gut microbiome (Lees et al. 2013). Elevated circulating hippuric acid was associated with greater microbiome diversity, higher intakes of fruit and whole grains, and reduced odds of having metabolic syndrome in the TwinsUK Study and an independent validation cohort (Pallister et al. 2017).
CKD is associated with dyslipidemia, including elevated TG levels, decreased HDL cholesterol, and variable levels of LDL cholesterol (Dincer et al. 2019). In the present study involving relatively healthy adults, there were no significant differences in total TG, HDL cholesterol, or LDL cholesterol between adults with and without CKD. However, those with CKD had elevated concentrations of many TG species at P < 0.05 but Q > 0.05. Decreased eGFR was associated with significantly elevated TG species at a FDR of < 0.05, notably those containing oleic acid (18:0), linoleic acid (18:2), and eicosatraenoic acid (20:4). The mass spectrometry platform used in the study cannot distinguish the remaining two fatty acid chains of the TG; most of the two chains in sum contained a total of 2–6 double bonds. Plasma TG are primarily synthesized in the liver from DG through diacylglycerol acyltransferase in the Kennedy pathway. There were 3 that were elevated in adults with CKD compared to those without CKD. The platform used in the study cannot distinguish between isomeric and isobaric species of PC and SM, however, it is notable that all species were unsaturated and contained 3–4 double bonds.
The strengths of this study include a well characterized group of participants from an established aging study that had highly standardized procedures for data collection and laboratory assessments, use of a targeted metabolomics platform, and a robust statistical approach that accounted for multiple comparisons to reduce the risk of false-positive results. The participants in the BLSA are more educated and healthier than the general population, thus, the results of the study cannot necessarily be extrapolated to the general population and other settings. There were no subjects with stage 4 or 5 CKD, thus this study could not characterize metabolites associated with more severe disease. The study has a cross-sectional design; thus, any direction of causality cannot necessarily be inferred between plasma metabolites and renal function. Longitudinal studies are needed in the future to determine whether the metabolites identified in the present study are predictive of decline in eGFR and the development of CKD.
5 Conclusions
We demonstrated that 22 plasma metabolites are associated with CKD and 52 plasma metabolites are associated with eGFR in community dwelling adults. The pattern of plasma metabolites study suggests alterations in the urea cycle, the arginine-nitric oxide pathway, the polyamine pathway, and short chain acylcarnitine metabolism in adults with CKD. Further studies are needed to determine whether alterations in these pathways predict the development or progression of CKD in adults.
Data availability
Data are available upon reasonable request.
References
Aguer, C., McCoin, C. S., Knotts, T. A., Thrush, A. B., Ono-Moore, K., McPherson, R., et al. (2015). Acylcarnitines: Potential implications for skeletal muscle insulin resistance. FASEB Journal, 29(1), 336–345. https://doi.org/10.1096/fj.14-255901.
Ajjarapu, A. S., Hinkle, S. N., Li, M., Francis, E. C., & Zhang, C. (2019). Dietary patterns and renal health outcomes in the general population: A review focusing on prospective studies. Nutrients, 11(8), 1877. https://doi.org/10.3390/nu11081877.
Baylis, C. (2008). Nitric oxide deficiency in chronic kidney disease. American Journal of Physiology. Renal Physiology, 294(1), F1–F9. https://doi.org/10.1152/ajprenal.00424.2007.
Benito, S., Sánchez-Ortega, A., Unceta, N., Jansen, J. J., Postma, G., Andrade, F., et al. (2018). Plasma biomarker discovery for early chronic kidney disease diagnosis based on chemometric approaches using LC-QTOF targeted metabolomics data. Journal of Pharmaceutical and Biomedical Analysis, 149, 46–56. https://doi.org/10.1016/j.jpba.2017.10.036.
Benito, S., Sánchez-Ortega, A., Unceta, N., Goicolea, M. A., & Barrio, R. J. (2019). LC-QQQ-MS routine analysis method for new biomarker quantification in plasma aimed at early chronic kidney disease diagnosis. Journal of Pharmaceutical and Biomedical Analysis, 169, 82–89. https://doi.org/10.1016/j.jpba.2019.02.042.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 298–300. https://doi.org/10.2307/2346101.
Boca, S. M., & Leek, J. T. (2018). A direct approach to estimating false discovery rates conditional on covariates. PeerJ, 6, e6035. https://doi.org/10.7717/peerj.6035.
Cañadas-Garre, M., Anderson, K., McGoldrick, J., Maxwell, A. P., & McKnight, A. J. (2019). Proteomic and metabolomic approaches in the search for biomarkers in chronic kidney disease. Journal of Proteomics, 193, 93–122. https://doi.org/10.1016/j.jprot.2018.09.020.
Centers for Disease Control and Prevention. (2019). Chronic kidney disease in the United States, Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. Retrieved August 3, 2020 from https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf.
Chen, H., Chen, L., Liu, D., Chen, D. Q., Vaziri, N. D., Yu, X. Y., et al. (2017). Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. Journal of Proteome Research, 16(4), 1566–1578. https://doi.org/10.1021/acs.jproteome.6b00956.
Chobanian, A. V., Bakris, G. L., Black, H. R., Cushman, W. C., Green, L. A., Izzo, J. L., et al. (2003). Seventh report of the Joint National Committee on prevention detection, evaluation, and treatment of high blood pressure. Hypertension (Dallas, Tex.: 1979), 42(6), 1206–1252. https://doi.org/10.1161/01.HYP.0000107251.49515.c2.
Cohen, E., Margalit, I., Shochat, T., Goldberg, E., & Krause, I. (2019). The relationship between the concentration of plasma homocysteine and chronic kidney disease: A cross sectional study of a large cohort. Journal of Nephrology, 32(5), 783–789. https://doi.org/10.1007/s40620-019-00618-x.
Dincer, N., Dagel, T., Afsar, B., Covic, A., Ortiz, A., & Kanbay, M. (2019). The effect of chronic kidney disease on lipid metabolism. International Urology and Nephrology, 51(2), 265–277. https://doi.org/10.1007/s11255-018-2047-y.
Dragsted, L. O. (2010). Biomarkers of meat intake and the application of nutrigenomics. Meat Science, 84(2), 301–307. https://doi.org/10.1016/j.meatsci.2009.08.028.
Folstein, M. F., Folstein, S. E., & McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. https://doi.org/10.1016/0022-3956(75)90026-6.
Fouque, D., Holt, S., Guebre-Egziabher, F., Nakamura, K., Vianey-Saban, C., Hadj-Aïssa, A., et al. (2006). Relationship between serum carnitine, acylcarnitines, and renal function in patients with chronic renal disease. Journal of Renal Nutrition, 16(2), 125–131. https://doi.org/10.1053/j.jrn.2006.01.004.
Gagnebin, Y., Julien, B., Belén, P., & Serge, R. (2018). Metabolomics in chronic kidney disease: Strategies for extended metabolome coverage. Journal of Pharmaceutical and Biomedical Analysis, 161, 313–325. https://doi.org/10.1016/j.jpba.2018.08.046.
Garg, U., & Smith, L. D. (2017). Biomarkers in inborn errors of metabolism. San Diego: Elsevier.
GBD Chronic Kidney Disease Collaboration. (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England), 395(10225), 709–733. https://doi.org/10.1016/S0140-6736(20)30045-3.
Goek, O. N., Prehn, C., Sekula, P., Römisch-Margl, W., Döring, A., Gieger, C., et al. (2013). Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrology, Dialysis, Transplantation, 28(8), 2131–2138. https://doi.org/10.1093/ndt/gft217.
Hagen, I. V., Helland, A., Bratlie, M., Midttun, Ø., McCann, A., Sveier, H., et al. (2020). TMAO, creatine and 1-methylhistidine in serum and urine are potential biomarkers of cod and salmon intake: A randomised clinical trial in adults with overweight or obesity. European Journal of Nutrition, 59(5), 2249–2259. https://doi.org/10.1007/s00394-019-02076-4.
Hocher, B., & Adamski, J. (2017). Metabolomics for clinical use and research in chronic kidney disease. Nature Reviews. Nephrology, 13(5), 269–284. https://doi.org/10.1038/nrneph.2017.30.
International Society of Nephrology, & Kidney Disease Improving Global Outcomes (KDIGO). (2013). Chapter 1: Definition and classification of CKD. Kidney International Supplements, 3(1), 19–62. https://doi.org/10.1038/kisup.2012.64.
Jha, V., Garcia-Garcia, G., Iseki, K., Li, Z., Naicker, S., Plattner, B., et al. (2013). Chronic kidney disease: Global dimension and perspectives. Lancet (London, England), 382(9888), 260–272. https://doi.org/10.1016/S0140-6736(13)60687-X.
Kim, R. B., Morse, B. L., Djurdjev, O., Tang, M., Muirhead, N., Barrett, B., et al. (2016). Advanced chronic kidney disease populations have elevated trimethylamine N-oxide levels associated with increased cardiovascular events. Kidney International, 89(5), 1144–1152. https://doi.org/10.1016/j.kint.2016.01.014.
Kövamees, O., Shemyakin, A., & Pernow, J. (2016). Amino acid metabolism reflecting arginase activity is increased in patients with type 2 diabetes and associated with endothelial dysfunction. Diabetes & Vascular Disease Research, 13(5), 354–360. https://doi.org/10.1177/1479164116643916.
Laidlaw, S. A., Berg, R. L., Kopple, J. D., Naito, H., Walker, W. G., & Walser, M. (1994). Patterns of fasting plasma amino acid levels in chronic renal insufficiency: Results from the feasibility phase of the Modification of Diet in Renal Disease Study. American Journal of Kidney Diseases, 23(4), 504–513. https://doi.org/10.1016/s0272-6386(12)80371-4.
Lees, H. J., Swann, J. R., Wilson, I. D., Nicholson, J. K., & Holmes, E. (2013). Hippurate: The natural history of a mammalian-microbial cometabolite. Journal of Proteome Research, 12(4), 1527–1546. https://doi.org/10.1021/pr300900b.
Levey, A. S., Coresh, J., Greene, T., Stevens, L. A., Zhang, Y. L., Hendriksen, S., et al. (2006). Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals of Internal Medicine, 145(4), 247–254. https://doi.org/10.7326/0003-4819-145-4-200608150-00004.
Li, R., Dai, J., & Kang, H. (2018). The construction of a panel of serum amino acids for the identification of early chronic kidney disease patients. Journal of Clinical Laboratory Analysis, 32(3), e22282. https://doi.org/10.1002/jcla.22282.
Long, Y., & Nie, J. (2016). Homocysteine in renal injury. Kidney Diseases (Basel, Switzerland), 2(2), 80–87. https://doi.org/10.1159/000444900.
Long, C. L., Haverberg, L. N., Young, V. R., Kinney, J. M., Munro, H. N., & Geiger, J. W. (1975). Metabolism of 3-methylhistidine in man. Metabolism: Clinical and Experimental, 24(8), 929–935. https://doi.org/10.1016/0026-0495(75)90084-0.
Midttun, Ø., Ulvik, A., Nygård, O., & Ueland, P. M. (2018). Performance of plasma trigonelline as a marker of coffee consumption in an epidemiologic setting. The American Journal of Clinical Nutrition, 107(6), 941–947. https://doi.org/10.1093/ajcn/nqy059.
Mitry, P., Wawro, N., Rohrmann, S., Giesbertz, P., Daniel, H., & Linseisen, J. (2019). Plasma concentrations of anserine, carnosine and pi-methylhistidine as biomarkers of habitual meat consumption. European Journal of Clinical Nutrition, 73(5), 692–702. https://doi.org/10.1038/s41430-018-0248-1.
Moaddel, R., Shardell, M., Khadeer, M., Lovett, J., Kadriu, B., Ravichandran, S., et al. (2018). Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl), 235(10), 3017–3030. https://doi.org/10.1007/s00213-018-4992-7.
Morris, S. M., Jr. (2016). Arginine metabolism revisited. The Journal of Nutrition, 146(12), 2579S-2586S. https://doi.org/10.3945/jn.115.226621.
National Kidney Foundation. (2012). KDOQI clinical practice guideline for diabetes and CKD: 2012 update. American Journal of Kidney Diseases, 60(5), 850–886. https://doi.org/10.1053/j.ajkd.2012.07.005.
Ninomiya, T., Kiyohara, Y., Kubo, M., Tanizaki, Y., Tanaka, K., Okubo, K., et al. (2004). Hyperhomocysteinemia and the development of chronic kidney disease in a general population: The Hisayama study. American Journal of Kidney Diseases, 44(3), 437–445. https://doi.org/10.1053/j.ajkd.2004.05.024.
Nkuipou-Kenfack, E., Duranton, F., Gayrard, N., Argilés, À., Lundin, U., Weinberger, K. M., et al. (2014). Assessment of metabolomic and proteomic biomarkers in detection and prognosis of progression of renal function in chronic kidney disease. PLoS ONE, 9(5), e96955. https://doi.org/10.1371/journal.pone.0096955.
Nonaka, H., Nakanishi, Y., Kuno, S., Ota, T., Mochidome, K., Saito, Y., et al. (2019). Design strategy for serine hydroxymethyltransferase probes based on retro-aldol-type reaction. Nature Communications, 10(1), 876. https://doi.org/10.1038/s41467-019-08833-7.
Pallister, T., Jackson, M. A., Martin, T. C., Zierer, J., Jennings, A., Mohney, R. P., et al. (2017). Hippurate as a metabolomic marker of gut microbiome diversity: Modulation by diet and relationship to metabolic syndrome. Scientific Reports, 7(1), 13670. https://doi.org/10.1038/s41598-017-13722-4.
Qi, S., Ouyang, X., Wang, L., Peng, W., Wen, J., & Dai, Y. (2012). A pilot metabolic profiling study in serum of patients with chronic kidney disease based on (1) H-NMR-spectroscopy. Clinical and Translational Science, 5(5), 379–385. https://doi.org/10.1111/j.1752-8062.2012.00437.x.
Radloff, L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. https://doi.org/10.1177/014662167700100306.
Reddy, Y. S., Kiranmayi, V. S., Bitla, A. R., Krishna, G. S., Rao, P. V., & Sivakumar, V. (2015). Nitric oxide status in patients with chronic kidney disease. Indian Journal of Nephrology, 25(5), 287–291. https://doi.org/10.4103/0971-4065.147376.
Rhee, E. P., Clish, C. B., Wenger, J., Roy, J., Elmariah, S., Pierce, K. A., et al. (2016). Metabolomics of chronic kidney disease progression: A case-control analysis in the chronic renal insufficiency cohort study. American Journal of Nephrology, 43(5), 366–374. https://doi.org/10.1159/000446484.
Rutkowsky, J. M., Knotts, T. A., Ono-Moore, K. D., McCoin, C. S., Huang, S., Schneider, D., et al. (2014). Acylcarnitines activate proinflammatory signaling pathways. American Journal of Physiology. Endocrinology and Metabolism, 306(12), E1378–E1387. https://doi.org/10.1152/ajpendo.00656.2013.
Schepers, E., Barreto, D. V., Liabeuf, S., Glorieux, G., Eloot, S., Barreto, F. C., et al. (2011). Symmetric dimethylarginine as a proinflammatory agent in chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN, 6(10), 2374–2383. https://doi.org/10.2215/CJN.01720211.
Sheffield-Moore, M., Dillon, E. L., Randolph, K. M., Casperson, S. L., White, G. R., Jennings, K., et al. (2014). Isotopic decay of urinary or plasma 3-methylhistidine as a potential biomarker of pathologic skeletal muscle loss. Journal of Cachexia, Sarcopenia and Muscle, 5(1), 19–25. https://doi.org/10.1007/s13539-013-0117-7.
Silva, R. E., Baldim, J. L., Chagas-Paula, D. A., Soares, M. G., Lago, J., Gonçalves, R. V., et al. (2018). Predictive metabolomic signatures of end-stage renal disease: A multivariate analysis of population-based data. Biochimie, 152, 14–30. https://doi.org/10.1016/j.biochi.2018.06.009.
Srithongkul, T., & Ungprasert, P. (2020). Coffee consumption is associated with a decreased risk of incident chronic kidney disease: A systematic review and meta-analysis of cohort studies. European Journal of Internal Medicine, 77, 111–116. https://doi.org/10.1016/j.ejim.2020.04.018.
Sun, C. Y., Lin, C. J., Pan, H. C., Lee, C. C., Lu, S. C., Hsieh, Y. T., et al. (2019). Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clinical Nutrition (Edinburgh, Scotland), 38(6), 2945–2948. https://doi.org/10.1016/j.clnu.2018.11.029.
Thomson, S. C., Deng, A., Bao, D., Satriano, J., Blantz, R. C., & Vallon, V. (2001). Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. The Journal of Clinical Investigation, 107(2), 217–224. https://doi.org/10.1172/JCI10963.
Tuck, M. K., Chan, D. W., Chia, D., Godwin, A. K., Grizzle, W. E., Krueger, K. E., et al. (2009). Standard operating procedures for serum and plasma collection: Early detection research network consensus statement standard operating procedure integration working group. Journal of Proteome Research, 8(1), 113–117. https://doi.org/10.1021/pr800545q.
Tuomainen, M., Lindström, J., Lehtonen, M., Auriola, S., Pihlajamäki, J., Peltonen, M., et al. (2018). Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutrition and Diabetes, 8(1), 35. https://doi.org/10.1038/s41387-018-0046-9.
Wagner-Golbs, A., Neuber, S., Kamlage, B., Christiansen, N., Bethan, B., Rennefahrt, U., et al. (2019). Effects of long-term storage at -80 °C on the human plasma metabolome. Metabolites, 9(5), 99. https://doi.org/10.3390/metabo9050099.
Wang, H., Hu, P., & Jiang, J. (2012). Measurement of 1- and 3-methylhistidine in human urine by ultra performance liquid chromatography-tandem mass spectrometry. Clinica Chimica Acta; International Journal of Clinical Chemistry, 413(1–2), 131–138. https://doi.org/10.1016/j.cca.2011.09.007.
Watanabe, H., Enoki, Y., & Maruyama, T. (2019). Sarcopenia in chronic kidney disease: Factors, mechanisms, and therapeutic interventions. Biological & Pharmaceutical Bulletin, 42(9), 1437–1445. https://doi.org/10.1248/bpb.b19-00513.
Wu, G. (2013). Amino acids: Biochemistry and nutrition. Boca Raton: CRC Press. Taylor & Francis Group.
Ye, L., & Mao, W. (2016). Metabonomic biomarkers for risk factors of chronic kidney disease. International Urology and Nephrology, 48(4), 547–552. https://doi.org/10.1007/s11255-016-1239-6.
Zhang, Z. H., Chen, H., Vaziri, N. D., Mao, J. R., Zhang, L., Bai, X., et al. (2016). Metabolomic signatures of chronic kidney disease of diverse etiologies in the rats and humans. Journal of Proteome Research, 15(10), 3802–3812. https://doi.org/10.1021/acs.jproteome.6b00583.
Zhou, L., Yan, N., Zhang, H., Zhou, X., Pu, Q., & Hu, Z. (2010). Microwave-accelerated derivatization for capillary electrophoresis with laser-induced fluorescence detection: A case study for determination of histidine, 1- and 3-methylhistidine in human urine. Talanta, 82(1), 72–77. https://doi.org/10.1016/j.talanta.2010.03.061.
Funding
This study was supported by the National Institutes of Health R01 AG027012, R01 AG057723, P30 AG021334 Johns Hopkins University Older Americans Independence Center, and the Intramural Research Program of the National Institute on Aging, Baltimore, Maryland.
Author information
Authors and Affiliations
Contributions
RDS and LF designed the study; YY and MZ created the dataset; YY analyzed the data; YY, RDS, MZ, RM, LF, and TKC interpreted the data: YY, RDS, MZ, and RM drafted the manuscript; all authors revised the manuscript; all authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the institutional review boards of the National Institute of Environmental Health Science (NIH, North Carolina) and the Johns Hopkins School of Medicine and conducted in accordance with the 1964 Helsinki Declaration.
Consent to participate
All participants signed an informed consent document.
Consent for publication
The funding agencies had no role in the research, manuscript, or publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamaguchi, Y., Zampino, M., Moaddel, R. et al. Plasma metabolites associated with chronic kidney disease and renal function in adults from the Baltimore Longitudinal Study of Aging. Metabolomics 17, 9 (2021). https://doi.org/10.1007/s11306-020-01762-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-020-01762-3