Abstract
Introduction
Three out of four people with diabetes will die of cardiovascular disease. However, the molecular mechanisms by which hyperglycemia promotes atherosclerosis, the major underlying cause of cardiovascular disease, are not clear.
Objectives
Three distinct models of hyperglycemia-associated accelerated atherosclerosis were used to identify commonly altered metabolites and pathways associated with the disease.
Methods
Normoglycemic apolipoprotein-E-deficient mice served as atherosclerotic control. Hyperglycemia was induced by multiple low-dose streptozotocin injections, or by introducing a point-mutation in one copy of insulin-2 gene. Glucosamine-supplemented mice, which experience accelerated atherosclerosis to a similar extent as hyperglycemia-induced models without alterations in glucose or insulin levels, were also included in the analysis. Untargeted plasma metabolomics were used to investigate hyperglycemia-associated accelerated atherosclerosis in three disease models. The effect of specific significantly altered metabolites on pro-atherogenic processes was investigated in cultured human vascular cells.
Results
Hyperglycemic and glucosamine-supplemented mice showed distinct metabolomic profiles compared to controls. Meta-analysis of three disease models revealed 62 similarly altered metabolite features (FDR-adjusted p < 0.05). Identification of shared metabolites revealed alterations in glycerophospholipid and sphingolipid metabolism, and pro-atherogenic processes including inflammation and oxidative stress. Post-multivariate and pathway analyses indicated that the glycosphingolipid pathway is strongly associated with hyperglycemia-induced accelerated atherosclerosis in these atherogenic mouse models. Glycosphingolipids induced oxidative stress and inflammation in cultured human vascular cells.
Conclusion
Glycosphingolipids are strongly associated with hyperglycemia-induced accelerated atherosclerosis in three distinct models. They also promote pro-atherogenic processes in cultured human cells. These results suggest glycosphingolipid pathway may be a potential therapeutic target to block or slow atherogenesis in diabetic patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Omics-based technologies have been used to map pathways and/or mechanisms involved in the etiology and pathogenesis of many human diseases. Metabolomics, the latest omics strategy, offers novel insights into disease mechanisms, as the level of specific metabolites provides a direct functional readout of the physiological or pathological state of an organism (Fiehn 2002; Patti et al. 2012). Recent advances in analytical and bioinformatic technologies allow for the characterization of thousands of metabolites, leading to the development of untargeted metabolomics. This type of approach involves the global analysis of the metabolome, which is the entire collection of metabolites in a biological specimen. Untargeted metabolomics enables novel and/or unanticipated discoveries linking molecular pathways to biological phenotypes and/or genotypes.
The prevalence of diabetes is increasing dramatically worldwide (Hossain et al. 2007; Patterson et al. 2009). Cardiovascular disease (CVD) is the leading cause of death in both type 1 and type 2 diabetes (Haffner et al. 1998; Lehto et al. 2000). In fact, approximately three out of four diabetic patients will die of CVD. Atherosclerosis, a chronic inflammatory disease of the medium-large arteries, is the major underlying cause of most CVDs (Falk 2006; Ross 1993). This disease is characterized by the accumulation of lipids and inflammatory factors in the arterial walls. Despite the progressive relationship between diabetes and CVD, the molecular mechanisms by which hyperglycemia, the defining characteristic of all types of diabetes mellitus, promotes atherosclerosis are not clear (Glass and Witztum 2001; Shalhoub et al. 2014). This has impaired the development of strategies to block or slow atherogenesis in diabetic patients.
Animal-based studies offer a great opportunity to gain insight into the pathophysiological mechanisms of a disease due to ease of genetic and environmental manipulation and relatively short time frame for disease development. The apolipoprotein E-deficient (ApoE−/−) mouse model was chosen as the control, as it is a well-established model in which all recognized stages of atherogenesis can be observed (Meir and Leitersdorf 2004; Whitman 2004). This mouse model also develops complex atherosclerotic lesions that are morphologically very similar to those observed in humans (Nakashima et al. 1993; Reddick et al. 1994). Hyperglycemia was induced by multiple low-dose streptozotocin (STZ) injections (Kunjathoor et al. 1996) or by introducing a point mutation in one copy of the insulin 2 gene (Ins2+/Akita) (Venegas-Pino et al. 2015). The glucosamine-supplemented ApoE−/− mouse model was also included. We have previously shown that ApoE−/− mice supplemented with glucosamine, a downstream metabolite of glucose, experience accelerated atherosclerosis to a similar extent as the hyperglycemia-induced models, without alterations in the levels of glucose or insulin (Dang et al. 2015). This mouse model serves as a valuable control for insulin in this study.
In this study, untargeted metabolomic techniques were used to investigate the molecular alterations by which STZ injection, glucosamine supplementation and Ins2+/Akita promote the accelerated development of atherosclerosis in ApoE−/− control mice. The use of multiple distinct models facilitates the identification of commonly altered metabolites and pathways, while avoiding model-specific artifacts.
2 Materials and methods
2.1 Animal models
This study used female mice to avoid the confounding effects of enhanced dyslipidemia observed in male Ins2+/Akita ApoE−/− mice, relative to male Ins2+/+ ApoE−/− mice (Venegas-Pino et al. 2015). 5- to 6-week-old female ApoE−/− mice (B6.129P2-ApoEtm1Unc) were randomly divided into three groups: control, STZ-injected or glucosamine-supplemented. Multiple low-doses (40 mg/kg body weight/day for 10 days) of STZ (Sigma-Aldrich) were injected intraperitoneally to induce hyperglycemia. Glucosamine (5% w/v, Sigma-Aldrich) was supplemented in drinking water, which was given ad libitum. Female ApoE−/− mice were crossed with male ApoE−/−:Ins2+/Akita mice to produce ApoE−/−:Ins2+/Akita offspring. All mice were given unrestricted access to water and standard chow diet (Harlan-Teklad) and were maintained on a 12-h light/dark cycle throughout the study. Mice were fasted for 6 h prior to sacrifice at and 15 weeks of age (n = 8/group). Fasting blood glucose was measured using an UltraMini blood glucose meter (OneTouch). Mice were anaesthetized with 3% isoflurane prior to blood collection (in K2EDTA tubes) by cardiac puncture. Mice were euthanized by cervical dislocation and vasculature was flushed with saline. Tissues were collected, snap-frozen in liquid nitrogen, and stored at − 80 °C. Fasting plasma insulin was measured by enzyme-linked immunosorbent assay (CrystalChem). Plasma total cholesterol and triglycerides were measured using Infinity reagents (Thermo-Scientific).
2.2 Histochemistry
Harvested hearts were fixed in 10% formalin. The hearts were cut and paraffin-embedded aortic sinus was sectioned at 5 µm/section, as previously described (Venegas-Pino et al. 2013). Atherosclerotic lesions were visualized by staining serial sections with Masson’s Trichrome (Sigma-Aldrich). Stained sections were imaged using a Leitz LABORLUX S microscope connected to a DP72 Olympus camera. Lesion areas at the aortic sinus and the ascending aorta were quantified using Image J (1.48v) software. Lesion volume was computed as the area under the curve of lesion area versus distance from the aortic sinus.
2.3 Metabolomic analysis
Sample preparation, chromatographic separation conditions, mass spectrometer parameters, and metabolomic data analysis were performed as previous described (Dang et al. 2016). Metabolite features were identified and reported according to the Metabolomics Standard Initiative (Sumner et al. 2007).
2.4 In vitro experiments
Human aortic smooth muscle cells (HASMC), human aortic endothelial cells (HAEC), and human monocytic (Thp-1) cells were treated with ceramide (C18, Avanti Lipids), glucosyl-ceramide (GluCer, C18, Avanti Lipids), or lactosyl-ceramide (LacCer, C18, Avanti Lipids) at various concentrations (1, 10, or 50 µM) for 24 h. Total RNA was extracted using TRIzol® Reagent (Invitrogen) according to the manufacturer’s protocol. Complementary DNA (cDNA) was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) according to the manufacturer’s protocol. Quantitative RT-PCR was performed on the StepOne Plus (Applied Biosystems). Relative quantitative analysis was performed by normalizing data to the β-actin reference gene. Fold changes in expression levels were calculated relative to the corresponding vehicle-treated cell type. A detailed procedure can be found in Supplementary Methods.
2.5 Statistical analysis
One-way ANOVA followed by Tukey’s HSD test was performed using GraphPad Prism (v6.01). Correlation analysis, hierarchical heat map, partial least squares discriminant analysis (PLS-DA) and the derived VIP score plot, pathway analysis, principal component analysis, and Random Forest analysis were performed using MetaboAnalyst 3.0 (Xia et al. 2015). The t-test (two-tailed, unpaired heteroscedastic) analysis with FDR-adjusted p-value cut-off of 0.05 was used to identify the differential features in the metabolomic analyses. Auto-scaling followed by log transformation was applied in all multivariate analyses. The multivariate model validation parameters, R2 (i.e. prediction accuracy) and Q2 (i.e. model robustness), were used to assess the fitness of the model. Pearson correlation and Ward clustering algorithm were used in correlation analysis and heat map.
3 Results
3.1 Hyperglycemia and glucosamine supplementation accelerate atherosclerotic development in ApoE−/− mice
Plasma insulin was significantly lower in multiple low-dose STZ-injected and Ins2+/Akita mice, relative to the control mice, at 15 weeks of age. Compared to the normoglycemic ApoE−/− control, STZ injections resulted in a 2.4-fold increase in fasting blood glucose, whereas the introduction of the Ins2+/Akita mutation resulted in a 1.8-fold increase in fasting blood glucose (Table 1). Glucosamine supplementation did not alter the level of circulating glucose or insulin in ApoE−/− mice, compared to the non-treated control. No significant differences were observed in plasma triglyceride or cholesterol levels in any of the experimental groups.
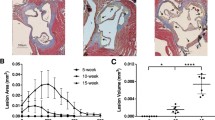
Both hyperglycemia and glucosamine supplementation significantly accelerated atherogenesis in ApoE−/− mice (Fig. 1). Atherosclerotic lesions in the aortic sinus and ascending aorta of STZ-injected mice were approximately 3.5-fold larger (p < 0.001) than those in the control group. Ins2+/Akita mutation (2.7-fold, p < 0.01) and glucosamine supplementation (2.7-fold, p < 0.05) resulted in a similar increase in atherosclerotic lesions in ApoE−/− mice, compared to the control.
Hyperglycemia and glucosamine supplementation accelerate the development of atherosclerosis in ApoE−/− mice. a Representative Masson’s trichrome stained images of aortic cross-sections of the control (Ctrl), glucosamine (GlcN)-supplemented, Ins2+/Akita and streptozotocin (STZ)-injected mice. Atherosclerotic lesions are highlighted by dotted lines. b Quantification of atherosclerotic lesion area from the aortic sinus and ascending aorta. c Lesion volumes were computed by calculating the area under the lesion area curve. n = 8/group, *p < 0.05, **p < 0.01 and ***p < 0.001 versus the control. Data are presented as mean ± SD
3.2 Metabolomics data analysis and quality control
We detected 4417 and 6224 metabolite features in negative and positive ionization modes in each plasma sample, respectively. Metabolomic data reduction (i.e. data clean-up) was performed by removing redundant (isotopes and adducts) and unreliable features (LC–MS peaks eluting in the ion-suppression regions). This left a total of 6369 metabolite features, with 2715 features in negative and 3654 features in positive ionization modes. Analysis of the quality control samples by principal component analysis showed a tight clustering of the pooled samples, indicating good instrumental reproducibility throughout the period of analysis (Supplementary Fig. S1).
3.3 Hyperglycemia and glucosamine supplementation induce similar changes in the plasma metabolomic profiles of ApoE−/− mice
PLS-DA score plots were constructed using the 6369 metabolite features detected. The comprehensive plasma metabolomic profiles of glucosamine-supplemented (Fig. 2a), Ins2+/Akita (Fig. 2b) and STZ-injected (Fig. 2c) mice were clearly differentiated from those of the controls. The magnitude of separation in the metabolomic profiles is depicted as component 1 in the PLS-DA score plot. A similar magnitude of separation (approximately 22%) and multivariate model robustness (Q2 = ~ 0.8) were observed in each treatment group, compared to the control. This suggests that glucosamine, chemically (STZ) and genetically (Ins2+/Akita)-induced hyperglycemia, each resulted in similar alterations in the plasma metabolomic profile of ApoE−/− mice.
Glucosamine and hyperglycemia induce changes in the plasma metabolomic profiles of ApoE−/− mice. PLS-DA score plots of the plasma metabolomic profiles of a glucosamine (GlcN)-supplemented, b Ins2+/Akita and c streptozotocin (STZ)-injected ApoE−/− mice versus the controls (Ctrl). R2 and Q2 indicate prediction accuracy and model robustness (after cross-validation) of the multivariate analysis, respectively. n = 8/group
3.4 Identification of commonly altered metabolite features in three mouse models of hyperglycemia-induced atherosclerosis
To identify the metabolite features that were commonly altered in each of the three models of hyperglycemia-induced atherosclerosis, we first performed pair comparison of each model group to the control (Fig. 3a–c). Next, a p-value filter step (FDR-adjusted p < 0.05) was applied to identify the significantly altered metabolite features in each pair comparison (Fig. 3d–f). The second-order comparison was then applied to identify similarly altered metabolite features between the three pair comparisons (Fig. 3g). The number of uniquely altered and shared features between the glucosamine-supplemented and two hyperglycemic models is summarized in a Venn diagram. The uniquely altered metabolite features in each model are model-specific and could potentially arise from the confounding effects of the treatment or mutation. To specifically focus on the common molecular alterations that are driving accelerated atherosclerosis in these three disease mouse models, the 62 shared metabolite features were identified for further exploration.
Identification of commonly altered metabolite features among three disease mouse models. a–c Heat map indicating the relative levels of the top 250 metabolite features (ranked by T-test) of the indicated pair groups of analysis. d–f T-test analysis was used to identify differential features (filtered by FDR-adjusted p-value smaller than 0.05) between the indicated groups. g Second-order analysis was applied to identify commonly altered metabolites between the three pair comparisons. Ctrl control, GlcN glucosamine, and STZ streptozotocin. n = 8/group
3.5 Metabolite identification of the commonly altered metabolites in three disease mouse models
A heat map of the 62 commonly altered metabolite features is shown in Fig. 4a. A total of 36 metabolites were identified (Supplementary Table S1), whereas 26 of the metabolite features remain unidentified. The 36 identified metabolites were associated with several metabolic pathways including inflammation, oxidative stress, glycerophospholipid, and sphingolipid metabolism (Supplementary Table S2). Mice with accelerated atherosclerosis show an upregulation in histamine biosynthesis which is associated with a systemic inflammatory immune activation. The level of several metabolites including oxidized fatty acids, oxidized cholesterol and S-(hydroxy-prostaglandin A1)-glutathione were augmented in all three experimental models versus the control, suggesting elevated levels of oxidative stress. We identified 14 and 12 altered metabolites from the glycerophospholipid and sphingolipid metabolism, respectively. These data suggest that these metabolic pathways are strongly associated with the pathogenesis of hyperglycemia-induced accelerated atherosclerosis.
Pathway analysis of the 62 commonly altered metabolites among the three mouse models of hyperglycemia-induced atherosclerosis. a Heat map indicating the relative levels of the 62 commonly altered metabolites. b Pathway analysis of the 36 identified metabolites. All matched pathways are plotted according to p-value from pathway enrichment analysis and pathway impact score from pathway topology analysis. Colour gradient and circle size indicate the significance of the pathway ranked by p-value (yellow: higher p-value and red: lower p-value) and pathway impact score (the larger the circle the higher the impact score), respectively. c PLS-DA score plots of control (Ctrl), glucosamine (GlcN)-supplemented, Ins2+/Akita and streptozotocin (STZ)-injected ApoE−/− mice, constructed based on the 36 identified metabolites. d Variable importance in the projection (VIP) score plot of the top 15 metabolites ranked by VIP scores. e A simplified schematic of the glycosphingolipid pathway
3.6 The glycosphingolipid pathway (sphingolipid metabolism) is strongly associated with hyperglycemia-induced atherosclerosis in the atherogenic mouse models
Pathway analysis was performed on the 36 identified metabolites. The results from integrating enrichment and pathway topology analyses were used to map out the identified metabolites into specific metabolic pathways (Fig. 4b). Sphingolipid metabolism was found to be among the top significantly affected metabolisms based on p-values (p < 2.4 × 10−8) and pathway impact scores (score = 0.38).
Select plasma metabolomic profiles, constructed based on the 36 identified metabolites, differentiate the three mouse models of accelerated atherosclerosis from the control (Fig. 4c). The variable importance in the projection (VIP) score plot highlights the top 15 metabolites ranked by VIP scores, which is a measure of a variable’s importance in the separation of the metabolomic profiles observed in a multivariate model (Fig. 4d). Additionally, we performed Random Forest classification on the 36 identified metabolites. Mean decrease in accuracy was used to rank metabolites based on their contributions to classification accuracy (Supplementary Fig. S2). There is a high degree of consistency (12 out of 15 metabolites) between the top-scoring metabolites identified by the VIP score and those identified by the mean decrease in accuracy score, confirming the importance of these metabolites in the pathology of hyperglycemia-induced atherosclerosis.
Several top-scoring metabolites in both analyses are from the glycosphingolipid pathway (which is a part of the sphingolipid metabolism), suggesting that these alterations are more likely to be actual biological changes, rather than random artifacts. As such, we continued to further explore this pathway in vitro. A simplified schematic of the glycosphingolipid pathway is shown in Fig. 4e.
3.7 Glycosphingolipid metabolites induce oxidative stress and increase levels of inflammatory cytokines in cultured human vascular cells
Atherosclerosis is an inflammatory disease and both endoplasmic reticulum (ER) stress and oxidative stress have a well-established causal role in atherosclerotic development and progression. Correlation analysis of the 36 identified metabolites showed a strong association between glycosphingolipids and metabolites involved in oxidative stress and inflammation pathways (Supplementary Fig. S3). To the best of our knowledge, there are no known circulating markers of ER stress. As such, the effect of glycosphingolipid metabolites on the activation of ER stress, oxidative stress, and inflammation was investigated in human vascular cell lines that are relevant to atherosclerosis, including macrophages, HAEC, and HASMC.
Markers of ER stress (78 and 94 kDa glucose-regulated proteins and C/EBP homologous protein), oxidative stress (catalase and glutathione peroxidase), and inflammation (IL-6 and IL-1β) were assessed. Ceramide, GluCer, and LacCer were each found to cause a significant increase in the expression levels of transcripts encoding antioxidant genes, including catalase (Fig. 5a–c) and glutathione peroxidase (Supplementary Fig. S4A-C), in all three cell types. The expression of inflammatory cytokines IL-6 (Fig. 5d–f) and IL-1β (Supplementary Fig. S4D-F) were also increased in all three cell types, upon treatment with ceramide, GluCer, and LacCer. All the effects of glycosphingolipid metabolites were in a dose–response manner. No significant effects were observed in the expression of ER stress response genes (data not shown). Together, these results suggest that glycosphingolipid metabolites are potential inducers of oxidative stress and inflammation in atherogenesis.
Glycosphingolipid metabolites induce oxidative stress and increase the levels of inflammatory cytokines in human vascular cells. Macrophages, human aortic endothelial cells (HAEC), and human aortic smooth muscle cells (HASMC) were incubated with ceramide, GluCer, or LacCer for 24 h. Expression levels of a–c catalase and d–f IL-6 were assessed by real-time PCR. Data are presented as mean ± SD. n = 5/group, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 versus the controls
4 Discussion
The pathophysiology of diabetes and atherosclerosis has previously been explored individually at the metabolomic level (Friedrich 2012; Goonewardena et al. 2010; Meikle and Summers 2017). However, the molecular mechanisms behind the complex interplay between diabetes and its macrovascular complications are not well understood. Murine models are currently preferred for atherosclerosis investigation because of advantages including ease of genetic manipulation, which allows for specific gene knockout or knockin through the whole-body or in specific tissue/cell type of interest. Currently, there is no ideal experimental model to study diabetic atherosclerosis, as each has its own advantages and disadvantages (King 2012; Wu and Huan 2007). To the best of our knowledge, this is the first study that involves the simultaneous comprehensive metabolomic analyses of three distinct murine models to facilitate the identification of common metabolic alteration(s), independent of model-specific changes or artifacts.
“Type 1-like” hyperglycemic mouse models were utilized in this study in order to minimize the potential confounding effects of obesity and insulin resistance that are associated with type 2 diabetes. The glucosamine-supplemented mouse model was included to account for potential confounding complications associated with insulinopenia. In each of the models used in this study, we have shown that accelerated atherosclerosis occurs independent of any changes in plasma triglyceride or cholesterol levels. Therefore, the accelerated progression of atherosclerosis in our mouse models can be attributed to increased levels of glucose and/or downstream metabolites of glucose (i.e. glucosamine). The two hyperglycemic mouse models exhibited similar alterations in the plasma metabolome of ApoE−/− mice, compared to glucosamine-supplemented mice. This suggests that elevated levels of glucosamine accelerate the progression of atherosclerosis, independent from the effect of insulin, which is consistent with hyperglycemia being an independent risk factor for CVD (Juutilainen et al. 2008; Seshasai et al. 2011).
Identification of the commonly altered metabolite features between the three experimental models revealed changes in glycerophospholipid and sphingolipid metabolism. Previously, alterations in these lipid pathways are associated with the pathogenesis of atherosclerosis in normoglycemic ApoE−/− mice (Dang et al. 2016). Recently, it has been shown that sphingolipids are elevated in human atherosclerotic plaques associated with symptoms (Edsfeldt et al. 2016). Another independent study revealed associations of sphingolipids with obesity and insulin resistance in young adults (Rauschert et al. 2016). Despite the progressive relationship between sphingolipid metabolism and the pathophysiology of atherosclerosis, the specific molecular mechanisms and metabolic pathways have not been investigated, especially in the context of hyperglycemia-associated atherosclerosis.
Glycosphingolipid was found to be strongly associated with the pathogenesis of hyperglycemia-induced atherosclerosis in our atherogenic mouse models. Glycosphingolipids are not only ubiquitous building blocks of eukaryotic cell membranes, but are also signalling molecules that regulate a diverse range of cellular processes (Zheng et al. 2006). Inhibiting the synthesis of this pathway has shown to improve glucose homeostasis in diabetic fatty rats (Zhao et al. 2007) and reduce atherosclerotic lesions in ApoE*3 Leiden mice (Bietrix et al. 2010). However, the specific roles of the glycosphingolipid pathway in the pathogenesis of hyperglycemia-induced accelerated atherosclerosis are not clear. In this study, glycosphingolipid metabolites were found to induce oxidative stress and inflammation in cultured human vascular cells. This suggests that these glycosphingolipid metabolites are not only strongly associated with the pathology of hyperglycemia-induced atherosclerosis, but may also contribute to the activation of pro-atherogenic processes. Future studies are required to determine if these lipids are sufficient to induce inflammation and oxidative stress (and promote atherosclerosis) in vivo.
This study utilizes a high-throughput, high-sensitivity, and comprehensive analytical system, which is able to simultaneously detect a wide range of polar and nonpolar metabolites in complex plasma samples (Chalcraft and McCarry 2013). The identification of a strong association between glycosphingolipids and the pathogenesis of hyperglycemia-induced atherosclerosis suggests that future studies should focus on lipidomics analysis of plasma samples using lipid-focused sample extraction and analytical techniques in order to increase the detection coverage of the lipidome.
In conclusion, we show that mice with accelerated atherosclerosis present with distinct metabolomic profiles containing elevated levels of circulating sphingolipid metabolites, especially glycosphingolipids. Additionally, treatment of human vascular cells with ceramide, GluCer, and LacCer induced oxidative stress and inflammation, suggesting a potential causal role in disease development. Our findings also provide the first evidence that glycosphingolipid metabolism is a potential link between hyperglycemia and accelerated atherosclerosis. This suggests that the glycosphingolipid pathway may be a potential therapeutic target to block or slow atherogenesis in diabetic patients.
References
Bietrix, F., Lombardo, E., Roomen, C. P. A. A., Van Ottenhoff, R., Vos, M., Rensen, P. C. N., et al. (2010). Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 leiden and low-density lipoprotein receptor -/- mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 931–937.
Chalcraft, K. R., & McCarry, B. E. (2013). Tandem LC columns for the simultaneous retention of polar and nonpolar molecules in comprehensive metabolomics analysis. Journal of Separation Science, 36, 3478–3485.
Dang, V. T., Beriault, D. R., Deng, A., Shi, Y., & Werstuck, G. H. (2015). Glucosamine-induced ER stress accelerates atherogenesis: A potential link between diabetes and cardiovascular disease. Journal of Molecular and Genetic Medicine, 9, 4.
Dang, V. T., Huang, A., Zhong, L. H., Shi, Y., & Werstuck, G. H. (2016). Comprehensive plasma metabolomic analyses of atherosclerotic progression reveal alterations in glycerophospholipid and sphingolipid metabolism in apolipoprotein E-deficient mice. Scientific Reports, 6, 35037.
Edsfeldt, A., Dunér, P., Ståhlman, M., Mollet, I. G., Asciutto, G., Grufman, H., et al. (2016). Sphingolipids contribute to human atherosclerotic plaque inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 36, 1132–1140.
Falk, E. (2006). Pathogenesis of atherosclerosis. Journal of the American College of Cardiology, 47, C7–C12.
Fiehn, O. (2002). Metabolomics-the link between genotypes and phenotypes. Plant Molecular Biology, 48, 155–171.
Friedrich, N. (2012). Metabolomics in diabetes research. Journal of Endocrinology, 215(1), 29–42.
Glass, C. K., & Witztum, J. L. (2001). Atherosclerosis: The road ahead review. Cell, 104, 503–516.
Goonewardena, S. N., Prevette, L. E., & Desai, A. A. (2010). Metabolomics and atherosclerosis. Current Atherosclerosis Reports, 12, 267–272.
Haffner, S. M., Lehto, S., Ronnemaa, T., Pyorala, K., & Laakso, M. (1998). Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. The New England Journal of Medicine, 339(4), 229–234.
Hossain, P., Kawar, B., & Nahas, E., M (2007). Obesity and diabetes in the developing world—A growing challenge. The New England Journal of Medicine, 356(3), 213–215.
Juutilainen, A., Lehto, S., Ronnemaa, T., Pyorala, K., & Laakso, M. (2008). Similarity of the impact of type 1 and type 2 diabetes on cardiovascular mortality in middle-aged subjects. Diabetes Care, 31(4), 714–719.
King, A. J. F. (2012). The use of animal models in diabetes research. British Journal of Pharmacology, 166, 877–894.
Kunjathoor, V. V., Wilson, D. L., & LeBoeuf, R. C. (1996). Increased atherosclerosis in streptozotocin-induced diabetic mice. Journal of Clinical Investigation, 97(7), 1767–1773.
Lehto, S., Rönnemaa, T., Pyörälä, K., & Laakso, M. (2000). Cardiovascular risk factors clustering with endogenous hyperinsulinaemia predict death from coronary heart disease in patients with Type II diabetes. Diabetologia, 43(2), 148–155.
Meikle, P. J., & Summers, S. A. (2017). Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nature Reviews Endocrinology, 13(2), 79–91.
Meir, K. S., & Leitersdorf, E. (2004). Atherosclerosis in the apolipoprotein E-deficient mouse a decade of progress. Arteriosclerosis, Thrombosis, and Vascular Biology, 24, 1006–1014.
Nakashima, Y., Plump, A. S., Raines, E. W., Breslow, J. L., & Ross, R. (1993). ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis, Thrombosis, and Vascular Biology, 14(1), 133–140.
Patterson, C. C., Dahlquist, G. G., Gyürüs, E., Green, A., & Soltész, G. (2009). Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: A multicentre prospective registration study. Lancet, 373(9680), 2027–2033.
Patti, G. J., Yanes, O., & Siuzdak, G. (2012). Metabolomics: The apogee of the omics trilogy. Nature Reviews: Molecular Cell Biology, 13(4), 263–269.
Rauschert, S., Uhl, O., Koletzko, B., Kirchberg, F., Mori, T. A., Huang, R., et al. (2016). Lipidomics reveals associations of phospholipids with obesity and insulin resistance in young adults. Journal of Clinical Endocrinology and Metabolism, 101, 871–879.
Reddick, R. L., Zhang, S. H., & Maeda, N. (1994). Atherosclerosis in mice lacking Apo E. Arteriosclerosis and Thrombosis, 14(1), 141–148.
Ross, R. (1993). The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature, 362(6423), 801–809.
Seshasai, S. R. K., Kaptoge, S., Thompson, A., Di Angelantonio, E., Gao, P., Sarwar, N., et al. (2011). Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England Journal of Medicine, 364(9), 829–841.
Shalhoub, J., Sikkel, M. B., Davies, K. J., Vorkas, P., Want, E. J., & Davies, A. H. (2014). Systems biology of human atherosclerosis. Vascular and Endovascular Surgery, 48(1), 5–17.
Sumner, L. W., Amberg, A., Barrett, D., Beale, M. H., Beger, R., Daykin, C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3(3), 211–221.
Venegas-Pino, D. E., Banko, N., Khan, M. I., Shi, Y., & Werstuck, G. H. (2013). Quantitative analysis and characterization of atherosclerotic lesions in the murine aortic sinus. Journal of Visualized Experiments, 82, 50933.
Venegas-Pino, D. E., Wang, P.-W., Stoute, H. K., Singh-Pickersgill, N. A., Hong, B. Y., Khan, M. I., et al. (2015). Sex-specific differences in an ApoE–/–:Ins2+/Akita mouse model of accelerated atherosclerosis. The American Journal of Pathology, 186, 67–77.
Whitman, S. C. (2004). A practical approach to using mice in atherosclerosis research. The Clinical Biochemist Reviews, 25, 81–93.
Wu, K. K., & Huan, Y. (2007). Diabetic atherosclerosis mouse models. Atherosclerosis, 191(2), 241–249.
Xia, J., Sinelnikov, I. V., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Research, 43, W251–W257.
Zhao, H., Przybylska, M., Wu, I., Zhang, J., Siegel, C., Komarnitsky, S., et al. (2007). Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes, 56, 1210–1218.
Zheng, W., Kollmeyer, J., Symolon, H., Momin, A., Munter, E., Wang, E., et al. (2006). Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochimica et Biophysica Acta, 1758, 1864–1884.
Acknowledgements
The authors would like to thank Dr. Peter Shi for providing the human cell lines for the in vitro experiments and the Centre for Microbial Chemical Biology at McMaster University for access to the Mass Spectrometer. This research was supported by operating grants from the Canadian Institutes of Health Research (MOP62910 and MOP142248) and the Heart and Stroke Foundation of Canada (G-17-0017029). V.T.D. is supported by an International Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
GHW and VTD conceived and designed the study. VTD and AH performed mouse experiments. VTD, LHZ and AD conducted and analyzed histochemistry and in vitro experiments. VTD performed all metabolomic experiments and analyses. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Vi Dang, Lexy Zhong, Aric Huang, Arlinda Deng and Geoff Werstuck declare no conflict of interest.
Ethical approval
All animal procedures were pre-approved by, and performed in accordance with, the McMaster University Animal Research Ethics Board and conform with the guidelines of the Canadian Council on Animal Care.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dang, V.T., Zhong, L.H., Huang, A. et al. Glycosphingolipids promote pro-atherogenic pathways in the pathogenesis of hyperglycemia-induced accelerated atherosclerosis. Metabolomics 14, 92 (2018). https://doi.org/10.1007/s11306-018-1392-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1392-2