Abstract
Introduction
Comparative metabolic profiling of different human cancer cell lines can reveal metabolic pathways up-regulated or down-regulated in each cell line, potentially providing insight into distinct metabolism taking place in different types of cancer cells. It is noteworthy, however, that human cell lines available from public repositories are deposited with recommended media for optimal growth, and if cell lines to be compared are cultured on different growth media, this introduces a potentially serious confounding variable in metabolic profiling studies designed to identify intrinsic metabolic pathways active in each cell line.
Objectives
The goal of this study was to determine if the culture media used to grow human cell lines had a significant impact on the measured metabolic profiles.
Methods
NMR-based metabolic profiles of hydrophilic extracts of three human pancreatic cancer cell lines, AsPC-1, MiaPaCa-2 and Panc-1, were compared after culture on Dulbecco’s Modified Eagle Medium (DMEM) or Roswell Park Memorial Institute (RPMI-1640) medium.
Results
Comparisons of the same cell lines cultured on different media revealed that the concentrations of many metabolites depended strongly on the choice of culture media. Analyses of different cell lines grown on the same media revealed insight into their metabolic differences.
Conclusion
The choice of culture media can significantly impact metabolic profiles of human cell lines and should be considered an important variable when designing metabolic profiling studies. Also, the metabolic differences of cells cultured on media recommended for optimal growth in comparison to a second growth medium can reveal critical insight into metabolic pathways active in each cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabolic profiling of human cell lines can be used to characterize their intrinsic metabolism, to compare the metabolic profiles of different cell lines, and to determine how cell metabolism changes in response to application of various treatments or specific therapies, in addition to many other applications (Bayet-Robert et al. 2010; Brougham et al. 2011; Cano et al. 2010; Cao et al. 2014; Chatterjee et al. 2017; Cuperlovic-Culf et al. 2014; Gierok et al. 2014; Lane et al. 2017; Lefort et al. 2014; MacIntyre et al. 2011; Maria et al. 2015; Morin et al. 2013; Pan et al. 2011, 2013; Righi et al. 2009; Santana-Filho et al. 2017; Schwarzfischer et al. 2017; Shao et al. 2014; Sorice et al. 2016; Spratlin et al. 2011; Teahan et al. 2011; Tiziani et al. 2009; Tripathi et al. 2012; Wallace et al. 2013; Watanabe et al. 2012; Wen et al. 2015; Yin et al. 2016). It is important to recognize that the choice of culture media has the potential to impact the measured metabolic profiles, and therefore to affect the conclusions drawn from such experiments, regardless of the metabolic profiling technique that is applied. Consequently, the choice of culture media is an important consideration for both NMR and LC/MS based metabolic profiling studies, and for any other metabolic or omics profiling technique that might be applied.
It is common for researchers to use the culture media recommended for optimal growth included in the repository record for the cell line when conducting metabolic profiling analyses of human cell lines (Cano et al. 2010; Cuperlovic-Culf et al. 2014; Lane et al. 2017; Morin et al. 2013; Pan et al. 2011, 2013; Shao et al. 2014; Teahan et al. 2011; Tiziani et al. 2009; Watanabe et al. 2012; Wen et al. 2015). As an example, the recommendation of DMEM as the optimal growth medium for the human pancreatic cancer cell line MiaPaCa-2 can be found at the following ATCC website link (https://www.atcc.org/Products/All/CRM-CRL-1420.aspx#culturemethod). In some cases, the conclusions inferred from a metabolic profiling study may be largely independent of the choice of culture media. For example, the choice of culture media may be of little consequence if one is comparing the effect that an enzyme inhibitor has on the metabolism of a given human cell line, as long as the comparison is performed using the same culture medium for the cells both in the presence and absence of the inhibitor. However, even this conclusion cannot be simply assumed without repeating the analysis with at least one alternative culture medium to ensure that the cell metabolism does not respond differently if the cells are cultured on a different medium. For example, the cells might respond differently to an inhibitor if they were grown under the stress of a minimal medium compared to growth on a rich medium. The choice of culture medium is critical when comparing the metabolism of two or more cell lines since intrinsic metabolic differences may be masked if the cells are grown on different medium recommended for optimal growth of each cell line. Despite its potential importance, the dependence of NMR-based metabolic profiling of human cell lines on the choice of culture media has not been thoroughly investigated in the literature.
In this study, we have determined how the metabolic profiles of three different human pancreatic cancer cell lines, Panc-1, MiaPaCa-2 and AsPC-1, depended on the culture media used for cell growth. Whereas the recommended culture medium from ATCC is DMEM for Panc-1 and MiaPaCa-2, the recommended culture medium for AsPC-1 is RPMI-1640. DMEM and RPMI-1640 differ significantly in their glucose and glutamine composition, with DMEM having a higher content of both glucose and glutamine. RPMI-1640 has more amino acids compared to DMEM. Each cell line was cultured on both RPMI-1640 and DMEM and their metabolic profiles determined using NMR spectroscopy. The metabolic profiles of each cell line grown on DMEM or RPMI-1640 were compared to assess how strongly the metabolic profiles depended on the choice of growth media. Pairwise comparisons of the metabolic profiles of the Panc-1, MiaPaCa-2 and AsPC-1 cell lines cultured on the same growth media were also performed to identify differences in intrinsic metabolism in each cell line.
2 Materials and methods
2.1 Cell cultures
Panc-1, MiaPaCa-2 and AsPC-1 were purchased from the American Type Culture Collection (ATCC, Manassas, VA). MiaPaCa-2, Panc-1 and AsPC-1 cells were grown on the high glucose Dulbecco’s Modified Eagle Medium (DMEM) (Thermofisher, Pittsburgh, PA) and on Roswell Park Memorial Institute medium (RPMI-1640) (Thermofisher, Pittsburgh, PA), both supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (ThermoFischer, Pittsburgh PA). Cells were harvested and extracted as previously described by Watanabe et al. (Watanabe et al. 2012).
2.2 Preparation of cell extracts for NMR analysis
Following chloroform/methanol extraction of 12 replicate samples of each cell line, the hydrophilic fractions were flash frozen using liquid nitrogen and dried using lyophilization (Labconco, Kansas City, MO). Hydrophilic extracts were resuspended in buffer and NMR spectroscopy was conducted as described by Watanabe et al. (2012) except the data were collected at a different temperature and a different spectral width was used for the 1D NOESY (noesygppr1d) experiments (Goodpaster et al. 2010, 2011; Watanabe et al. 2012). NMR spectra were recorded at 600 MHz at 298 K. The one-dimensional first increments of 1H NOESY NMR experiments were recorded with a spectral width of 17.0 ppm, an acquisition time of 3.2 s and a recycle time of 4 s. The 90° pulse widths, ranging between 7.9 and 8.5 µs, were determined using the Topspin 3.2.1 (Bruker Biospin, Billerica, MA) automatic pulse calculation experiment (pulsecal). The 2D 1H-1H TOCSY was collected using the TOCSY (mlevgpph19) pulse sequence at 298 K, 32 scans, a 1.5 s recycle delay with acquisition times of 0.17 s (F2) and 0.042 s (F1) and a TOCSY mixing time of 0.06 s. Spectral widths were set to 10 ppm for both the direct and indirect dimensions.
2.3 Data analysis
Statistical significance analyses and principal components analyses (PCA) were conducted using AMIX (Bruker BioSpin, Billerica, MA) as previously described by Goodpaster et al. (2010) (Goodpaster and Kennedy 2011), except that manual bucketing was used to avoid peaks being divided across multiple buckets, which can occur with automated simple rectangular bucketing. Manual buckets were identified for any resonance present in spectra of samples cultured on either DMEM or RPMI-1640. Buckets with p values less than the Bonferroni corrected α-value (αBC) were considered significant (Table 1). The loadings plot buckets were color-coded based on p values as follows black (> α), blue (α–αBC), green (αBC–10−5), yellow (10−6–10−7), orange (10−8–10−9), red (10−10–0), where α indicates the uncorrected critical value = 0.05 and αBC indicates the Bonferonni-corrected α value, equal to α divided by the number of manual buckets created for the spectrum. Values in parentheses indicate the range of p values for a loading with that color, e.g. yellow (10−6–10−7) indicates that all loadings with p values with exponents of 10−6 or 10−7 will be colored yellow and loadings with p values with exponents of 10−8 or 10−9 will be colored orange, with no gap in p values. PLS-DA was performed using the SIMCA-P ver. 11.0 software package (Umetrics, Umea, Sweden) as described by Chihanga et al. (2017).
2.4 Identification of metabolites
Resonances of metabolites that had statistically significant different concentrations in samples cultured on the two different culture media based on the statistical significance analysis method introduced by (Goodpaster et al. 2010) metabolites were identified using ChenomX NMR Suite software 8.1 (ChenomX Inc., Edmonton, Alberta, Canada) augmented with the HMDB metabolite package, and reviewed and confirmed by 2D 1H-1H-TOCSY NMR experiments. The Minimum Standards Initiative assignment levels for all identified metabolites are presented in the Online Resource 1, Table S-1.
2.5 Pathway analysis involving metabolites that had significantly different concentrations in media and cell line comparisons
The complete list of metabolites identified to have significantly different concentrations in the media comparisons or cell line comparisons (Online Resource 1, Table S-2) was submitted to the MetaboAnalyst 3.6 software server (Xia et al. 2009, 2012, 2015; Xia and Wishart 2011a, b, 2016). The pathway analysis (targeted) module was used to generate the pathway analysis results.
3 Results and discussion
3.1 Study design
Twelve biological replicates were prepared for each cell line for each culture media to increase the statistical sampling power for metabolic profiling comparisons. A total of 72 samples were analyzed using 1H NMR spectroscopy. A summary of the experimental design is presented in the Online Resource 1, Fig. S-1.
3.2 NMR spectral analysis
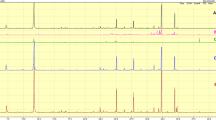
One dimensional 1H NMR spectroscopy data were collected and used to define the metabolic profiles of each cell line. Representative NMR spectra of hydrophilic extracts of each cell line are shown in Fig. 1. Metabolites identified as being significantly different in the comparisons are listed in Table 2. Entire spectrum integration was used to generate total intensity plots for each spectrum (Online Resource 1, Fig. S-2) using Topspin 2.1.1 prior to PCA and statistical analysis. The total intensity plots were used to determine if there was experimental evidence to justify normalization prior to analysis. Normalization of the NMR spectra to total intensity can be used to correct for different numbers of cells harvested from the Petri dish of each biological replicate cultured over the same period of time. Different numbers of cells can occur in Petri dishes cultured over the same period of time due to small growth variations in biological replicates, due to small differences in inoculation cell numbers or volume, differences in intrinsic doubling of different cell lines, or due to differential growth rates when cells are grown on different growth media. Based on the total intensity analysis, all spectra were normalized to total intensity to correct for differences in the number of cells in Petri dishes prior to comparisons.
3.3 Simultaneous PCA cluster analysis of all three cell lines
In order to gain insight into the global differences in the metabolic profiles of the three cell lines cultured on the various culture media, PCA was performed on the three cell lines cultured on DMEM (Fig. 2a), for the three cell lines cultured on RPMI-1640 medium (Fig. 2b), for the three cell lines cultured on their respective recommended media (Fig. 2c), and finally, for three cell lines cultured on both culture media (Fig. 2d). The PCA scores plots exhibited complete cluster separation of the three cell lines when cultured on DMEM (Fig. 2a) and when cultured on their respective recommended media (Fig. 2c), which indicated distinct metabolic profiles for each cell line when cultured with those growth media. When the cells were cultured on RPMI-1640, the metabolic profiles of the Panc-1 and MiaPaCa-2 cells did not separate into distinct clusters, however, they did separate from the AsPC-1 cells (Fig. 2b). When all cell lines were compared on all culture media, the Panc-1 cells cultured on DMEM were strongly separated from all other cells cultured on either growth media along PC1 (Fig. 2d), whereas the Panc-1 and MiaPaCa-2 cells cultured on RPMI were overlapped along both PC1 and PC2 but separated from AsPC-1 cells cultured on RPMI-1640 and AsPC-1 and MiaPaCa-2 cells cultured on DMEM along the PC2 direction (Fig. 2d).
PCA scores plots for various comparisons of all three Panc-1, MiaPaCa-2 and AsPC-1 cell lines. PCA scores plot for the three-way comparison of Panc-1, MiaPaCa-2 and AsPC-1 cells cultured on (a) DMEM, (b) RPMI-1640, (c) their respective recommended media, and (d) the six-way comparison of all cell lines cultured on both DMEM and RPMI-1640
3.4 Comparison of Panc-1 grown on DMEM and RPMI-1640
One-dimensional 1H NMR spectra were initially analyzed using unbiased and untargeted multivariate statistical analysis. The PCA scores plot for Panc-1 grown on DMEM and RPMI-1640 showed cluster separation without overlap at the 95% confidence intervals (Fig. 3a and Online Resource 1, Table S-3). The corresponding PCA loadings plot (Online Resource 1, Fig. S3A) enabled identification of those NMR resonances whose variance was responsible for group separation in the PCA scores plot in Fig. 3a. The intensities of 128 out of 174 bucketed resonances were statistically different between the DMEM and RPMI-1640 groups (Table 1). These resonances belonged to 21 metabolites with acetate, alanine, aspartate, ethanol, isoleucine, lactate, succinate, valine, leucine, tyrosine and phenylalanine higher in concentration in RPMI-1640 cells and creatine, glutamate, glutathione, glycerophosphocholine, glycine, myoinositol, NAD+, phosphorylcholine, and taurine higher in concentration in DMEM cells (Table 2). 84 buckets remained un-identified due to database limitations and overlap and 18 out of these 84 buckets fell into the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot for the comparison, shown in Fig. 3b, showed strong separation along t[1] with R2Y(cum) = 0.999 and Q2(cum) = 0.996. The VIP scores in descending magnitude are listed in Online Resource 3, Table S-5, with all VIP scores greater than 1.0 colored red. The metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis.
PCA and PLS-DA scores plots of media comparisons for Panc-1, MiaPaCa-2 and AsPC-1 cell lines. PC1 versus PC2 scores plots for growth media comparisons of hydrophilic extracts of (a) Panc-1, (c) MiaPaCa-2 and (e) AsPC-1. The 95% confidence interval for each clusters is indicated by an oval line. The t[1] versus t[2] PLS-DA scores plots analyses of (b) Panc-1, (d) MiaPaCa-2 and (f) AsPC-1. The ellipse represents the Hotelling T2 95% confidence interval
Comparison of the composition of the DMEM and RPMI-1640 media provided a starting place for interpreting the difference in the metabolic profiles. DMEM has double the concentration of glucose and glutamine compared to RPMI-1640 (Online Resource 1, Table S-6). On the other hand, RPMI-1640 contains arginine, asparagine, aspartic acid, glutamic acid, histidine and proline (Online Resource 1, Table S-6), which are all absent in DMEM. Complete culture media composition is included in Online Resource 1, Table S-6. Another foundation facilitating interpretation of the metabolic profiling differences came from identification of the metabolic pathways involving the metabolites identified to have significantly different concentrations in the various cell lines depending on the culture media based on the MetaboAnalyst 3.6 software package. The resulting analysis identified that the 23 metabolites were involved in 43 different metabolic pathways (Fig. 4; Online Resource 4, Tables S-7 and Online Resource 5, Tables S-8).
Plot of the metabolic pathway analysis based on metabolites that are significantly different between cell line comparisons. The significance of the metabolic pathways is indicated along the y-axis. The impact of the metabolic pathways is plotted along the x-axis. The plot was generated using the MetaboAnalyst 3.6 software. The numbers correspond to metabolic pathways of decreasing significance and are detailed in Online Resource 4
DMEM is the recommended culture medium for Panc-1 cells. Optimum cell growth in Panc-1 cells, presumably driven by higher glucose in DMEM, appeared to also drive lipid synthesis (pathway 29 in Fig. 4) as indicated by higher concentrations of glycero-phosphocholine and phosphorylcholine (Table 2), which are building blocks for fatty acids synthesis essential for membrane synthesis during cell growth (Morin et al. 2013; Röhrig and Schulze 2016; Santoyo-Ramos et al. 2012). NAD+ was in higher concentration in Panc-1 cells grown in DMEM. NAD+ is synthesized using either niacinamide or tryptophan as starting materials (Cantó et al. 2015) and both of these metabolites were present in higher concentration in DMEM. The higher concentration of NAD+ in DMEM grown Panc-1 cells appeared to reflect the higher relative abundance of niacinamide and tryptophan in the DMEM medium feeding NAD+ synthesis. Taurine was higher in DMEM cultures of Panc-1 (Table 2) and not present in either growth medium. Taurine is involved in nitrogen metabolism and taurine and hypotaurine metabolism (pathways 2 and 3 in Fig. 4) and is reported to play a role in membrane stabilization (Schaffer et al. 1989), which could play a role in supporting rapid cell proliferation. Taurine is synthesized from byproducts of cysteine degradation (Stipanuk et al. 2006; Vitvitsky et al. 2011). Both RPMI-1640 and DMEM contain cysteine but cysteine metabolism seemed to be lower in RPMI-1640 cultured Panc-1 based on the lower concentrations of taurine and glutathione observed in Panc-1 cells grown on RPMI-1640 (Table 2) and both taurine and glutathione are dependent on cysteine for their production (Stipanuk et al. 2006; Vitvitsky et al. 2011; Wu et al. 2004).
Panc-1 cells had higher concentrations of several amino acids when grown on RPMI-1640, including alanine, aspartate, isoleucine, valine, leucine, tyrosine and phenylalanine. These amino acids all fall into the most significant metabolic pathway identified by MetaboAnalyst (pathway 1, Fig. 4), which corresponded to aminoacyl-tRNA biosynthesis activity in support of protein synthesis required for cell proliferation. Many of these amino acids show up in other of the most significant metabolic pathways identified by the MetaboAnalyst analysis and can be inspected in Fig. 4 and in Online Resources 4 and 5. Tyrosine and phenylalanine were in higher concentration in Panc-1 cells grown in RPMI-1640 even though phenylalanine was present at about four times higher concentration in DMEM medium and tyrosine was about at equal concentrations in both media. Higher tyrosine levels in RPMI-1640 Panc-1 were also consistent with increased protein synthesis demands of Panc-1 cells in RPMI-1640, and the response of Panc-1 cells attempting to meet those demands by upregulating synthesis of these amino acids.
Panc-1 cells also had significantly higher levels of lactate and succinate when cultured on RPMI-1640 (Table 2), with neither present in the culture media. Lactate is an end product of glycolysis converted from pyruvate by lactate dehydrogenase (Rogatzki et al. 2015). Higher levels of lactate indicated a higher state of glycolysis in Panc-1 cells grown on RPMI-1640 compared to DMEM. Higher concentrations of alanine, produced from pyruvate by transamination (Rogatzki et al. 2015), were consistent with higher glycolytic activity in RPMI-1640 cells. Succinate concentrations were also higher in Panc-1 cells grown on RPMI-1640. Succinate, a metabolic intermediate of the citric acid cycle, normally accumulates and is concentrated in mitochondria. Succinate is also known to be an oncometabolite that accumulates in the cytoplasm either in the presence of mutations to succinate dehydrogenase or in response to pseudohypoxia (Jain et al. 2013; Sciacovelli and Frezza 2016). Higher levels of succinate in Panc-1 cells grown on RPMI-1640 possibly indicated a higher state of pseudohypoxia compared to growth on DMEM. Ethanol was also higher in Panc-1 cells grown on RPMI-1640 (Table 2). Since ethanol is produced from conversion of pyruvate to acetaldehyde by pyruvate decarboxylase, which is then converted to ethanol by alcohol dehydrogenase, there seemed to be higher conversion of pyruvate to ethanol rather than consumption of pyruvate for generation of ATP via the citric acid cycle when Panc-1 cells were grown in RPMI-1640. As a result of less pyruvate entering the citric acid cycle from glycolysis and having reduced glutamine entering the TCA cycle (Daye and Wellen 2012a; Li et al. 2016) there was a decrease in the TCA cycle metabolic intermediates such as succinate and glutamate (Table 2), and this was further driven by the absence of sodium pyruvate in RPMI-1640 media (Online Resource 1, Table S-6).
3.5 Comparison of MiaPaCa-2 cells grown on DMEM and RPMI-1640
The PCA scores plot for MiaPaCa-2 grown on DMEM and RPMI-1640 (Fig. 3c) showed cluster separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). The intensities of 110 out of 150 bucketed resonances were statistically different between the DMEM and RPMI-1640 groups (Table 1) as shown in the corresponding PCA loadings plot (Online Resource 1, Fig. S-3B). Acetate, alanine, aspartate, glycerophosphocholine, ethanol, isoleucine, succinate, valine, leucine, tyrosine, phenylalanine had higher concentrations in RPMI-1640 cells and creatine, glutamate, glutathione, glycine, lactate, myoinositol, NAD+, phosphorylcholine, and UDP-N-acetyl-glucosamine had higher concentrations in DMEM cells (Table 2). 70 buckets remained un-identified due to database limitations and overlap, 6 out of 70 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 3d) showed strong separation along t[1] with R2Y(cum) = 0.992 and Q2(cum) = 0.966. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis.
The recommended media for MiaPaCa-2 cell culture is DMEM. The metabolic profile differences between MiaPaCa-2 cells grown on DMEM compared to growth on RPMI-1640 was very similar to those observed for Panc-1 cells. MiaPaCa-2 cells also had higher concentrations of several amino acids when grown on RPMI-1640, including alanine, isoleucine, valine, leucine, tyrosine and phenylalanine. Again, these amino acids all fall into the most significant metabolic pathway identified by MetaboAnalyst (pathway 1, Fig. 4), which corresponded to aminoacyl-tRNA biosynthesis activity in support of protein synthesis required for cell proliferation. Lactate, succinate, and alanine were significantly higher in MiaPaCa-2 cultured on RPMI-1640 (Table 2) indicating growth primarily driven by glycolysis and glutaminolysis. The lower glucose concentration in the RPMI-1640 medium (Online Resource 1, Table S-6) could promote activation of gluconeogenesis pathways to compensate for lower glucose as a means of supporting rapid cell division in MiaPaCa-2 cancer cells (Balsa-Martinez and Puigserver 2015). Activation of gluconeogenesis pathways could also explain the increase in non-essential amino acid concentrations such as alanine and tyrosine, which would support anaplerosis in low glucose and glutamine environments (Balsa-Martinez and Puigserver 2015).
MiaPaCa-2 cells had higher concentrations of phosphorylcholine when grown on DMEM compared to RPMI-1640, consistent with higher fatty acid synthesis activity in DMEM needed to support the higher proliferation rates of these cells (Bao et al. 2017; Gradiz et al. 2016). DMEM and RPMI-1640 both contain tyrosine and phenylalanine and both were higher in MiaPaCa-2 cultured on RPMI-1640 compared to DMEM, possibly to meet the demands of higher protein synthesis activity.
MiaPaCa-2 cells grown on DMEM had higher concentrations of UDP-N-acetyl-glucosamine compared to RPMI-1640 (Table 2). The hexosamine biosynthetic pathway (HBP) generates UDP-N-acetyl-glucosamine via the reaction of fructose-6-phosphates and glutamine (Santoyo-Ramos et al. 2012; Sethi and Vidal-Puig 2010). As glucose is converted to fructose-6-phosphate via glycolysis and continues on to the production of pyruvate, the HBP can also be activated depending on the concentration of glucose and glutamine present (Santoyo-Ramos et al. 2012; Sethi and Vidal-Puig 2010). The lower levels of both glutamine and glucose in the RPMI-1640 media would result lower activation of the HBP and reduced amounts of UDP-N-acetyl-glucosamine. Lin et al. (2016) showed that the surface marker CD133 had an influence on the HBP production of UDP-N-acetyl-glucosamine in hepatic cancer stem cells (Lin et al. 2016). CD133 seemed to only be expressed in a subpopulation of cells and this subpopulation could be influenced by glucose and glutamine concentration (Lin et al. 2016). This study highlighted the influence that one surface marker can have in a subpopulation of cells (Lin et al. 2016). Although MiaPaCa-2 does not express CD133, other markers could similarly influence the HBP in MiaPaCa-2 cells (Gradiz et al. 2016).
3.6 Comparison of AsPC-1 grown on DMEM and RPMI-1640
The PCA scores plot for AsPC-1 cells grown on DMEM and RPMI-1640 showed separation without overlap at the 95% confidence intervals (Fig. 3e). The intensities of 44 out of 77 bucketed resonances were statistically different in the PCA loadings plot (Online Resource 1, Fig. S-3C) between DMEM and RPMI-1640 groups (Online Resource 1, Table S-3). Creatine, aspartate, glycerophosphocholine, glutathione, NAD+, phosphorylcholine, and ethanol were higher in RPMI-1640 cells and acetate, alanine, succinate, valine, leucine, glutamate, glycine, lactate, tyrosine, phenylalanine and myoinositol were higher in DMEM cells (Table 2). 12 buckets remained un-identified due to database limitations and overlap (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 3f) showed strong separation along t[1] with R2Y(cum) = 0.991 and Q2(cum) = 0.959. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis.
RPMI-1640 is the recommended media for AsPC-1 cell culture. Given that RPMI-1640 has only half the glucose concentration as DMEM, AsPC-1 cells growth in RPMI-1640 could activate gluconeogenic-like pathways to support optimal cell growth, and RPMI-1640 also contains amino acids that are absent in DMEM (Online Resource 1, Table S-6), which are useful for anabolic reaction that support gluconeogenesis (Balsa-Martinez and Puigserver 2015; Pignatelli et al. 1990) in conjunction with glycolysis and glutaminolysis (Li et al. 2016). Ethanol was much higher (> 6 ×) in AsPC-1 cells grown on RPMI-1640, consistent with a higher state of glycolysis (Pathway 7 in Fig. 4) generating excess pyruvate, which can then be converted to ethanol. Most amino acids were higher in AsPC-1 cultured on DMEM (Table 2) consistent with requirements for protein synthesis supporting cell proliferation. Tyrosine and phenylalanine were higher in AsPC-1 cells cultured on RPMI-1640 despite DMEM containing roughly four times the amount of tyrosine and phenylalanine compared to RPMI-1640 and yet the optimal AsPC-1 cell growth is reported to occur on RPMI-1640. The higher AsPC-1 cellular content of tyrosine and phenylalanine appeared to reflect higher cellular uptake of tyrosine and phenylalanine from DMEM media that is not consumed as rapidly for protein synthesis and cellular growth/division as in RPMI-1640, resulting in higher steady-state concentrations of tyrosine and phenylalanine in AsPC-1 cells cultured on DMEM.
NAD+ concentrations were higher in AsPC-1 cells cultured on RPMI-1640 (Table 2). NADH plays a pivotal role in redox reactions in cellular metabolism. NADH is also utilized in anabolic reactions including gluconeogenesis. NAD+ is not present in either of the growth media. NAD+ is synthesized in a salvage pathway starting from niacinamide. Niacinamide is present at about four times higher concentrations in DMEM compared to RPMI-1640. However, optimal growth of AsPC-1 cells occurs on RPMI-1640. The higher concentrations of NAD+ in RPMI-1640 cells can be rationalized from the fact that RPMI-1640 media contains about four times less glucose compared to DMEM, but the AsPC-1 cells grow more rapidly on RPMI-1640 media. This would cause more rapid depletion of glucose in the RPMI-1640 media, producing stress resulting in stimulation of gluconeogenesis, which would require activation of NADH synthesis required to support gluconeogenesis, leading to higher NAD+ concentrations in AsPC-1 cells grown on RPMI-1640.
3.7 Comparisons of Panc-1 and AsPC-1 cells
The PCA scores plot for comparison of Panc-1 and AsPC-1 grown on DMEM media (Fig. 5a) showed separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). In the PCA loadings plot (Online Resource 1, Fig. S-4A), 93 resonances out of 134 bucketed resonances were significantly different between Panc-1 and AsPC-1 cells (Table 1). Glutamate, creatine, glutathione, myoinositol, NAD+, phosphorycholine, taurine and glycerophosphocholine had higher concentrations in Panc-1 cells whereas acetate, alanine, aspartate, ethanol, glycine, isoleucine, lactate and succinate had higher concentrations in AsPC-1 cells (Table 3). 57 buckets remained un-identified due to database limitations and overlap, and 2 out of 57 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 5b) showed strong separation along t[1] with R2Y(cum) = 0.996 and Q2(cum) = 0.98. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis.
PCA of Panc-1 versus AsPC-1 comparisons. PCA PC1 versus PC2 scores plots for growth media comparisons of hydrophilic extracts of Panc-1 versus AsPC-1 grown on (a) DMEM, (c) RPMI-1640, and (e) Panc-1/DMEM and AsPC-1/RPMI-1640. PLS-DA t[1] versus t[2] scores plots for growth media comparisons of hydrophilic extracts of Panc-1 versus AsPC-1 grown on (b) DMEM, (d) RPMI-1640, and (f) Panc-1/DMEM and AsPC-1/RPMI-1640
The largest fold-change was observed for succinate, which was > 10× higher in AsPC-1 cells. This indicated much higher alanine, aspartate and glutamate metabolism (pathway 4, Fig. 4) in AsPC-1 cells compared to Panc-1 cells. Panc-1 exhibited evidence of higher membrane and energy molecule synthesis demands. This was supported by the higher concentrations of glutamate, myoinositol, phosphorycholine, glycerophosphocholine. Glutamate is a product of the conversion of glutamine by glutaminase and is recognized as essential step in intermediate production for cell growth (Daye and Wellen 2012a). A higher concentration of glutamate pointed to more aggressive cell proliferation. This coupled with higher concentrations of glycerophosphocholine and phosphorylcholine, which are essential for membrane synthesis (pathway 28 in Fig. 4), and myoinositol, a product of glucose-6-phosphate conversion and a precursor for phospholipid biosynthesis, all essential for cell membrane synthesis, would support Panc-1 having a shorter doubling time and being a more aggressive cancer cell growth compared to AsPC-1 (Deer et al. 2010).
The PCA scores plot for comparison of Panc-1 and AsPC-1 grown on RPMI-1640 media (Fig. 5c) showed separation without overlap at the 95% confidence (Online Resource 1, Table S-3). In the PCA loadings plot (Online Resource 1, Fig. S-4B), 103 resonances out of 134 bucketed resonances were significantly different between the Panc-1 and AsPC-1 cells (Table 1). Acetate, alanine, glycerophosphocholine, phosphorylcholine, creatine, isoleucine, lactate, leucine, phenylalanine, taurine, tyrosine and valine were higher in Panc-1 cells and aspartate, ethanol, glutathione, myoinositol and succinate were higher in AsPC-1 cells (Table 3). 62 buckets remained un-identified due to database limitations and overlap, 24 out of 62 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 5d) showed strong separation along t[1] with R2Y(cum) = 0.998 and Q2(cum) = 0.989. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis. A detailed discussion of the comparisons of the metabolic profiles of AsPC-1 and Panc-1 cells cultured on RPMI-1640 media is included in Online Material 1, Analysis S-1.
The PCA scores plot for the comparison of Panc-1 and AsPC-1 grown on their recommended media, DMEM and RPMI-1640, respectively (Fig. 5e) showed separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). In the PCA loadings plot (Online Resource 1, Fig. S-4C), 103 resonances out of 134 resonances were different between the Panc-1 and AsPC-1 cells (Table 1). 68 buckets remained un-identified due to database limitations and overlap, 6 out of 68 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 5f) showed strong separation along t[1] with R2Y(cum) = 0.996 and Q2(cum) = 0.98. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis. A detailed discussion of the comparisons of the metabolic profiles of AsPC-1 and Panc-1 cells cultured on their respective recommended media is included in Online Material 1, Analysis S-2.
3.8 Comparisons of Panc-1 and MiaPaCa-2 cells
The PCA scores plot for the comparison of Panc-1 and MiaPaCa-2 grown on their recommended media, both DMEM, (Fig. 6a) showed separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). In the corresponding PCA loadings plot (Online Resource 1, Fig. S-5A), 158 out of 212 bucketed resonances were different between the Panc-1 and MiaPaCa-2 samples grown on DMEM media. Acetate, alanine, aspartate, choline, ethanol, glycine, lactate, leucine, NAD+, succinate and UDP-N-acetylglucosamine had higher concentrations in MiaPaCa-2 cells whereas glutathione, glycerophosphocholine, isoleucine, phosphorylcholine, taurine, tyrosine, uracil and valine were higher in Panc-1 cells (Table 3). 92 buckets remained un-identified due to database limitations and overlap, 10 out of 92 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 6b) showed strong separation along t[1] with R2Y(cum) = 0.998 and Q2(cum) = 0.988. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis.
PCA of Panc-1 versus MiaPaCa-2 comparisons. PCA PC1 versus PC2 scores plots for growth media comparisons of hydrophilic extracts of Panc-1 versus MiaPaCa-2 grown on (a) DMEM and (c) RPMI-1640. PLS-DA t[1] versus t[2] scores plots for growth media comparisons of hydrophilic extracts of Panc-1 versus MiaPaCa-2 grown on (b) DMEM and (d) RPMI-1640
Metabolites necessary for protein synthesis, alanine, glycine and leucine, were at higher concentrations in MiaPaCa-2 cells indicating higher protein synthesis demands in those cells. Metabolites that drive glycolysis such as succinate, a known oncometabolite (Jain et al. 2013) and aspartate, which is a component in the malate-aspartate shuttle (Yang et al. 2015), were also significantly higher in MiaPaCa-2 cells, which would induce higher glycolytic rates to meet cell energy demands (Wheaton and Chandel 2011). The largest difference was the presence of UDP-N-acetylglucosamine in the MiaPaCa-2, which indicated HBP activity (Santoyo-Ramos et al. 2012) and its absence in Panc-1 cells (Table 3). Though both cells were cultured in media containing glucose and glutamine (Online Resource 1, Table S-6), which influence the HBP pathway, the pathway appeared to only be active in MiaPaCa-2 cells. Taurine, which is synthesized from cysteine (Vitvitsky et al. 2011), was only identifiable in Panc-1 cells, and this coupled with the higher concentration glutathione (Wu et al. 2004), which is also synthesized from cysteine and is a sink for reactive oxygen species (Amores-Sánchez and Medina 1999), indicated a difference in cysteine metabolism in these two cell lines, specifically it indicated activity of taurine synthesis in Panc-1 cells but not MiaPaCa-2 cells. Though both cells lines had access to cysteine in DMEM (Online Resource 1, Table S-4), there was a marked difference in cysteine metabolism in these two cell lines.
The PCA scores plot for comparison of Panc-1 and MiaPaCa-2 grown on RPMI-1640 media (Fig. 6c) showed separation without overlap at the 95% (Online Resource 1, Table S-3). In the corresponding PCA loadings plot (Online Resource 1, Fig. S-5B), 102 out of 188 bucketed resonances were identified as having intensities that were statistically significant between the Panc-1 and MiaPaCa-2 samples grown on RPMI-1640 media (Table 3). Alanine, ethanol, NAD+, succinate and UDP-N-acetylglucosamine were higher in MiaPaCa-2 cells whereas acetate, aspartate, creatine, glutathione, isoleucine, lactate, leucine, tyrosine, taurine, and valine were higher in Panc-1 cells. 1 bucket remained un-identified due to database limitations and overlap, 1 out of 56 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 6d) showed strong separation along t[1] with R2Y(cum) = 0.977 and Q2(cum) = 0.952. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis. A detailed discussion of the comparisons of the metabolic profiles of Panc-1 and MiaPaCa-2 cells cultured on RPMI-1640 media is included in Online Material 1, Analysis S-3.
3.9 Comparisons of MiaPaCa-2 and AsPC-1 cells
The PCA scores plot for comparison of MiaPaCa-2 and AsPC-1 cells grown on DMEM media (recommended media for MiaPaCa-2 cells) (Fig. 7a) showed separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). In the corresponding loadings plot (Online Resource 1, Fig. S-6A), 178 out of 264 bucketed resonances were different between MiaPaCa-2 and AsPC-1 cells (Table 1). Choline, ethanol, glycine, NAD+, UDP-N-acetylglucosamine were higher in MiaPaCa-2 cells and acetate, alanine, aspartate, creatine, glycerylphosphocholine, isoleucine, lactate, phenylalanine, succinate, tyrosine, uracil and valine were higher in concentration in AsPC-1 cells (Table 3). 119 buckets remained un-identified due to database limitations and overlap, 22 out of 119 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 7b) showed strong separation along t[1] with R2Y(cum) = 0.998 and Q2(cum) = 0.983. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis.
PCA of MiaPaCa-2 versus AsPC-1 comparisons. PCA PC1 versus PC2 scores plots for comparisons of hydrophilic extracts of MiaPaCa-2 versus AsPC-1 grown on (a) DMEM, (c) RPMI-1640, and (e) MiaPaCa-2/DMEM and AsPC-1/RPMI-1640. PLS-DA t[1] versus t[2] scores plots for comparisons of hydrophilic extracts of MiaPaCa-2 versus AsPC-1 grown on (b) DMEM, (d) RPMI-1640, and (f) MiaPaCa-2/DMEM and AsPC-1/RPMI-1640
Succinate was nearly 8 × higher in AsPC-1 cells indicating much higher activity of alanine, aspartate and glutamate metabolism (pathway 4 in Fig. 4) in these cells when cultured on DMEM. UDP-N-acetylglucosamine was only present in MiaPaCa-2 cells as the high glutamine and glucose DMEM media suppressed hexosamine biosynthesis in AsPC-1 cells (Santoyo-Ramos et al. 2012). Choline was elevated in MiaPaCa-2 cells indicating higher membrane synthesis activity in these cells. Also, the higher glucose and glutamine in the DMEM medium (Online Resource 1, Table S-6) seemed to drive protein synthesis in AsPC-1 cells, which was indicated by higher intracellular concentrations of several amino acids including alanine, aspartate, isoleucine, phenylalanine, tyrosine, and valine (Table 3).
The PCA scores plot (PC1 vs PC2) for the comparison of MiaPaCa-2 and AsPC-1 grown on RPMI-1640 media (recommended media for AsPC-1 cells) (Fig. 7c) showed separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). In the PCA loadings plot (Online Resource 1, Fig. S-6B), 205 resonances out of 264 bucketed resonances were identified as having changes in intensities that were deemed statistically significant (Table 1). Acetate, alanine, choline, glycerophosphocholine, glycine, isoleucine, leucine, phenylalanine, phosphorylcholine, tyrosine, UDP-N-acetylglucosamine and valine were higher in concentration in MiaPaCa-2 cells and aspartate, creatine, ethanol, glutathione, lactate, succinate and uracil were higher in the AsPC-1 cells (Table 3). 146 buckets remained un-identified due to database limitations and overlap, 35 out of 146 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 7d) showed strong separation along t[1] with R2Y(cum) = 0.997 and Q2(cum) = 0.983. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis. A detailed discussion of the comparisons of the metabolic profiles of MiaPaCa-2 and AsPC-1 cells cultured on RPMI-1640 media is included in Online Material 1, Analysis S-4.
The PCA scores plot for the comparison of MiaPaCa-2 and AsPC-1 grown on their recommended media, DMEM and RPMI-1640, respectively, (Fig. 7e) showed separation without overlap at the 95% confidence intervals (Online Resource 1, Table S-3). In the corresponding PCA loadings plot (Online Resource 1, Fig. S-6C), 99 out of 150 bucketed resonances were different between MiaPaCa-2 and AsPC-1 cells (Table 3). Glycine, leucine, NAD+, phenylalanine, tyrosine and UDP-N-acetylglucosamine were higher in MiaPaCa-2/DMEM cells and acetate, aspartate, creatine, ethanol, glutathione, glycerophosphocholine, lactate, uracil and valine were higher in AsPC-1/RPMI-1640 cells (Table 3). 48 buckets remained un-identified due to database limitations and overlap, 9 out of 48 of these buckets fell in the most significant p value range (10−10–0) (Goodpaster et al. 2010) (Online Resource 2, Table S-4). The PLS-DA scores plot (Fig. 7f) showed strong separation along t[1] with R2Y(cum) = 0.998 and Q2(cum) = 0.997. The VIP scores are listed in Online Resource 3, Table S-5. Metabolites identified as significant based on having VIP scores greater than 1.0 were generally consistent with those identified from the PCA and statistical significance analysis. A detailed discussion of the comparisons of the metabolic profiles of MiaPaCa-2 and AsPC-1 cells cultured on their respective recommended media is included in Online Material 1, Analysis S-5.
3.10 Comparison with prior studies of Panc-1, MiaPaCa-2 and AsPC-1
The metabolic profiles of Panc-1, MiaPaCa-2 and AsPC-1 cells grown on their optimal growth media have been previously compared by Watanabe et al. (2012). In their study, lipopolysaccharide biosynthesis was reported to be significantly higher in MiaPaCa-2 cells as indicated by the unique presence of UDP-N-acetylglucosamine (Watanabe et al. 2012). Our results also identified UDP-N-acetylglucosamine in MiaPaCa-2 cells but did not identify it Panc-1 and AsPC-1 cells (Table 3) consistent with the prior study. Watanabe et al. (2012) also reported that higher concentrations of aspartate were correlated to glutamine metabolism and that AsPC-1 had the highest concentration of aspartate followed by Panc-1 and MiaPaCa-2, and this trend was also identified in our results (Table 3). Watanabe et al. (2012) reported that acetate was significantly higher in Panc-1 cells in comparison to either AsPC-1 or MiaPaCa-2 cells. Here, MiaPaCa-2 and AsPC-1 had equal and higher concentrations of acetate compared to Panc-1 cells (Table 3). Watanabe et al. (2012) reported that creatine concentration was higher in Panc-1 compared to Miapaca-2 and AsPC-1 cells and this was consistent with our findings (Table 3). Watanabe et al. (2012) reported that choline metabolism, which is essential for membrane synthesis, was upregulated in cancer cells in comparison to their healthy control. Specifically, glycerophosphocholine was reported to be upregulated in all three cancer cells whereas phosphorylcholine was only in higher concentration in AsPC-1 and MiaPaCa-2 (Watanabe et al. 2012). In the study reported here, phosphorylcholine and glycerophosphocholine were identified and both metabolites were highest in Panc-1 followed by MiaPaCa-2 and AsPC-1 when grown on their recommended media (Table 3). Watanabe et al. (2012) reported lower concentrations of amino acids in cancer cells in comparison to their healthy controls, however no comparison with a non-cancerous control was performed in this study. Amino acids such as valine, glycine, alanine, leucine and isoleucine were identified in all three cell lines by Watanabe et al. (2012). In the results reported here, all these amino acids were also identified plus tyrosine and phenylalanine (Table 3). Some discrepancies between the studies should be noted, for example whereas UDP-N-acetylglucosamine was identified and confirmed in only MiaPaCa-2 cells and absent in other cell lines in this study (Tables 2, 3), Watanabe et al. (2012) identified UDP-N-acetylglucosamine in all three cancer cell lines. Taurine was not identified in Panc-1 cells or the other cell lines in the study reported by Watanabe et al. (2012), which could be due to database limitations at that time. Other than the few discrepancies noted here, the previously reported results appear to be largely consistent with the comparisons reported here.
4 Conclusions
The data presented here illustrate that the choice of culture medium in cell-line based metabonomics studies is a critical factor that must be carefully considered as a part of study design. For the three pancreatic cancer cell lines investigated, we found that the choice of culture media strongly influenced the measured metabolic profiles. When cell lines were not grown on their recommended media, there tended to be a decrease in most identified metabolites, implying reduced metabolic activity of the corresponding biochemical pathways compared to culture on the media recommended for optimal growth. However, cell culture on media with a significantly higher nutrient content did not universally correlate with increased metabolic activity. This was illustrated with AsPC-1, which when cultured on the richer DMEM medium, exhibited a metabolic profile less indicative of cell proliferation compared to AsPC-1 grown on the leaner RPMI-1640 medium recommended for its optimal growth. Growing all cell lines to be compared on the same culture media might seem logical but when one cell line prefers an alternative culture medium for optimal growth, due to unanticipated preferences for certain critical biochemical pathway activities, this can also significantly affect the comparison and interpretation of the respective metabolic profiles. How well or poorly each cell line grows on a specific culture media may reflect the genetic differences that arise as a consequence of disease. It is also important to be aware that the same conclusions apply to other omics studies that are used to compare human cancer cell lines.
Clearly, the conclusions reached here regarding the impact of the choice of culture media on measured metabolomes of the various cell lines should be expected to extend to other “omics” studies of human cancer cell lines as well. For example, the transcriptomes or proteomes of human cancer cell lines should also be expected to depend strongly on the culture conditions. Therefore, the choice of culture media should also be carefully considered when designing any omics studies with the goal of comparing different cell lines and it should be assumed that the choice of culture media will potentially influence the transcriptomes or proteomes. It should be noted that the choice of culture medium should not simply be considered a complication or confounding variable in omics studies. Rather, comparisons of the same human cancer cell lines cultured on multiple growth media can potentially be used in omics investigations to facilitate investigation and characterization of the activity of specific metabolic pathways.
Some pathways were not strongly influenced by culture media and possibly reflected unique intrinsic metabolic differences between the cell lines. For example, the HBP appeared to be active only in MiaPaCa-2 cells and its activity in MiaPaCa-2 cells was observed regardless of the culture media. Despite the HBP being sensitive to glutamine and glucose concentrations, the HBP was still active when MiaPaCa-2 cells were grown on RPMI-1640 even though RPMI-1640 had lower concentrations of both substrates. Panc-1 also exhibited uniquely higher metabolism of cysteine, which was present in both culture media, but taurine was only synthesized in Panc-1 cells. Though the taurine concentration was lower when Panc-1 cells were cultured on RPMI-1640, the pathway appeared to remain active. Higher concentrations of cholines were found in Panc-1 cells regardless of the growth media used for cell culture, when compared to MiaPaCa-2 and AsPC-1 cells. This indicated that Panc-1 had higher membrane synthesis demands and those biosynthetic pathways appeared to be preferentially activated in Panc-1 cells. Aspartate, which we previously discussed, and succinate were higher in AsPC-1 cells possibly indicating a higher glycolytic rate. Succinate an oncometabolite that activates HIF-1 (Jain et al. 2013), which upregulates glycolysis, would also result in high concentrations of lactate as a consequence of the Warburg effect.
Prior NMR based metabolic profiling comparison of the MiaPaCa-2, Panc-1 and AsPC-1 cell lines previously reported by Watanabe et al. (Watanabe et al. 2012) were generally consistent with the results reported here. Watanabe et al. (2012) showed an overall increase in the choline synthesis required for membrane synthesis and cell replication. They also identified varying concentration of amino acids with all three cancer cells grown on their recommended media. Another hall-mark of their study was a higher concentration of glutathione across all three cell lines compared to their healthy control. They showed that MiaPaCa-2 had the highest concentration of glutathione followed by Panc-1 and AsPC-1, whereas our study showed AsPC-1 having the highest concentration followed by Panc-1 and MiaPaCa-2.
In conclusion, our results illustrate that the metabolic profiles of human cancer cell lines depend strongly on the culture medium used to grow the cells. Consequently, our results indicate that when doing a comparative study of the metabolic profiles of multiple cells lines, it is essential to give careful consideration to the choice and selection of the culture medium use to grow each cell line. When different media must be used when comparing two or more cell lines, we recommend to first do an initial assessment of the metabolic profile of each cell line on their respective recommended media. We then strongly recommend that at least one other alternative medium be tested for each cell line to determine if it is possible to use a common culture media for comparison of the metabolic profiles of pairs of human cancer cell lines. Our results also demonstrate that examination of the metabolic profile of a cell line grown on more than once culture medium can reveal important insight into metabolic pathways that are up-regulated or down-regulated in the cell line independent of the culture media. This information can indicate metabolic pathways that are a signature of the particular cell line under investigation. Importantly, these conclusions are not limited to NMR-based metabolic profiling studies, but apply generally to any metabolic profiling study regardless of the technique being used to measure the metabolic profiles, as well as to other “omics” studies as well.
References
Amores-Sánchez, M. I., & Medina, M. (1999). Glutamine, as a precursor of glutathione, and oxidative stress. Molecular Genetics and Metabolism, 67(2), 100–105. https://doi.org/10.1006/mgme.1999.2857.
Balsa-Martinez, E., & Puigserver, P. (2015). Cancer cells hijack gluconeogenic enzymes to fuel cell growth. Molecular Cell, 60(4), 509–511. https://doi.org/10.1016/j.molcel.2015.11.005.
Bao, B., Azmi, A. S., Aboukameel, A., Ahmad, A., Bolling-Fischer, A., Sethi, S., et al. (2017). Pancreatic cancer stem-like cells display aggressive behavior mediated via activation of FoxQ1. The Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M113.532887.
Bayet-Robert, M., Loiseau, D., Rio, P., Demidem, A., Barthomeuf, C., Stepien, G., & Morvan, D. (2010). Quantitative two-dimensional HRMAS 1H-NMR spectroscopy-based metabolite profiling of human cancer cell lines and response to chemotherapy. Magnetic Resonance in Medicine, 63(5), 1172–1183. https://doi.org/10.1002/mrm.22303.
Brougham, D. F., Ivanova, G., Gottschalk, M., Collins, D. M., Eustace, A. J., O’Connor, R., et al. (2011). Artificial neural networks for classification in metabolomic studies of whole cells using 1H nuclear magnetic resonance. Journal of Biomedicine & Biotechnology, 2011, 158094. https://doi.org/10.1155/2011/158094.
Cano, K. E., Li, Y.-J., & Chen, Y. (2010). NMR metabolomic profiling reveals new roles of SUMOylation in DNA damage response. Journal of Proteome Research, 9(10), 5382–5388. https://doi.org/10.1021/pr100614a.
Cantó, C., Menzies, K. J., & Auwerx, J. (2015). NAD+ metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metabolism, 22, 31–53. https://doi.org/10.1016/j.cmet.2015.05.023.
Cao, M. D., Lamichhane, S., Lundgren, S., Bofin, A., Fjøsne, H., Giskeødegård, G. F., & Bathen, T. F. (2014). Metabolic characterization of triple negative breast cancer. BMC Cancer, 14(1), 941. https://doi.org/10.1186/1471-2407-14-941.
Chatterjee, N., Yang, J., Yoon, D., Kim, S., Joo, S.-W., & Choi, J. (2017). Differential crosstalk between global DNA methylation and metabolomics associated with cell type specific stress response by pristine and functionalized MWCNT. Biomaterials, 115, 167–180. https://doi.org/10.1016/j.biomaterials.2016.11.005.
Chihanga, T., Ma, Q., Nicholson, J. D., Ruby, H. N., Edelmann, R. E., Devarajan, P., & Kennedy, M. A. (2017). NMR spectroscopy and electron microscopy identification of metabolic and ultrastructural changes to the kidney following ischemia reperfusion injury. American Journal of Physiology - Renal Physiology. https://doi.org/10.1152/ajprenal.00363.2017.
Cuperlovic-Culf, M., Touaibia, M., St-Coeur, P.-D., Poitras, J., Morin, P., & Culf, A. S. (2014). Metabolic effects of known and novel HDAC and SIRT inhibitors in glioblastomas independently or combined with temozolomide. Metabolites, 4(3), 807–830. https://doi.org/10.3390/metabo4030807.
Daye, D., & Wellen, K. E. (2012a). Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Seminars in Cell and Developmental Biology, 23(4), 362–369. https://doi.org/10.1016/j.semcdb.2012.02.002.
de Santana-Filho, A. P., Jacomasso, T., Riter, D. S., Barison, A., Iacomini, M., Winnischofer, S. M. B., & Sassaki, G. L. (2017). NMR metabolic fingerprints of murine melanocyte and melanoma cell lines: Application to biomarker discovery. Scientific Reports, 7, 42324. https://doi.org/10.1038/srep42324.
Deer, E. L., Gonzalez-Hernandez, J., Coursen, J. D., Shea, J. E., Ngatia, J., Scaife, C. L., et al. (2010). Phenotype and genotype of pancreatic cancer cell lines. Pancreas, 39(4), 425. https://doi.org/10.1097/mpa.0b013e3181c15963.
Gierok, P., Harms, M., Richter, E., Hildebrandt, J.-P., Lalk, M., Mostertz, J., & Hochgräfe, F. (2014). Staphylococcus aureus alpha-toxin mediates general and cell type-specific changes in metabolite concentrations of immortalized human airway epithelial cells. PLoS ONE, 9(4), e94818. https://doi.org/10.1371/journal.pone.0094818.
Goodpaster, A. M., & Kennedy, M. A. (2011). Quantification and statistical significance analysis of group separation in NMR-based metabonomics studies. Chemometrics and Intelligent Laboratory Systems, 109(2), 162–170. https://doi.org/10.1016/j.chemolab.2011.08.009.
Goodpaster, A. M., Ramadas, E. H., & Kennedy, M. A. (2011). Potential effect of diaper and cotton ball contamination on NMR-and LC/MS-based metabonomics studies of urine from newborn babies. Analytical Chemistry, 83(3), 896–902.
Goodpaster, A. M., Romick-Rosendale, L. E., & Kennedy, M. A. (2010). Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Analytical Biochemistry, 401(1), 134–143. https://doi.org/10.1016/j.ab.2010.02.005.
Gradiz, R., Silva, H. C., Carvalho, L., Botelho, M. F., & Mota-Pinto, A. (2016). MIA PaCa-2 and PANC-1—Pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Scientific Reports, 6(1), 21648. https://doi.org/10.1038/srep21648.
Jain, M., Kami, K., Ueno, Y., Naraoka, H., Tomita, M., & Nishioka, T. (2013). Oncometabolites: Linking altered metabolism with cancer. Science, 336(6084), 1040–1044. https://doi.org/10.1172/JCI67228.
Lane, A. N., Tan, J., Wang, Y., Yan, J., Higashi, R. M., & Fan, T. W.-M. (2017). Probing the metabolic phenotype of breast cancer cells by multiple tracer stable isotope resolved metabolomics. Metabolic Engineering. https://doi.org/10.1016/j.ymben.2017.01.010.
Lefort, N., Brown, A., Lloyd, V., Ouellette, R., Touaibia, M., Culf, A. S., & Cuperlovic-Culf, M. (2014). 1H NMR metabolomics analysis of the effect of dichloroacetate and allopurinol on breast cancers. Journal of Pharmaceutical and Biomedical Analysis, 93, 77–85. https://doi.org/10.1016/j.jpba.2013.08.017.
Li, C., Zhang, G., Zhao, L., Ma, Z., & Chen, H. (2016). Metabolic reprogramming in cancer cells: Glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer. World Journal of Surgical Oncology, 14(1), 15. https://doi.org/10.1186/s12957-016-0769-9.
Lin, S.-H., Liu, T., Ming, X., Tang, Z., Fu, L., Schmitt-Kopplin, P., et al. (2016). Regulatory role of hexosamine biosynthetic pathway on hepatic cancer stem cell marker CD133 under low glucose conditions. Scientific Reports, 6(1), 21184. https://doi.org/10.1038/srep21184.
MacIntyre, D. A., Melguizo Sanchís, D., Jiménez, B., Moreno, R., Stojkovic, M., & Pineda-Lucena, A. (2011). Characterisation of human embryonic stem cells conditioning media by 1H-nuclear magnetic resonance spectroscopy. PLoS ONE, 6(2), e16732. https://doi.org/10.1371/journal.pone.0016732.
Maria, R. M., Altei, W. F., Andricopulo, A. D., Becceneri, A. B., Cominetti, M. R., Venâncio, T., & Colnago, L. A. (2015). Characterization of metabolic profile of intact non-tumor and tumor breast cells by high-resolution magic angle spinning nuclear magnetic resonance spectroscopy. Analytical Biochemistry, 488, 14–18. https://doi.org/10.1016/j.ab.2015.07.015.
Morin, P. J., Ferguson, D., LeBlanc, L. M., Hébert, M. J. G., Paré, A. F., Jean-François, J., et al. (2013). NMR metabolomics analysis of the effects of 5-lipoxygenase inhibitors on metabolism in glioblastomas. Journal of Proteome Research, 12(5), 2165–2176. https://doi.org/10.1021/pr400026q.
Pan, X., Wilson, M., McConville, C., Arvanitis, T. N., Griffin, J. L., Kauppinen, R. A., & Peet, A. C. (2013). Increased unsaturation of lipids in cytoplasmic lipid droplets in DAOY cancer cells in response to cisplatin treatment. Metabolomics, 9(3), 722–729. https://doi.org/10.1007/s11306-012-0483-8.
Pan, X., Wilson, M., Mirbahai, L., McConville, C., Arvanitis, T. N., Griffin, J. L., et al. (2011). In vitro metabonomic study detects increases in UDP-GlcNAc and UDP-GalNAc, as early phase markers of cisplatin treatment response in brain tumor cells. Journal of Proteome Research, 10(8), 3493–3500. https://doi.org/10.1021/pr200114v.
Pignatelli, M., Durbin, H., Bodmer, W. F., Hu, S., Klug, T., Zurawski, V., et al. (1990). Carcinoembryonic antigen functions as an accessory adhesion molecule mediating colon epithelial cell-collagen interactions. Proceedings of the National Academy of Sciences USA, 87(4), 1541–1545. https://doi.org/10.1073/pnas.87.4.1541.
Righi, V., Roda, J. M., Paz, J., Mucci, A., Tugnoli, V., Rodriguez-Tarduchy, G., et al. (2009). 1H HR-MAS and genomic analysis of human tumor biopsies discriminate between high and low grade astrocytomas. NMR in Biomedicine, 22(6), 629–637. https://doi.org/10.1002/nbm.1377.
Rogatzki, M. J., Ferguson, B. S., Goodwin, M. L., & Gladden, L. B. (2015). Lactate is always the end product of glycolysis. Frontiers in Neuroscience, 9(FEB), 1–7. https://doi.org/10.3389/fnins.2015.00022.
Röhrig, F., & Schulze, A. (2016). The multifaceted roles of fatty acid synthesis in cancer. Nature Reviews Cancer, 16(11), 732–749. https://doi.org/10.1038/nrc.2016.89.
Santoyo-Ramos, P., Cristina, M., & Robles-Flores, M. (2012). The role of O-Linked β-N-acetylglucosamine (GlcNAc) modification in cell signaling. In Glycosylation. InTech. https://doi.org/10.5772/47874.
Schaffer, S. W., Allo, S., Harada, H., Stroo, W., Azuma, J., & Hamaguchi, T. (1989). Mechanism underlying the membrane-stabilizing activity of taurine. In H. Iwata, J. B. Lombardini & T. Segawa (Eds.), Taurine and the heart. Developments in cardiovascular medicine (Vol. 93). Boston: Springer.
Schwarzfischer, P., Reinders, J., Dettmer, K., Kleo, K., Dimitrova, L., Hummel, M., et al. (2017). Comprehensive metaboproteomics of Burkitt’s and diffuse large B-cell lymphoma cell lines and primary tumor tissues reveals distinct differences in pyruvate content and metabolism. Journal of Proteome Research, 16(3), 1105–1120. https://doi.org/10.1021/acs.jproteome.6b00164.
Sciacovelli, M., & Frezza, C. (2016). Oncometabolites: Unconventional triggers of oncogenic signalling cascades. Free Radical Biology & Medicine, 100, 175–181. https://doi.org/10.1016/j.freeradbiomed.2016.04.025.
Sethi, J. K., & Vidal-Puig, A. (2010). Wnt signalling and the control of cellular metabolism. Biochemical Journal, 427(1). Retrieved May 20, 2017 from http://www.biochemj.org/content/427/1/1.
Shao, W., Gu, J., Huang, C., Liu, D., Huang, H., Huang, Z., et al. (2014). Malignancy-associated metabolic profiling of human glioma cell lines using 1H NMR spectroscopy. Molecular Cancer, 13, 197. https://doi.org/10.1186/1476-4598-13-197.
Sorice, A., Siano, F., Capone, F., Guerriero, E., Picariello, G., Budillon, A., et al. (2016). Potential anticancer effects of polyphenols from chestnut shell extracts: Modulation of cell growth, and cytokinomic and metabolomic profiles. Molecules, 21(10), 1411. https://doi.org/10.3390/molecules21101411.
Spratlin, J. L., Pitts, T. M., Kulikowski, G. N., Morelli, M. P., Tentler, J. J., Serkova, N. J., & Eckhardt, S. G. (2011). Synergistic activity of histone deacetylase and proteasome inhibition against pancreatic and hepatocellular cancer cell lines. Anticancer Research, 31(4), 1093–103. Retrieved June 8, 2017 from http://www.ncbi.nlm.nih.gov/pubmed/21508352.
Stipanuk, M. H., Dominy, J. E., Lee, J., & Coloso, R. M. (2006). Mammalian cysteine metabolism: New insights into regulation. The Journal of Nutrition, 136, 1652–1659.
Teahan, O., Bevan, C. L., Waxman, J., & Keun, H. C. (2011). Metabolic signatures of malignant progression in prostate epithelial cells. The International Journal of Biochemistry & Cell Biology, 43(7), 1002–1009. https://doi.org/10.1016/j.biocel.2010.07.003.
Tiziani, S., Lodi, A., Khanim, F. L., Viant, M. R., Bunce, C. M., & Günther, U. L. (2009). Metabolomic profiling of drug responses in acute myeloid leukaemia cell lines. PLoS ONE, 4(1), e4251. https://doi.org/10.1371/journal.pone.0004251.
Tripathi, P., Kamarajan, P., Somashekar, B. S., Mackinnon, N., Chinnaiyan, A. M., Kapila, Y. L., et al. (2012). Delineating metabolic signatures of head and neck squamous cell carcinoma: Phospholipase A2, a potential therapeutic target. International Journal of Biochemistry and Cell Biology, 44, 1852–1861. https://doi.org/10.1016/j.biocel.2012.06.025.
Vitvitsky, V., Garg, S. K., & Banerjee, R. (2011). Taurine biosynthesis by neurons and astrocytes. The Journal of biological chemistry, 286(37), 32002–32010. https://doi.org/10.1074/jbc.M111.253344.
Wallace, M., Whelan, H., & Brennan, L. (2013). Metabolomic analysis of pancreatic beta cells following exposure to high glucose. Biochimica et Biophysica Acta (BBA): General Subjects, 1830(3), 2583–2590. https://doi.org/10.1016/j.bbagen.2012.10.025.
Watanabe, M., Sheriff, S., Lewis, K. B., Cho, J., Tinch, S. L., Balasubramaniam, A., & Kennedy, M. A. (2012). Metabolic profiling comparison of human pancreatic ductal epithelial cells and three pancreatic cancer cell lines using NMR based metabonomics HHS public access. Journal of Molecular Biomarkers & Diagnosis. https://doi.org/10.4172/2155-9929.S3-002.
Wen, H., Xu, W. J., Jin, X., Oh, S., Phan, C. H. D., Song, J., et al. (2015). The roles of IP3 receptor in energy metabolic pathways and reactive oxygen species homeostasis revealed by metabolomic and biochemical studies. Biochimica et Biophysica Acta (BBA): Molecular Cell Research, 1853(11), 2937–2944. https://doi.org/10.1016/j.bbamcr.2015.07.020.
Wheaton, W. W., & Chandel, N. S. (2011). Hypoxia. 2. Hypoxia regulates cellular metabolism. American Journal of Physiology: Cell Physiology, 300(3). Retrieved July 20, 2017 from http://ajpcell.physiology.org/content/300/3/C385.
Wu, G., Fang, Y.-Z., Yang, S., Lupton, J. R., & Turner, N. D. (2004). Glutathione metabolism and its implications for health. The Journal of Nutrition, 134(3), 489–492. Retrived July 25, 2017 from http://www.ncbi.nlm.nih.gov/pubmed/14988435.
Xia, J., Mandal, R., Sinelnikov, I., Broadhurst, D., & Wishart, D. S. (2012). MetaboAnalyst 2.0—A comprehensive server for metabolomic data analysis. Nucleic Acids Research, 40, W127–W133.
Xia, J., Psychogios, N., Young, N., & Wishart, D. S. (2009). MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Research, 37, W652–W660.
Xia, J., Sinelnikov, I., Han, B., & Wishart, D. S. (2015). MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Research, 43, W251–W257.
Xia, J., & Wishart, D. S. (2011a). Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nature Protocols, 6(6), 743–760.
Xia, J., & Wishart, D. S. (2011b). Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Current Protocols in Bioinformatics. https://doi.org/10.1002/0471250953.bi1410s34.
Xia, J., & Wishart, D. S. (2016) Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Current Protocols in Bioinformatics, 55, 14.10.1–14.10.91.
Yang, H., Zhou, L., Shi, Q., Zhao, Y., Lin, H., Zhang, M., & Zhao, S. (2015). SIRT 3-dependent GOT 2 acetylation status affects the malate–aspartate NADH shuttle activity and pancreatic tumor growth. The EMBO Journal, 34(8), 1110–1125.
Yin, T., Zhang, Z., Cao, B., Duan, Q., Shi, P., Zhao, H., et al. (2016). Bmi1 inhibition enhances the sensitivity of pancreatic cancer cells to gemcitabine. Oncotarget, 7(24), 37192–37204. https://doi.org/10.18632/oncotarget.9293.
Acknowledgements
The instrumentation used in this work was obtained with the support of Miami University and the Ohio Board of Regents with funds used to establish the Ohio Eminent Scholar Laboratory where the work was performed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Research involving human and animal participants
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chihanga, T., Hausmann, S.M., Ni, S. et al. Influence of media selection on NMR based metabolic profiling of human cell lines. Metabolomics 14, 28 (2018). https://doi.org/10.1007/s11306-018-1323-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1323-2