Abstract
Cheese intake has been shown to decrease total cholesterol and LDL cholesterol concentrations when compared to butter of equal fat content. An untargeted metabolite profiling may reveal exposure markers of cheese but may also contribute with markers which can help explain how the intake of cheese affects cholesterol concentrations. Twenty-three subjects collected 2 × 24 h urine samples after 6 weeks of cheese and 6 weeks of butter intake with equal amounts of fat in a cross-over intervention study. The samples were analyzed by UPLC-QTOF/MS. A two-step univariate data analysis approach using linear mixed model was applied separately for positive and negative ionization mode: In the first step a total of 44 features related to treatment were identified and in the second step 36 of these features were related to total cholesterol concentrations. Cheese intake resulted in increased urinary indoxyl sulfate, xanthurenic acid, tyramine sulfate, 4-hydroxyphenylacetic acid, isovalerylglutamic acid and several acylglycines including isovalerylglycine, tiglylglycine and isobutyrylglycine when compared to butter intake of equal fat content. The biological mechanisms of action linking the metabolites to cholesterol concentrations need to be further explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Saturated fat intake is recommended to be lowered due to the increase of plasma low-density (LDL) cholesterol concentrations and thereby the risk of lifestyle diseases such as cardiovascular diseases (CVDs) (Lichtenstein et al. 2006; Mensink and Katan 1992). Results from several intervention studies have established that saturated fatty acid intake increases LDL cholesterol concentrations (Mensink et al. 2003). Cheese is a dietary product commonly known to have a high content of saturated fatty acids. Despite of this, correlation studies suggest that cheese intake does not increase the risk of CVD (Artaud-Wild et al. 1993; Moss and Freed 2003; Seely 1988). Also, some prospective cohort studies find no association between cheese intake and CVD risk or sometimes even report a decreased risk with cheese intake (Gartside et al. 1998; Larsson et al. 2012; Sonestedt et al. 2011; van Aerde et al. 2013; von Ruesten et al. 2013). Furthermore, in three human intervention studies cheese intake was shown to decrease total cholesterol and/or LDL cholesterol concentrations when compared to butter (Biong et al. 2004; Hjerpsted et al. 2011; Tholstrup et al. 2004). Butter intake with equal amounts of fat was used as control in these studies because cheese and butter have similar fatty acid composition and therefore would be expected to increase cholesterol concentrations similarly. However, as fat from cheese seems to lower cholesterol concentrations compared to fat from butter the possible mechanisms of action would be highly relevant to elucidate. Some possible mechanisms of action have already been suggested including the high calcium of cheese and the fermentation products; however, this has not been confirmed (Hjerpsted et al. 2011; Huth and Park 2012). To investigate possible mechanisms, an untargeted metabolomics approach may be used. Untargeted metabolomics is a rapidly evolving method aiming at measuring all metabolites in a biological fluid making it possible to explore metabolites that are coming from or affected by the diet. In this way cheese intake can be investigated in an explorative manner compared to classical approaches usually conducted in nutrition research. The use of untargeted metabolite profiling may reveal exposure markers of cheese but may also contribute with markers which can help to explain how the intake of cheese affects cholesterol concentrations. To our knowledge, this has not been examined previously.
Several types of biological samples can be used for metabolomics studies, however, plasma and urine are most often used as they are relatively easy to collect (Álvarez-Sánchez et al. 2010). Also, in urine a greater diversity of metabolites are derived from food (Primrose et al. 2011). As metabolomics studies result in a high number of features, these studies should be as controlled as possible and because of the high inter-individual variations in human studies a cross-over design is preferred compared to parallel studies.
The present study aimed at comparing markers of cheese and butter intake by profiling samples from a human randomized cross-over intervention, with 2x6 weeks duration. An additional aim was to find markers that can help explain how the intake of cheese affects cholesterol concentrations in the study. An untargeted profiling by Ultra-Performance Liquid Chromatography coupled to Quadrupole Time-Of-Flight Mass Spectrometry (UPLC-QTOF/MS) was employed for maximal sensitivity. We report here for the first time potential novel markers of intake as well as markers related to the physiological effects of cheese compared with butter.
2 Methods
2.1 Subjects and study design
Details of participant recruitment, exclusion criteria and test diets has been described previously (Hjerpsted et al. 2011). Twenty-three men and women in the age from 22 to 70 participated in the part of the study described here. These were the subjects who agreed to hand in 4 × 24 h urine collections during the intervention. Subjects received oral and written information about the study before giving written informed consent and were instructed to keep their physical activity level and weight throughout the study.
A randomized, cross-over intervention, with a 2 weeks run-in period of habitual diet was conducted. Each intervention period lasted 6 weeks and was separated by 2 weeks washout period of habitual diet. The energy needs of each subject was calculated using the Commission of the European communities (Commission of the European Communities 1993) and WHO/FAO/UNU (World Health Organization 1985) prediction equations for basal energy expenditure multiplied with the physical activity level. The test diets were to substitute ~13 % of energy of the subject’s daily fat intake. The test food items were hard, yellow cheese named ‘Samsø’ and salted butter produced from cow’s milk. The fatty acid composition of the cheese and butter was similar (Hjerpsted et al. 2011). No other dairy products were allowed during the intervention periods except a maximum of 6 cl. low fat milk (0.5 % fat, for coffee, tea etc.) per day which had to be consumed each day throughout the entire intervention. The subject could choose to consume none, 1.5, 3, 4.5 or 6 cl. of low-fat milk per day. The remaining diet was self-selected. The study was carried out at Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen and approved by the Danish National Committee on Biomedical Research Ethics, report no. H–B-2009-052 in accordance with the Helsinki declaration. The study was registered at the data base ClinicalTrials.gov, the U.S. National Institutes of Health; Protocol ID: A308, ClinicalTrial.gov ID: NCT01140165, working title: ‘Cheese and human health’.
2.2 Sample collections
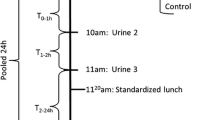
Twenty-four hours urine samples were collected throughout the last two days of each intervention period (a total of 48 h) into sterile containers and stored at 5 °C during the collection period. After each 24 h collection urine samples were portioned into 2 ml Cryo tubes (NUNC, Roskilde, Denmark) and frozen at -80 °C until analyses. During the urine collections subjects were instructed to ingest a tablet of 80 mg para-aminobenzoic acid (PABA) three times a day with breakfast, lunch and dinner. PABA content was measured using a colorimetric method on a spectrophotometer (Evolution 300, Thermo Scientific, UK). A recovery of >85 % PABA in the urine was considered complete (Bingham and Cummings 1983). In addition, a 1 % minor recovery was accepted for each decennium from age 30 (Jakobsen et al. 2003), thus a recovery between 81 and 85 % were considered complete depending on age. Three subjects were excluded from the analyses due to incomplete urine collections, leaving 20 subjects (13 men, 7 women) for the analyses. Baseline characteristics of these 20 subjects are given as supplementary material (Online Resource 1).
The cholesterol sampling and the result of the cholesterol concentrations from the intervention study has been published elsewhere (Hjerpsted et al. 2011).
2.3 UPLC-QTOF/MS analyses
Preparation of samples was conducted by the following procedure: Samples were thawed and centrifuged at approximately 2700 RCF for 4 min at 4 °C. Hereafter, 150 µl of each urine sample were transferred to a 96-well sample collection plate (Waters, Milford, MA) and diluted with 150 µl aqueous 5 % acetonitrile/methanol (70/30, v/v). Pooled urine samples and standard mix was added to individual wells in the 96-well sample collection plate for quality control (QC samples) such as mass error and retention time shifts during the run. Average of QCs and samples were very similar across all markers as shown by the PCA plots in supplementary figure SF1 and SF2, and the deviation between QCs was less that 7.5 % on average. Samples from the same person were randomized within the plate to minimize any possible systematic variation through run-time. All samples were analyzed using a Waters QTOF Premier Mass Spectrometer combined with an Ultra Performance Liquid Chromatograph (Waters) as described previously (Barri et al. 2012).
In short, the column and pre-column for the UPLC were HSS T3 C18 columns (particle size: 1.7 µm, internal diameter: 2.1 mm and length: 10 and 1 cm, respectively).
The mobile phase included 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile/methanol (70/30, v/v, B). A flow gradient and a concentration gradient of mobile phase was applied from 95 % A and 5 % B at a flow of 0.5 ml/min and reached 100 % B within 4.5 min and remained at this level for the next minute while flow increased to 1.2 ml/min and these conditions were kept until 7 min. The injection volume was 5 μl.
Electrospray ionization (ESI, Waters) was performed in both positive and negative mode at a probe voltage of 3.2 and 2.8 kV, respectively. The m/z range was from 50 to 1,000 Da. For accurate mass calibration, infusion of leucine encephalin was applied as lock-mass solution. Samples were kept sealed in an autosampler at 4 °C throughout the analysis. Furthermore, A and B were degassed by ultrasonication to minimize redox reactions of sensitive compounds.
2.4 Data preprocessing
Before data preprocessing the raw spectra were converted to netCDF files by the DataBridge software (Waters). Data preprocessing was performed using MZmine 2 (Pluskal et al. 2010). Data in positive and negative ESI mode were processed separately, using a subset of data from both modes to optimize the parameters. Each step and applied parameters are given as supplementary material (Online Resource 2).
Data (m/z values, retention times and peak area) were imported into MATLAB (version 7.11.0.584 (R2010b), MathWorks Inc., Massachusetts, USA) for further processing. To remove noise, the percent rule (Bijlsma et al. 2006) was applied on data with the following modifications. The threshold was set to 5 as the threshold could not be 0 due to the gap filling in MZmine. If at least 60 % of the measurements from a detected feature were not above 5 in at least one of the treatments, the feature was removed as being noise rather than an actual metabolite. Also, features with a retention time below 0.5 min. were considered noise. To adjust for different urine concentrations data were normalized to a total of 1,000 for each sample across the features.
2.5 Data analyses
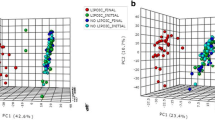
Initially, data were visually examined by principal components analyses (PCA) performed in the PLS-toolbox for MATLAB (version 6.0.1, Eigenvector Research Inc., Massachusetts, USA) resulting in 2-dimensional scores plots showing similar samples clustering together whereas disparate samples are farther apart. Data were mean centered prior to the analysis.
For univariate analysis, the mean was taken for the two measurements of each metabolite from each of the two consecutive 24 h collections. A two-step statistical screening procedure was applied to identify metabolites that could possibly be correlated with cholesterol. The key idea was to link two sets of univariate analyses. At first metabolites that showed a treatment effect were identified and subsequently it was evaluated whether or not these candidate metabolites were correlated with total cholesterol.
More specifically, in the first step a linear mixed model was fitted for each metabolite (3,027 models in total in positive ESI mode and 2,587 in negative ESI mode). The model included the treatment and was additionally adjusted for age, body mass index, gender, smoking as well as period effects. As repeated measurements were obtained from each subject random effects were also included in the model to account for inter-subject variability that could not be explained by the explanatory variables included. The p values for testing the treatment difference equal to 0 obtained for all metabolites were Bonferroni-adjusted to control the overall risk of a false positive finding (controlling the family-wise error rate); this means a total of 3,027 and 2,587 p values, in positive and negative ESI mode, respectively, were subjected to Bonferroni adjustment. Model validation was carried out by visual inspection of (automatically generated and stored) residual plots and normal probability plots for all linear mixed model fits that identified significant treatment differences. Metabolites with a significant treatment difference were included in the second step of the univariate analysis.
In the second step a linear mixed model with total cholesterol as outcome was fitted for each of the metabolites selected in the first step. This model included as fixed effects the raw residuals which were obtained from the corresponding linear mixed models run in the first step but without treatment effect (these residuals reflect the metabolite-specific treatment effect that potentially may explain the total cholesterol level), and adjustment for age, body mass index, gender, smoking as well as period effects was carried out. Like in the first step subjects-specific random effects were also included.
The p values for evaluating the metabolite-specific treatment effect on cholesterol (testing a slope coefficient equal to 0) were again Bonferroni adjusted. The metabolites that were also significant in the second step were prioritized for identification.
The univariate two-step procedure was applied separately to samples in positive ESI mode and negative ESI mode. The statistical environment R was used for the analysis (R Core Team 2013).
2.6 Identification
Initial identification was carried out by mass search in the Human Metabolome Database (Wishart et al. 2013). Further identification was performed by UPLC-QTOF/MS experiments where fragmentations of the metabolites were compared with that of standards of the candidate molecule and key structural isomers. Literature searches were made for verification and to find possible biological links to cholesterol concentrations. The identification was differentiated at four levels according to Sumner et al. (2007). Therefore, compounds identified by matching retention time and mass spectra of candidate molecules with that of authentic standards were designated level 1 identifications; compounds identified by MS/MS spectra similar to online database spectra were designated as level 2 identifications, compounds identified by characteristic physiochemical properties of a compound class were designated as level 3 identifications and finally compounds related to cheese or butter but not identified were designated as level 4 identifications.
The following standards acquired from Sigma-Aldrich (Schnelldorf, Germany) were analyzed by UPLC-QTOF/MS together with samples for level 1 identifications: 3- and 5-hydroxyanthranilic acid, tyramine, 3- and 4-hydroxyphenylacetic acid, 4,8-dihydroxyquinoline-2-carboxylic acid, 2-methoxynaphthalene, and xanthurenic acid.
2.7 Syntheses of standards
2.7.1 Isovalerylglycine and tiglylglycine
Isovaleric acid (1.473 g, 14.42 mmol), or tiglic acid (58 mg, 0.58 mmol) was added with acetic anhydride (46 mg, 0.44 mmol) and NaOH (6.59 mg, 0.165 mmol). The mixture was stirred at 35 °C for 1 h and then glycine (48.28 mg 0.64 mmol) was added, and the mixture was stirred at 35 °C for additionally 3 h. The crude mixture was allowed to cool to room temperature and diluted 1,500 times before being subjected to the same UPLC-QTOF/MS analysis used for the urine samples.
2.7.2 Valerylglycine and isobutyrylglycine
To a solution of valeric acid anhydride (50 mg, 0.26 mmol) or isobutyric acid anhydride (40 mg, 0.254 mmol) in water (0.3 ml) was added glycine (21 mg, 0.28 mmol) and NaOH (20 mg, 0.5 mmol). The mixtures were stirred at r.t. for 18 h and diluted 1,000 times before being subjected to UPLC-MS.
2.7.3 Isovalerylglutamic acid
To isovaleric acid (0.278 g, 2.72 mmol) was added acetic anhydride (46 mg, 0.44 mmol) and NaOH (6.90 mg, 0.173 mmol). The mixture was stirred at 35 °C for 1 h. To the mixture was then added glutamic acid (67.49 mg, 0.46 mmol), and the mixture was stirred at 35 °C for 3 h. The crude mixture was allowed to cool to r.t. and diluted 1,500 times before being subjected to UPLC-MS.
2.7.4 Tyramine sulfate and indoxyl sulfate
Sulfation of tyramine or indoxyl with the use of rat liver S9 enzymes and PAPS was performed as described previously (Rago et al. 2013).
2.7.5 Unknowns
Several syntheses were performed in order to identify potential unknowns. These syntheses also included analogs to rule out alternative identifications. These are detailed in supplementary materials (Online Resource 3).
3 Results
After data preprocessing a total of 3,413 features where detected in ESI positive mode and 3,156 features in ESI negative mode. After removal of noise and irrelevant features by the percent rule 3,027 features were included for further analyses in positive ESI mode and 2,587 where included in negative ESI mode.
Principal component analyses of these features did not result in any clear separation of the features according to treatment (figure not shown).
The univariate data analysis resulted in 16 features related to treatment in ESI positive mode and 13 of these were related to total cholesterol concentrations according to our statistical procedure. The same analyses for ESI negative mode resulted in 28 features and 23 of these were related to total cholesterol concentrations. Thus, a total of 44 features were related to treatment and 36 of these features were related to total cholesterol concentrations.
Several of the related compounds, including a number of acylglycine and sulfate conjugates were tentatively identified based on their fragmentation patterns and subsequently identified by matching retention times and mass spectra after synthesis of standards (Online Resource 2). Most metabolites identified were related to the intake of cheese and to lower total cholesterol concentrations. All metabolites including their retention time, mass, level of identification and p values from the statistical analyses are listed in Table 1.
4 Discussion
This study was conducted to investigate the differences in urinary metabolites excreted after intake of equal amounts of fat from cheese and butter and attempted to relate these metabolites to cholesterol concentrations. Because cheese and butter have similar fatty acid composition butter intake with equal amounts of fat was used as control. The possible metabolites verified with an authentic standard (level 1 identification) were all increased with cheese intake. They are discussed in the following with regard to their origin and metabolism.
Indoxyl sulfate is synthesized in the liver from indole, which is a metabolite produced from the essential amino acid tryptophan by the intestinal flora (Brunet et al. 2003). Also, xanthurenic acid (XA) is produced when tryptophan is catabolized via the kynurenine pathway. When tryptophan is taken as a supplement, the urinary excretion of XA is increased relatively to the amount of tryptophan ingested (Hiratsuka et al. 2013).
The tryptophan content in cheese is much higher than in butter (340 mg/100 g vs. 7 mg/100 g) (Saxholt et al. 2008) and therefore the finding of indoxyl sulfate and XA as markers of cheese intake could be expected. Yet, any possible relation of indoxyl sulfate and XA with cholesterol is not known. Tryptophan itself has been shown to decrease cholesterol concentrations in broiler chicks (Emadi et al. 2011) and together with lysine this has also been suggested in humans (Raja and Jarowski 1975). However, a recent cross-over intervention study in Japanese women found no effect on the lipid profile with tryptophan supplementation up to 5 g/d (Hiratsuka et al. 2013).
The two markers tyramine sulfate and 4-hydroxyphenylacetic acid are both deriving from tyrosine. The tyrosine content in cheese is much higher (1,400 mg/100 g) than in butter (21 mg/100 g) (Saxholt et al. 2008). However, tyramine is formed by microbial decarboxylation of tyrosine during cheese ripening and the content varies in different types of cheese depending on e.g. pasteurization, microflora and ripening period (Curtin and McSweeny 2004; Linares et al. 2012). Besides pasteurization, starter culture and ripening period, storage conditions may also affect the content of tyramine in cheese. The participants of our study received the cheese from the department each week, or in a few cases every second week, for storage and consumption at home. Some participants liked the cheese better at room temperature, therefore storage conditions may have affected the tyramine content of the cheese since higher temperatures increase the content of tyramine (Linares et al. 2012). In addition, the cheese was delivered at the department in a sliced form which may also increase the content of tyramine as the free surfaces of cheese have been found to contain more tyramine (Linares et al. 2012; Komprda et al. 2008). Tyramine sulfate was previously found in urine samples of depressed patients after an oral load of tyramine (Steinberg et al. 1993).
Oxidative deamination of tyramine catalyzed by monoamine oxidase produces 4-hydroxyphenylacetic acid which is the main degradation pathway for tyramine in humans (Fox et al. 2000). However, 4-hydroxyphenylacetic acid has also been found as a product of tyrosine catabolism under simulated Cheddar cheese ripening conditions (Gummalla and Broadbent 2001) although the bacterial strains used were different from the ones used for the production of the cheese investigated in our study. Thus, 4-hydroxyphenylacetic acid may occur naturally in cheese which has also been confirmed in a study analyzing the content of phenols in some cheeses (Gkouma et al. 2011). Excess tyrosine has been found to increase cholesterol concentrations in rats (Nagaoka et al. 1986) but any possible link between tyramine sulfate or 4-hydroxyphenylacetic acid and the lowering of cholesterol concentrations with cheese intake is uncertain.
Isobutyric acid is an aroma compound formed during fermentation and is a well-known constituent of many cheeses (Yvon and Rijnen 2001). It could additionally result from degradation of valine. The glycine conjugate identified in urine after ingestion of cheese seems to be a major excretion product in this study. Isovalerylglycine is a byproduct of the catabolism of another branched-chain amino acid, leucine, which is also abundant in cheese. Isovalerylglycine was associated with decreased total cholesterol concentrations (p = 0.0004), which is in accordance with studies of rodents finding decreased cholesterol concentrations with leucine supplementation (Torres-Leal et al. 2011; Zhang et al. 2007). Little is known about the molecular mechanism resulting in decreased cholesterol concentrations with leucine intake and whether these results are applicable to humans will depend on further studies. However, our findings of high concentrations of isovalerylglycine with cheese intake is very interesting as it may suggest that less leucine from cheese is converted to 3-hydroxy-3-methylglutaryl coenzyme A, a direct precursor of cholesterol, but that it is instead converted to isovalerylglycine. Furthermore, we found tiglylglycine which is a byproduct of the catabolism of isoleucine. Tiglylglycine is increased in urine of patients with disorders of propionate metabolism or with β-ketothiolase deficiency (Bennett et al. 1994). In general, the many acylglycines excreted in urine after cheese intake and their inverse correlation with plasma cholesterol is interesting. Glycine conjugation of the acyl coenzyme A (acyl-CoA) esters of xenobiotics by glycine N-acyltransferase results in formation of these acylglycines and liberation of coenzyme A, a central metabolic hub (Badenhorst et al. 2013). The increased concentration of acylglycines found in urine suggest an up-regulation of hepatic or renal glycine N-acyltransferases catalyzing this process in order to liberate sufficient coenzyme A (CoA) for lipid processing. We speculate based on our current findings that limitation of CoA due to sequestering after high exposure to branched aliphatic acids might decrease the rate of cholesterol synthesis because acetoacetyl-CoA cannot be formed efficiently. It is also possible that the normal catabolic pathway of the amino acids is inadequate due to the very high amino acid intake with cheese resulting in these acylglycines being formed as an excretory pathway. The possible biological link between the many acylglycines excreted and cholesterol lowering with cheese intake needs to be further studied. Alternatively, glycine conjugates are caused by increased availability of glycine with cheese intake, either directly from the glycine content of cheese or from the conversion of the high serine content of cheese to glycine (Badenhorst et al. 2013). In this case there may be no direct link with the effects on plasma cholesterol.
Our study has several strengths. The analytical tool used was UPLC-QTOF/MS which is believed to be a powerful tool in metabolomics studies, among others, due to the high sensitivity (Zhang et al. 2012). Furthermore, the study was a cross-over intervention which should reduce inter-individual variation as the participants receive all treatments compared to parallel intervention studies where subjects are randomly assigned to a treatment group or a control group. Although many acute exposure metabolites can be found in spot urine samples (Primrose et al. 2011), the use of 2 × 24 h urine samples after 6 weeks of cheese and butter intake have several advantages. This includes the chance of finding late eluting metabolites. Also, acute and habitual metabolites deriving from the cheese and butter intake can be found in 24 h urine samples independently of the time since consumption. In addition, the collection of urine samples after 6 weeks of intake makes it possible to find metabolites related to cheese and butter intake but also related to cholesterol concentrations as it reaches a steady state level after ~2 week (Keys et al. 1957; Mattson and Grundy 1985) and therefore any possible metabolites related to the cholesterol concentrations may first appear at this point in time. Furthermore, urine collections for 2 × 24 h decreased day-to-day variations and the intake of PABA ensured complete urine collections.
Although the present study had a cross-over design, the diet provided was not fully controlled and the participants where free-living. Therefore, the remaining diet and the different lifestyle of the participants may have affected the results. Additionally, the participants varied in age (from 22 to 70 years), gender and weight making it a very heterogeneous group in this respect. The metabolic and transport processes occurring in e.g. a person of 22 years and a person of 70 years may differ somewhat both in rate and in metabolite responses. This has for instance been shown for PABA, which is excreted more slowly with age (Jakobsen et al. 2003). The strict statistical demands used in this study with Bonferroni correction may have been too high a threshold for an explorative setting so that we may have missed additional explanatory markers related to other pathways, however setting this high threshold should ascertain that we have focused on the most important markers and pathways. Most of the metabolites associated with cheese intake were also inversely correlated with total cholesterol concentrations and any biological links between specific cheese-related metabolites and cholesterol may therefore be spurious. Additional studies with cheese components may help to identify which metabolites are related to total cholesterol concentrations. It could be interesting to investigate the relation of the features related to treatment with e.g. LDL or HDL cholesterol concentrations instead of total cholesterol. However, in the current study the features identified were related to total cholesterol as the effect of cheese intake on total cholesterol, LDL and HDL cholesterol concentrations were similar (cheese intake decreased the concentration of all) and we believe that the study should have been larger to be able to differentiate between the subclasses.
Cheese is a complex product that can be produced in several ways. The many different kinds of cheese may affect the metabolome differently. The cheese investigated in this study was a hard yellow cheese and the findings may not be applicable to other kinds of cheese. However, some of the compounds observed, including short-chain branched acid metabolites, aromatic amino acid metabolites and tyrosol may be quite common metabolites for a variety of cheeses.
To our knowledge this is the first study investigating metabolites excreted in urine after the intake of cheese and butter in humans. Our findings suggest that several possible metabolites deriving from the high protein content of cheese can be found in urine after cheese intake when compared to equal amounts of fat from butter. Tyramine-related metabolites may be specific to cheese intake but would probably vary considerably, depending of the cheese consumed. Furthermore, several acylglycines where found in urine after cheese intake. The exact biological mechanism potentially linking these metabolites with cholesterol concentrations needs to be further explored.
Abbreviations
- CVD:
-
Cardiovascular disease
- ESI:
-
Electrospray ionization
- LDL:
-
Low-density lipoprotein
- PABA:
-
Para-aminobenzoic acid
- RT:
-
Retention time
- UPLC-QTOF/MS:
-
Ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry
- XA:
-
Xanthurenic acid
References
Álvarez-Sánchez, B., Priego-Capote, F., & de Luque Castro, M. D. (2010). Metabolomics analysis I. Selection of biological samples and practical aspects preceding sample preparation. Trends in Analytical Chemistry, 29(2), 111–119.
Artaud-Wild, S. M., Connor, S. L., Sexton, G., & Connor, W. E. (1993). Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. A paradox. Circulation, 88, 2771–2779.
Badenhorst, C. P., van der Sluis, R., Erasmus, E., & van Dijk, A. A. (2013). Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation. Expert Opinion on Drug Metabolism and Toxicology, 9(9), 1139–1153.
Barri, T., Holmer-Jensen, J., Hermansen, K., & Dragsted, L. O. (2012). Metabolic fingerprinting of high-fat plasma samples processed by centrifugation- and filtration-based protein precipitation delineates significant differences in metabolite information coverage. Analytica Chimica Acta, 718, 47–57.
Bennett, M. J., Powell, S., Swartling, D. J., & Gibson, K. M. (1994). Tiglylglycine excreted in urine in disorders of isoleucine metabolism and the respiratory chain measured by stable isotope dilution GC-MS. Clinical Chemistry, 40(10), 1879–1883.
Bijlsma, S., Bobeldijk, I., Verheij, E. R., et al. (2006). Large-scale human metabolomics studies: A strategy for data (pre-) processing and validation. Analytical Chemistry, 78(2), 567–574.
Bingham, S., & Cummings, J. H. (1983). The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clinical Science (London), 64(6), 629–635.
Biong, A. S., Muller, H., Sejleflot, I., Veierod, M. B., & Pedersen, J. I. (2004). A comparison of the effects of cheese and butter on serum lipids, haemostatic variables and homocysteine. British Journal of Nutrition, 92, 791–797.
Brunet, P., Dou, L., Cerini, C., & Berland, Y. (2003). Protein-bound uremic retention solutes. Advances in Renal Replacement Therapy, 10(4), 310–320.
Commission of the European Communities. (1993). Reports of the Scientific Committee on Food: Nutrient and energy intakes for the European Community. Luxembourg: Office for Official Publications of the European Communities.
Curtin, A. C., & McSweeny, P. L. H. (2004). Catabolism of amino acids in cheese during ripening. In P. F. Fox, et al. (Eds.), Cheese: Chemistry, physics and microbiology (Third ed., Vol. 1, pp. 435–454). California: Elsevier Ltd.
Emadi, M., Jahanshiri, F., Kaveh, K., et al. (2011). Nutrition and immunity: The effects of the combination of arginine and tryptophan on growth performance, serum parameters and immune response in broiler chickens challenged with infectious bursal disease vaccine. Avian Pathology, 40(1), 63–72.
Fox, P. F., Guinee, T. P., Cogan, T. M., & McSweeny, P. L. (2000). Fundamentals of cheese science (1st ed.). Gaithersburg, Maryland: Aspen Publishers Inc.
Gartside, P. S., Wang, P., & Glueck, C. J. (1998). Prospective assessment of coronary heart disease risk factors: The NHANES I epidemiologic follow-up study (NHEFS) 16-year follow-up. Journal of the American College of Nutrition, 17(3), 263–269.
Gkouma, M., Cornu, A., Massouras, T., Lemaire, M., & Graulet, B. (2011). Comparative analysis of milk and cheese produced in the Pélardon PDO production zone based on their phenolic profiles. In M. Coppa (Ed.), Proceedings of the 10th international meeting on mountain cheese. Italy: University of Turin.
Gummalla, S., & Broadbent, J. R. (2001). Tyrosine and phenylalanine catabolism by Lactobacillus cheese flavor adjuncts. Journal of Dairy Science, 84(5), 1011–1019.
Hiratsuka, C., Fukuwatari, T., Sano, M., et al. (2013). Supplementing healthy women with up to 5.0 g/d of l-tryptophan has no adverse effects. Journal of Nutrition, 143(6), 859–866.
Hjerpsted, J., Leedo, E., & Tholstrup, T. (2011). Cheese intake in high amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. American Journal of Clinical Nutrition, 94, 1479–1484.
Huth, P. J., & Park, K. M. (2012). Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Advances in Nutrition, 3(3), 266–285.
Jakobsen, J., Pedersen, A. N., & Ovesen, L. (2003). Para-aminobenzoic acid (PABA) used as a marker for completeness of 24 hour urine: Effects of age and dosage scheduling. European Journal of Clinical Nutrition, 57(1), 138–142.
Keys, A., Anderson, J. T., & Grande, F. (1957). Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet, 2(NOV16), 959–966.
Komprda, T., Burdychova, R., Dohnal, V., et al. (2008). Tyramine production in Dutch-type semi-hard cheese from two different producers. Food Microbiology, 25(2), 219–227.
Larsson, S. C., Virtamo, J., & Wolk, A. (2012). Dairy consumption and risk of stroke in Swedish women and men. Stroke, 43(7), 1775–1780.
Lichtenstein, A. H., Appel, L. J., Brands, M., et al. (2006). Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation, 114(1), 82–96.
Linares, D. M., Del, R. B., Ladero, V., et al. (2012). Factors influencing biogenic amines accumulation in dairy products. Frontiers in Microbiology, 3, 180.
Mattson, F. H., & Grundy, S. M. (1985). Comparison of effects of dietary saturated, mono-unsaturated, and poly-unsaturated fatty-acids on plasma-lipids and lipoproteins in man. Journal of Lipid Research, 26(2), 194–202.
Mensink, R. P., & Katan, M. B. (1992). Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arteriosclerosis and Thrombosis, 12, 911–919.
Mensink, R. P., Zock, P. L., Kester, A. D., & Katan, M. B. (2003). Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. American Journal of Clinical Nutrition, 77(5), 1146–1155.
Moss, M., & Freed, D. (2003). The cow and the coronary: Epidemiology, biochemistry and immunology. International Journal of Cardiology, 87, 203–216.
Nagaoka, S., Masaki, H., Aoyama, Y., & Yoshida, A. (1986). Effects of excess dietary tyrosine or certain xenobiotics on the cholesterogenesis in rats. Journal of Nutrition, 116(5), 726–732.
Pluskal, T., Castillo, S., Villar-Briones, A., & Oresic, M. (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics, 11, 395.
Primrose, S., Draper, J., Elsom, R., et al. (2011). Metabolomics and human nutrition. British Journal of Nutrition, 105(8), 1277–1283.
Rago, D., Kristensen, M., Gürdeniz, G., et al. (2013). A LC-MS metabolomics approach to investigate the effect of raw apple intake in the rat plasma metabolome. Metabolomics, 9, 1202–1215.
Raja, P. K., & Jarowski, C. I. (1975). Utility of fasting essential amino acid plasma levels in formulation of nutritionally adequate diets IV: Lowering of human plasma cholesterol and triglyceride levels by lysine and tryptophan supplementation. Journal of Pharmaceutical Sciences, 64(4), 691–692.
Saxholt, E., Christensen, A. T., Møller, A., Hartkopp, H. B., Hess Ygil, K. and Hels, O. H. Danish Food Composition Databank, revision 7. [version 7]. 2008. Department of Nutrition, National Food Institute, Technical University of Denmark. July 12, 2013.
Seely, S. (1988). Diet and coronary arterial disease: A statistical study. International Journal of Cardiology, 20, 183–192.
Sonestedt, E., Wirfalt, E., Wallstrom, P., et al. (2011). Dairy products and its association with incidence of cardiovascular disease: The Malmo diet and cancer cohort. European Journal of Epidemiology, 26(8), 609–618.
Steinberg, J. L., Orsulak, P. J., Raese, J. D., et al. (1993). Effects of tricyclic antidepressant treatment on tyramine-O-sulfate excretion in depressed patients. Journal of Affective Disorders, 27(1), 29–34.
Sumner, L. W., Amberg, A., Barrett, D., et al. (2007). Proposed minimum reporting standards for chemical analysis. Metabolomics, 3(3), 211–221.
Tholstrup, T., Hoy, C. E., Andersen, L. N., Christensen, R. D. K., & Sandstrom, B. (2004). Does fat in milk, butter and cheese affect blood lipids and cholesterol differently? Journal of the American College of Nutrition, 23(2), 169–176.
Torres-Leal, F. L., Fonseca-Alaniz, M. H., Teodoro, G. F., et al. (2011). Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutrition and Metabolism (London), 8(1), 62.
van Aerde, M. A., Soedamah-Muthu, S. S., Geleijnse, J. M., et al. (2013). Dairy intake in relation to cardiovascular disease mortality and all-cause mortality: The Hoorn Study. European Journal of Nutrition, 52(2), 609–616.
von Ruesten, A., Feller, S., Bergmann, M. M., & Boeing, H. (2013). Diet and risk of chronic diseases: Results from the first 8 years of follow-up in the EPIC-Potsdam study. European Journal of Clinical Nutrition, 67(4), 412–419.
Wishart, D.S., Jewison, T., Guo, A.C. et al. (2013). HMDB 3.0–The human metabolome database in 2013. Nucleic acids research, 41, (Database issue) D801-D807 Accessed July 9, 2013.
World Health Organization. (1985). Energy and protein requirements. WHO, Geneva: Report of a joint FAO/WHO/UNU expert consultation.
Yvon, N., & Rijnen, L. (2001). Cheese flavour formation by amino acid catabolism. International Dairy Journal, 11, 185–201.
Zhang, Y., Guo, K., LeBlanc, R. E., et al. (2007). Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes, 56(6), 1647–1654.
Zhang, A., Sun, H., Wang, P., Han, Y., & Wang, X. (2012). Modern analytical techniques in metabolomics analysis. Analyst, 137(2), 293–300.
Acknowledgments
We would like to thank Sarah Schultz John for preparing the samples for analysis, Ümmühan Celik for preprocessing of data in MZmine, Maj-Britt Scmidt Andersen and Daniella Rago for laboratory assistance and Göezde Gürdeniz and Jan Stanstrup for sharing scripts in MATLAB. The research was supported by the Danish Dairy Research Foundation, DK-8260 Viby J, Denmark and the Dairy Research Institute, Rosemont, IL, USA.
Conflicts of interest
Julie Bousgaard Hjerpsted, Christian Ritz, Simon Stubbe Schou, Tine Tholstrup, Lars Ove Dragsted declared that they have no conflict of interest.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants being included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hjerpsted, J.B., Ritz, C., Schou, S.S. et al. Effect of cheese and butter intake on metabolites in urine using an untargeted metabolomics approach. Metabolomics 10, 1176–1185 (2014). https://doi.org/10.1007/s11306-014-0657-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-014-0657-7