Abstract
Lysophosphatidylcholines (lysoPCs) are a class of compounds that have a constant polar head, and fatty acyls of different chain lengths, position, degrees of saturation, and double bond location in human plasma. LysoPCs levels can be a clinical diagnostic indicator that reveals pathophysiological changes. In this work, a method was developed to discriminate between different types of lysoPCs using reversed phase ultra-performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry, using mass spectrometry MSE. Isomeric lysoPCs were distinguished based on retention time and the peak intensity ratio of product ions, and 14 pairs of lysoPCs regioisomers were identified in human plasma. The plasma samples of 12 lung cancer patients and 12 healthy persons were collected and analyzed by principal component analysis to generate metabolic profiles of the identified lysoPCs. Both electrospray ionization ESI+ and ESI− results showed that all lung cancer patients had the same five lysoPC metabolic abnormalities, specifically in sn-1 lyso16:0, sn-2 lysoPC 16:0, sn-1 lysoPC 18:0, sn-1 lysoPC 18:1 and sn-1 lysoPC 18:2. Thus, the function of isomers with different fatty acyl positions may be related to lung cancer, and this may help elucidate the mechanism of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabonomics is a key method in systems biology that examines the dynamics of metabolic profiles during disease onset. In clinical trials, the primary goals of metabonomics are searching for, and identifying disease biomarkers (Jia et al. 2007; Lenz and Wilson 2007; Lu and Xu 2008; Wang et al. 2005). Using multivariate statistical methods such as principal component analysis (PCA), metabonomics processes data from information-rich analytical techniques and generates spectral patterns for evaluation. However, comprehensive investigation of the metabolome is complicated by its complexity and dynamic nature, and many unknown variables can introduce errors in sample comparison. In addition, unknown biomarker identification is complicated by the lack of good analytical techniques and standards, especially information such as structures, that are needed to identify minor compounds (Lenz and Wilson 2007; Lu and Xu 2008; Theodoridis et al. 2008). Knowing that a class of compounds is involved in a disease from the beginning of a search, can simplify metabonomics and make the process more effective.
Lysophosphatidylcholines (lysoPCs) are products or metabolites of phosphatidylcholines (PCs), which are structural components of animal cell membranes. The structure of lysoPCs is a constant polar head, and fatty acyls that differ in chain length, position, and degree of saturation. LysoPCs occur in two forms, with the fatty acyl groups at positions 1 (sn-1) or 2 (sn-2) on the glycerol backbone. The sn-1 lysoPCs are produced by phospholipase A2 and the sn-2 lysoPCs by phospholipase A1 (Hirano et al. 2004; Thies et al. 1992). The two lysoPC forms are found in different ratios in vivo, which would influence their removal from the blood, and their uptake, acylation and catabolism in tissues (Thies et al. 1992; Morash et al. 1989; Besterman and Domanico 1992). Consequently, these isomers have a biological significance.
Electrospray ionization (ESI) mass spectrometry (MS) coupled with reversed phase high performance liquid chromatography (RPLC) has been used to analyze lysoPCs and regioisomers in complex soybean extracts (Fang et al. 2003). This method combines chromatographic resolution with the rich structural information and sensitivity of MS and MS/MS. RPLC separates lysoPC regioisomers into individual molecular species (Fang et al. 2003; Adlercreutz and Wehtje 2001; Creer and Gross 1985), while MS determines the acyl chain regioisomers by comparison of the intensity ratio of characteristic fragment ions (Han and Gross 1996). These work showed that lysoPCs can be well separated and detected based on RPLC/MS. Until recently, no studies have used this method to investigate their changes for possible use in lung cancer diagnosis using plasma. Besides, UPLC/MSE (Plumb et al. 2006) plays an important role in generating molecular fragment information for biomarker structure elucidation, however, no work has used this method to identify lysoPC regioisomers.
To our knowledge, previous reversed-phase chromatography systems used in metabonomic analysis identified only the number of the carbons in the acyl groups, without specifying the sn-position (Sutphen et al. 2004; Yin et al. 2006; Jia et al. 2008; Cai et al. 2009a; Chen et al. 2009). However, lysoPCs with different sn-positions are metabolites in different pathways. Therefore, these regioisomers are expected to have different biological and biophysical properties. For disease studies, the position of the acyl group in lysoPC markers is necessary to understand their biological roles. However, few studies have used this method to identify regioisomers, or to investigate their changes for possible use in lung cancer diagnosis using plasma.
Previous studies have shown that lysoPC in vivo can be used to synthesis PC in the endoplasmic reticulum of the liver or kidney, using acyl-(coenzyme A) as a donor (Gurr et al. 2002). Hence, in metabonomics studies of liver and kidney diseases, such as chronic hepatitis B (Yang et al. 2006) and diabetes mellitus (Zhang et al. 2009a), lysoPCs have been used as markers. Recently, Shindou et al. (Nakanishi et al. 2006; Shindou et al. 2009) found that lysoPC can be converted to PC by lysoPC acyltransferases (LPCATs) in the pulmonary surfactant, where they decrease lung surface tension, performing an essential function for respiration. The incidence of lung disease activity would inevitably lead to LPCATs dysfunction, which arise the diversity and content changes of lysoPCs. LysoPCs are also recognized as potent mediators of cell activation (Fukushima et al. 1998; Hla et al. 2001; Samuelsson et al. 1987). Thus, circulating of lysoPCs may be an important contributing factor for lung function abnormalities, and lysoPCs may be useful as disease markers. As the lung cancer metabonomics research began rather late and the mechanism of lung cancer is not yet clear, there are not any effective treatments to hold back and treat lung cancer disease.
In this study, we analyzed lysoPCs in human plasma, and developed a method using ultra-performance RPLC coupled to quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF) to identify lysoPCs and their isomers. This method was applied to a metabonomics study on lung cancer patients and healthy people, discovering abnormalities in five lysoPCs, including isomers.
2 Experimental
2.1 Sample collection and preparation
The fasting blood sample (2 ml) was added to an EDTA-containing tube to inhibit phospholiphase activity and deposited at room temperature for 30 min, then centrifuged at 3000 rpm at 4°C for 10 min. The supernatant was transferred to a 1.5 ml microtube (Axygen, Central Avenue, Union City, CA 94587, USA) and stored at −80°C until assayed. For the analysis of plasma phospholipids, the sample was thawed at room temperature. Lipids were extracted from human plasma by precipitating protein with 4:1 (acetonitrile/human plasma) and centrifuging at 14,000g for 10 min. The supernatant was lyophilized and dissolved in 100 µl solvent (4:1, acetonitrile/water) before injected into the UPLC/Q-TOF system. Three microlitre of each sample was injected onto the column. For metabonomics study, plasma samples were collected from 12 healthy controls (aged from 52 to 73 years), 12 patients with squamous cell carcinoma (aged from 59 to 69 years) before breakfast. And these samples were treated in the same way.
2.2 UPLC/Q-TOF analysis
Sample analysis was performed on a Waters ACQUITY Ultra Performance LC system (Waters, Milford, MA) which was coupled with a Q-TOF premier (Waters MS Technologies, Manchester, UK). And the column (ACQUITY UPLC BEH C18 1.7 µm, 2.1 × 100 mm) temperature was maintained at 30°C for all of the analysis. The inject volume was 3 µl. The column was eluted with a liner gradient of 5–100% B, where A = 0.1% formic acid and B = acetonitrile. The gradient duration was 30 min at a flow rate of 0.35 ml/min−1. All the samples were injected in a random order. A blank was analyzed between every three samples to avoid carry over. The standard sample consisted of 10 µg/ml sn-1 lysoPC 18:1 in 80% acetonitrile/water solution was analyzed before and after experiment.
Mass spectrometry was operated in both negative and positive ion electrospray mode in V optics mode. The capillary voltage and the cone voltage were set at 3.0 kV and 40 V for positive electrospray mode and 2.5 kV and 40 V for negative electrospray mode, respectively. The nebulization gas was maintained at 800 l/h−1 at a temperature of 350°C, the cone gas was set to 50 l/h−1, and the source temperature was set to 120°C. The MCP detector voltage was set to 1600 V. The data acquisition rate was set to 0.5 s with a 0.02 s interscan delay. The scan range was from 50 to 1000 m/z. Data was collected in centroid mode. All analyses were acquired by using the lock spray to ensure accuracy and reproducibility; leucine-enkephalin was used as the lock mass at a concentration of 50 pg ml−1 and flow rate 0.02 ml min−1, generating an ion at 556.2695 Da in ESI+ mode and an ion at 554.2771 Da in ESI− mode. The lock spray frequency was set at 25 s. MSE analysis was performed on a Waters Q-Tof Premier mass spectrometer set at 5 eV for low collision energy and 25 eV for high collision energy.
2.3 Data extraction and analysis
The raw data were analyzed by the MarkerLynx applications manager version 4.0 (Waters, Manchester, UK) first. The software uses ApexTrack peak detection to integrate peaks in UPLC/MS data (Cai et al. 2009b; Idborg et al. 2005). After data acquisition Waters MarkerLynx software, it was exported to a CSV file, which includes the information of the m/z, retention time, height, and area of the extracted ion chromatograms (XIC) peaks. Then the 28 target ions sets were selected and fed to SIMCA-P softwares from Umetrics (Sweden) for further analysis.
3 Results and discussion
3.1 Sample preparation and analysis
LysoPCs were extracted from plasma by precipitating with acetonitrile (Rainville et al. 2007; Bruce et al. 2008; Want et al. 2006). Considering the migration of acyl groups in lyso-phospholipids under some conditions, all the samples were stored at 4° exacted simultaneously and analyzed immediately after treatment. The migration wasn’t observed on the chromatography by using the standard of sn-1 lysoPC 18:1 before and after the whole experiment.
For sample analysis, a UPLC column with sub-2-um porous stationary phase particles was used to obtain better quality and higher throughput. A full scan of human plasma samples was performed in both positive and negative modes. Base peak intensity (BPI) chromatograms from a healthy male volunteer are shown in Fig. 1.
3.2 Identification of endogenous metabolites
Identifying lysoPCs and regioisomers requires detailed examination of the MS/MS information. For lysoPC MS/MS analysis, we used MSE(Bateman et al. 2007), a technique in which precursor and fragment mass spectra are simultaneously acquired by alternating between high and low collision energy during a single chromatographic run. Performance was tested with UPLC coupled to a Q-tof Premier mass spectrometer, with electrospray in both positive and negative MSE mode. Due to the increased efficiency of the column packed with 1.7 um particles, narrower peaks could be obtained. For this reason, MSE could offer the MS fragmentation of every peak. Headgroup-specific fragmentation of lysoPCs resulted in two diagnostic fragment ions of m/z 184.1 and 104.1 in positive mode (data not shown), as discussed previously (Rainville et al. 2007; Liebisch et al. 2002).
Lysophospholipids exist in two forms in vivo, sn-1 lysoPC and sn-2 lysoPC. As regioisomers, they have the same fragmentation ions because of their closely related structures, so discrimination is difficult. Sn-1 and sn-2 lysoPC can be distinguished with a collision-induced dissociation (CID) pathway for the regioisomers using ESI-MS, and analyzing the peak intensity ratio of the product ions at m/z 104 and 147, generated from [M + Na]+ in the positive-ion mode (Han and Gross 1996). However, subsequent research found that the regioisomers can yield different fragments. For example, LC-MS/MS analysis of a lysoPC standard mixture resulted in cleavage of sn-1 lysoPC, yielding prominent phosphocholine cations (m/z 184) with more than 80% relative intensity (Fang et al. 2003). In contrast, phosphocholine cations from the sn-2 lyso-PC regioisomer were of low prominence. To distinguish the regioisomers in our experimental conditions, we used the ratio of fragmentation ions at m/z 184.1 and 104.1, and the retention time. The proposed high-energy CID major pathway in positive mode is in Fig. 2.
Using the characteristics of lysoPC fragmentation, and the principles of MSE, we generated an identification process for lysoPCs in plasma. First, extracted ion chromatograms (XIC) of fragmentation ions at m/z 184.1 and 104.1 m/z were used to determine the retention time for lysoPC peaks. Next, the exact molecular weights were calculated from the retention time peaks that gave the adduct molecular ion peak in low-energy positive and negative conditions. The mass of fatty acid fragments in high-energy negative conditions indicated the number of carbons, and the intensity ratio of m/z 184.1 and 104.1 fragmentation ions discriminated between the sn-1 and sn-2 isomers, since the ratio of the ion pair is more than 1 for sn-1, and less than 1 for the sn-2 isomer. This process identified 28 types of lysoPCs in human plasma (Table 1). The high separating ability of UPLC allowed baseline separation of individual lysoPCs (Table 1, second column), demonstrating the accuracy of mass fragments for each isomer.
Figure 3 shows the XIC of m/z 184.1 and m/z 104.1 fragmentation ions. The first peak, with a retention time of 15.72 min (Fig. 3c, Peak 1), showed m/z 468.3162 and 490.2823 in the low collision-energy mass spectrum. The difference between the two is 21.9661, which plus H molecular weight is 22.9740 Da, which indicated that the latter was [M + Na]+ and the molecular mass could be 467.3083 Da. In negative mode, peak 1 gave m/z 452.2878 and 512.2995 in the low-energy condition. These two ions were inferred to be [M-CH3]− and [M + HCOO]−, respectively, by exact mass results. An abundant fatty acid fragment, [C13H27COO]− was observed at m/z 227.1980 in the high-energy negative conditions. Accordingly, [M + H]+, the metabolite with m/z 468.3162,was identified as C14:0 lysoPC.
a Electrospraypositive total ionchromatogram (TIC) of UPLC/MS separation of analytesextracted from the supernatant of protein precipitated human plasma from a healthy control; b Electrospraypositive extracted ion chromatogram (XIC) of m/z 184.1 and 104.1 of UPLC/MS separation of lysoPCs. c the magnification of the XIC with retention time from 14 to 24 min
To discriminate between the sn-1 and sn-2 acyl chain positions, high collision-energy mass spectra were analyzed further. It should be noted that, these analyses also can be produce [M + Na] + and they are fragmented at the same time with [M + H]+ in the MSE mode. However, the abundance of [M + Na]+ is less than 10% of [M + H]+ in low-energy positive conditions (Fig. 4), and the relative influence can be ignored. The fragments of sn-1 lysoPC 18:1 were almost unanimouslyin between the MSE mode and traditional MS/MS mode (data not shown). For this reason, the influence about fragments of [M + Na]+ or other low abundance of ion were not considered. Besides,the powerful chromatographic resolution has ensured the base line separation of the main lysoPCs, such as lysoPC 18:0, 18:1, 18:2 and their corresponding isomers (Fig. 5 and Table 1, second column). As the result, the relative intensities of fragments in high-energy positive conditions were less affected, and the intensity ratio of m/z 184.1 and 104.1 fragmentation ions can be used for isomer assignment.
Comparison of the isomers from a plasma extract of a control (peak 1 and 2 in Fig. 2) with different acylchain position in both positive and negative modes with the utility of MSE. a, e Low collision energy mass spectra of peak 1and 2 in positive mode. b, f High collision energy mass spectra of peak 1 and 2 in positive mode. c, g Low collision energy mass spectra of peak 1 and 2 in negative mode. d, h High collision energy mass spectra of peak 1 and 2 in negative mode
In high-energy positive conditions, ion intensity ratios clearly showed that peak 1 was sn-2 lysoPC, and peak 2 was sn-1 lysoPC. Structures are in Fig. 4. Other lysoPCs compounds were identified in a similar manner. A standard of sn-1 lysoPC 18:1 was used to confirm the accuracy of molecular weight, retention time and fragment ion identification in the positive mode. Qualitative results for 28 endogenous metabolites are in Table 1, and indicate that lysoPCs are always present in the human plasma in the form of regioisomers.
The retention times for both the sn-1 and sn-2 isomers increased by acyl chain, with the acyl chain from the sn-2 to the sn-1 position having the greatest effect on retention time. Previous studies found that, in reversed-phase mode, lysoPC isomers containing an acyl substituent at the sn-2 position eluted before their 1-acyl lysoPC counterparts (Creer and Gross 1985), and the elution order from these studies confirmed our identification.
3.3 LysoPCs profiling of human plasma for classification between healthy people and lung cancer patients
Based on successful identification of lysoPCs, plasma lysoPCs from 12 lung cancer patients and 12 healthy volunteers were investigated, to find potential disease biomarkers and reveal pathophysiological changes in lung cancer. Since environmental differences and variations in diet (Solanky et al. 2003), sex (Cai et al. 2009b), estrus cycle (Bollard et al. 2001), lifestyle (Jernstrom et al. 2003), time of sampling (Beckwith-Hall et al. 2002), and drug dosing vehicle (Beckwith-Hall et al. 2002) can all influence the metabolome, a rigorous sampling selection was performed. Plasma was collected before breakfast in the same pathological type in same geographic region, and healthy controls and patients were selected to have similar ages, from 52 to 73 years. Raw data completed with pretreatment and PCA analysis was exported to SIMCA-P for further analysis. According to retention time and MS data, only the ion intensity of 28 target variables, including retention time and m/z pairs for each metabolite peak from the dataset, were arranged as columns of a matrix, with the 24 samples as matrix rows. This matrix was subjected to PCA analysis, and statistical analysis with the SIMCA-P to obtain potential biomarkers.
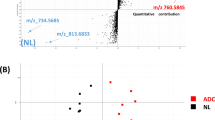
The PCA plot showed clear separation between patients and controls using the first two components (Fig. 6). This unsupervised PCA analysis showed a trend in lysoPC changes between patients and controls. Components that play important roles in the separation were picked out according to the parameter VIP (Variable Importance in the Projection). Both ESI+ and ESI− showed the same five VIP value, specifically sn-1 lysoPC 16:0, sn-2 lysoPC 16:0, sn-1 lysoPC 18:0, sn-1 lysoPC 18:1 and sn-1 lysoPC 18:2. The five lysoPCs were obviously decreased in lung cancer patients, and they showed that not all regioisomers correlated to lung cancer. Only lysoPC 16:0 regioisomers showed metabolic abnormality in lung cancer, sn-1 lysoPC 18:0 and sn-1 lysoPC 18:1 showed large changes (Fig. 7), and sn-2 lysoPC 18:0 and sn-2 lysoPC 18:1 showed little change. LysoPCs have been reported to be markers for diabetes (Zhang et al. 2008, 2009a), atherosclerosis (Zha et al. 2009; Zhang et al. 2009b), ovarian cancer (Sutphen et al. 2004), intestinal fistulas (Yin et al. 2008), chronic renal failure (Jia et al. 2008) and liver cancer (Chen et al. 2009; Yang et al. 2004), but the acyl position of the lysoPCs was not considered in all of these studies, and individual lysoPC were not identified as markers. Because lysoPCs are markers for many diseases, changes in one or two lysoPC regioisomers may not be clear, causing the lysoPC to lose its specificity as a disease marker. However, we hypothesize that isomer ratios might be different in the diseases for which lysoPCs are markers. Whether individual lysoPC levels change between healthy people and patients needs to be investigated. Our results support the proposal that individual lysoPC isomers play different essential roles in lung cancer patients and healthy people.
3.4 Biomarkers related to lung cancer
When identifying markers, the reasons for abnormal in vivo changes must be explained. Pulmonary surfactant contains mainly dipalmitoyl-PC and some of PCs with oleic acid (18:1), linoleic acid (18:2), linolenic acid (18:3) at the sn-2 position (Nakanishi et al. 2006; Bernhard et al. 2004; Postle et al. 2007). The conversion of these species into the disaturated forms implies a phospholipase A2 step to remove the unsaturated fatty acid. The resulting lysoPC can be reacylated with acyl-CoA esters, as first described by Lands (Lands 1958, 1960). The necessary enzyme is reported to be present in lung (Lands 1960; Garcia et al. 1975; Longmore et al. 1979; Ohta et al. 1972).
One factor in the decrease of sn-1 lysoPCs may be explained by the remodeling pathway (Lands’ cycle). In lung cancer, cell proliferation is associated with a high metabolic state, and abnormal expression of LPCAT1 results in a decrease in sn-1 lysoPC 16:0. The decrease of other sn-1 lysoPCs might be explained in the same way. We also observed a decrease in sn-2 lysoPC 16:0, however the mechanism in our case was not clear and further studies will be needed to confirm the relationship.
4 Conclusions
Many species of lysoPCs were found in human plasma in the form of regioisomers. We identified 28 plasma lysoPC species by UPLC/Q-TOF using the MSE method. The plasma lysoPCs from 12 patients with lung cancer and 12 healthy volunteers were investigated showing that different lysoPC acyl positions may be related to pathophysiological changes in lung cancer.
References
Adlercreutz, D., & Wehtje, E. (2001). A simple HPLC method for the simultaneous analysis of phosphatidylcholine and its partial hydrolysis products 1-and 2-acyl lysophosphatidylcholine. Journal of the American Oil Chemists Society, 78(10), 1007–1011.
Bateman, K. P., Castro-Perez, J., Wrona, M., Shockcor, J. P., Yu, K., Oballa, R., et al. (2007). MSE with mass defect filtering for in vitro and in vivo metabolite identification. Rapid Communications in Mass Spectrometry, 21(9), 1485–1496.
Beckwith-Hall, B. M., Holmes, E., Lindon, J. C., Gounarides, J., Vickers, A., Shapiro, M., et al. (2002). NMR-based metabonomic studies on the biochemical effects of commonly used drug carrier vehicles in the rat. Chemical Research in Toxicology, 15(9), 1136–1141.
Bernhard, W., Pynn, C. J., Jaworski, A., Rau, G. A., Hohlfeld, L. M., Freihorst, J., et al. (2004). Mass spectrometric analysis of surfactant metabolism in human volunteers using deuteriated choline. American Journal of Respiratory and Critical Care Medicine, 170(1), 54–58.
Besterman, J. M., & Domanico, P. L. (1992). Association and metabolism of exogenously-derived lysophosphatidylcholine by cultured-mammalian-cells—Kinetics and mechanisms. Biochemistry, 31(7), 2046–2056.
Bollard, M. E., Holmes, E., Lindon, J. C., Mitchell, S. C., Branstetter, D., Zhang, W., et al. (2001). Investigations into biochemical changes due to diurnal variation and estrus cycle in female rats using high-resolution H-1 NMR spectroscopy of urine and pattern recognition. Analytical Biochemistry, 295(2), 194–202.
Bruce, S. J., Jonsson, P., Antti, H., Cloarec, O., Trygg, J., Marklund, S. L., et al. (2008). Evaluation of a protocol for metabolic profiling studies on human blood plasma by combined ultra-performance liquid chromatography/mass spectrometry: From extraction to data analysis. Analytical Biochemistry, 372(2), 237–249.
Cai, S., Huo, T. G., Li, N., Xiong, Z. L., & Li, F. M. (2009a). Lysophosphatidylcholine-biomarker of Metformin action: studied using UPLC/MS/MS. Biomedical Chromatography, 23(7), 782–786.
Cai, X., Zou, L., Dong, J., Zhao, L., Wang, Y., Xu, Q., et al. (2009b). Analysis of highly polar metabolites in human plasma by ultra-performance hydrophilic interaction liquid chromatography coupled with quadrupole-time of flight mass spectrometry. Analytica Chimica Acta, 650(1), 10–15.
Chen, J., Wang, W., Lv, S., Yin, P., Zhao, X., Lu, X., et al. (2009). Metabonomics study of liver cancer based on ultra performance liquid chromatography coupled to mass spectrometry with HILIC and RPLC separations. Analytica Chimica Acta, 650(1), 3–9.
Creer, M. H., & Gross, R. W. (1985). Separation of isomeric lysophospholipids by reverse phase Hplc. Lipids, 20(12), 922–928.
Fang, N. B., Yu, S. G., & Badger, T. M. (2003). LC-MS/MS analysis of lysophospholipids associated-with soy protein isolate. Journal of Agricultural and Food Chemistry, 51(23), 6676–6682.
Fukushima, N., Kimura, Y., & Chun, J. (1998). A single receptor encoded by vzg-1/lp(A1)/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proceedings of the National Academy of Sciences of the United States of America, 95(11), 6151–6156.
Garcia, A., Newkirk, J. D., & Mavis, R. D. (1975). Lung surfactant synthesis—Ca++-dependent microsomal phospholipase A2 in lung. Biochemical and Biophysical Research Communications, 64(1), 128–135.
Gurr, M. I., Harwood, J. L., & Frayn, K. N. (2002). Lipid biochemistry: An introduction. Oxford: Blackwell Science.
Han, X. L., & Gross, R. W. (1996). Structural determination of lysophospholipid regioisomers by electrospray ionization tandem mass spectrometry. Journal of the American Chemical Society, 118(2), 451–457.
Hirano, K., Matsui, H., Tanaka, T., Matsuura, F., Satouchi, K., & Koike, T. (2004). Production of 1,2-didocosahexaenoyl phosphatidylcholine by bonito muscle lysophosphatidylcholine/transacylase. Journal of Biochemistry, 136(4), 477–483.
Hla, T., Lee, M. J., Ancellin, N., Paik, J. H., & Kluk, M. J. (2001). Lysophospholipids—Receptor revelations. Science, 294(5548), 1875–1878.
Idborg, H., Zamani, L., Edlund, P. O., Schuppe-Koistinen, I., & Jacobsson, S. P. (2005). Metabolic fingerprinting of rat urine by LC/MS Part 2. Data pretreatment methods for handling of complex data. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 828(1–2), 14–20.
Jernstrom, H., Klug, T. L., Sepkovic, D. W., Bradlow, H. L., & Narod, S. A. (2003). Predictors of the plasma ratio of 2-hydroxyestrone to 16 alpha-hydroxyestrone among pre-menopausal, nulliparous women from four ethnic groups. Carcinogenesis, 24(5), 991–1005.
Jia, L. W., Chen, J., Yin, P. Y., Lu, X., & Xu, G. W. (2008). Serum metabonomics study of chronic renal failure by ultra performance liquid chromatography coupled with Q-TOF mass spectrometry. Metabolomics, 4(2), 183–189.
Jia, L. W., Wang, C., Zhao, S. M., Lu, X., & Xu, G. W. (2007). Metabolomic identification of potential phospholipid biomarkers for chronic glomerulonephritis by using high performance liquid chromatography-mass spectrometry. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 860(1), 134–140.
Lands, W. E. M. (1958). Metabolism of glycerolipides—Comparison of lecithin and triglyceride synthesis. Journal of Biological Chemistry, 231(2), 883–888.
Lands, W. E. M. (1960). Metabolism of glycerolipids. 2. Enzymatic acylation of lysolecithin. Journal of Biological Chemistry, 235(8), 2233–2237.
Lenz, E. M., & Wilson, I. D. (2007). Analytical strategies in metabonomics. Journal of Proteome Research, 6, 443–458.
Liebisch, G., Drobnik, W., Lieser, B., & Schmitz, G. (2002). High-throughput quantification of lysophosphatidylcholine by electrospray ionization tandem mass spectrometry. Clinical Chemistry, 48(12), 2217–2224.
Longmore, W. J., Oldenborg, V., & Vangolde, L. M. G. (1979). Phospholipase-A2 in rat-lung microsomes—Substrate-specificity towards endogenous phosphatidylcholines. Biochimica et Biophysica Acta, 572(3), 452–460.
Lu, X., & Xu, G. (2008). LC-MS metabonomics methodology in biomarker discovery. Methods in Pharmacology and Toxicology (pp. 291–315). Totowa: Humana Press Inc.
Morash, S. C., Cook, H. W., & Spence, M. W. (1989). Lysophosphatidylcholine as an intermediate in phosphatidylcholine metabolism and glycerophosphocholine synthesis in cultured-cells—An evaluation of the roles of 1-Acyl-Lysophosphatidylcholine and 2-Acyl-Lysophosphatidylcholine. Biochimica et Biophysica Acta, 1004(2), 221–229.
Nakanishi, H., Shindou, H., Hishikawa, D., Harayama, T., Ogasawara, R., Suwabe, A., et al. (2006). Cloning and characterization of mouse lung-type acyl-CoA: Lysophosphatidylcholine acyltransferase 1 (LPCAT1)—Expression in alveolar type II cells and possible involvement in surfactant production. Journal of Biological Chemistry, 281(29), 20140–20147.
Ohta, M., Hasegawa, H., & Ohno, K. (1972). Calcium independent phospholipase-A2 activity in rat lung supernatant. Biochimica et Biophysica Acta, 280(4), 552–558.
Plumb, R. S., Johnson, K. A., Rainville, P., Smith, B. W., Wilson, I. D., Castro-Perez, J. M., et al. (2006). UPLC/MSE; a new approach for generating molecular fragment information for biomarker structure elucidation (vol 20, pg 1989, 2006). Rapid Communications in Mass Spectrometry, 20(14), 1989–1994.
Postle, A. D., Wilton, D. C., Hunt, A. N., & Attard, G. S. (2007). Probing phospholipid dynamics by electrospray ionisation mass spectrometry. Progress in Lipid Research, 46(3–4), 200–224.
Rainville, P. D., Stumpf, C. L., Shockcor, J. P., Plumb, R. S., & Nicholson, J. K. (2007). Novel application of reversed-phase UPLC-oaTOF-MS for lipid analysis in complex biological mixtures: A new tool for lipidomics. Journal of Proteome Research, 6, 552–558.
Samuelsson, B., Dahlen, S. E., Lindgren, J. A., Rouzer, C. A., & Serhan, C. N. (1987). Leukotrienes and lipoxins—Structures, biosynthesis, and biological effects. Science, 237(4819), 1171–1176.
Shindou, H., Hishikawa, D., Harayama, T., Yuki, K., & Shimizu, T. (2009). Recent progress on acyl CoA: Lysophospholipid acyltransferase research. Journal of Lipid Research, 50, S46–S51.
Solanky, K. S., Bailey, N. J. C., Beckwith-Hall, B. M., Davis, A., Bingham, S., Holmes, E., et al. (2003). Application of biofluid H-1 nuclear magnetic resonance-based metabonomic techniques for the analysis of the biochemical effects of dietary isoflavones on human plasma profile. Analytical Biochemistry, 323(2), 197–204.
Sutphen, R., Xu, Y., Wilbanks, G. D., Fiorica, J., Grendys, E. C., LaPolla, J. P., et al. (2004). Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiology Biomarkers & Prevention, 13(7), 1185–+.
Theodoridis, G., Gika, H. G., & Wilson, I. D. (2008). LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. Trac-Trends in Analytical Chemistry, 27(3), 251–260.
Thies, F., Delachambre, M. C., Bentejac, M., Lagarde, M., & Lecerf, J. (1992). Unsaturated fatty-acids esterified in 2-Acyl-1-Lysophosphatidylcholine bound to albumin are more efficiently taken up by the young-rat brain than the unesterified form. Journal of Neurochemistry, 59(3), 1110–1116.
Wang, C., Kong, H. W., Guan, Y. F., Yang, J., Gu, J. R., Yang, S. L., et al. (2005). Plasma phospholipid metabolic profiling and biomarkers of type 2 diabetes mellitus based on high-performance liquid chromatography/electrospray mass spectrometry and multivariate statistical analysis. Analytical Chemistry, 77(13), 4108–4116.
Want, E. J., O’Maille, G., Smith, C. A., Brandon, T. R., Uritboonthai, W., Qin, C., et al. (2006). Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Analytical Chemistry, 78(3), 743–752.
Yang, J., Xu, G. W., Zheng, Y. F., Kong, H. W., Pang, T., Lv, S., et al. (2004). Diagnosis of liver cancer using HPLC-based metabonomics avoiding false-positive result from hepatitis and hepatocirrhosis diseases. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences, 813(1–2), 59–65.
Yang, J., Zhao, X. J., Liu, X. L., Wang, C., Gao, P., Wang, J. S., et al. (2006). High performance liquid chromatography-mass spectrometry for metabonomics: Potential biomarkers for acute deterioration of liver function in chronic hepatitis B. Journal of Proteome Research, 5(3), 554–561.
Yin, P. Y., Zhao, X. J., Li, Q. R., Wang, J. S., Li, J. S., & Xu, G. W. (2006). Metabonomics study of intestinal fistulas based on ultraperformance liquid chromatography coupled with Q-TOF mass spectrometry (UPLC/Q-TOF MS). Journal of Proteome Research, 5(9), 2135–2143.
Zha, W., A, J. Y., Wang, G., Yan, B., Gu, S., Zhu, X., et al. (2009). Metabonomic characterization of early atherosclerosis in hamsters with induced cholesterol. Biomarkers, 14(6), 372–380.
Zhang, F. X., Jia, Z. H., Gao, P., Kong, H. W., Li, X., Chen, J., et al. (2009a). Metabonomics study of atherosclerosis rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Talanta, 79(3), 836–844.
Zhang, J., Yan, L., Chen, W., Lin, L., Song, X., Yan, X., et al. (2009b). Metabonomics research of diabetic nephropathy and type 2 diabetes mellitus based on UPLC-oaTOF-MS system. Analytica Chimica Acta, 650(1), 16–22.
Zhang, J., Yan, L. J., Lin, L., Chen, W. G., Song, X. Y., Yan, X. M., et al. (2008). Metabonomics research of diabetes mellitus and diabetic nephropathy based on UPLC-oaTOF-MS system. Chemical Journal of Chinese Universities-Chinese, 29(11), 2171–2173.
Acknowledgements
We acknowledge the grant of Project of National Science Foundation of China (20805046 and 30770646) and Project of International Cooperation Plan from Ministry of Science and Technology of China (2007DFC30550).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dong, J., Cai, X., Zhao, L. et al. Lysophosphatidylcholine profiling of plasma: discrimination of isomers and discovery of lung cancer biomarkers. Metabolomics 6, 478–488 (2010). https://doi.org/10.1007/s11306-010-0215-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-010-0215-x