Abstract
In this study data generated by 1H NMR spectroscopy were combined with chemometrics to analyse beef samples aged over a 21 day period. In particular, the amino acids, of which 12 were identified were found to increase over the ageing period with samples matured for 3 days having notably lower concentrations than carcasses aged for 21 days. This is believed to be a result of increased proteolysis within the muscle. This novel approach of using high resolution NMR spectrometry to analyse beef samples has not previously been reported and these findings demonstrate the potential of this technique linked with HPLC to be used as a suitable method for profiling meat samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Beef is stored at refrigeration temperatures, usually in vacuum packs, for periods of between 7 and 28 days before retail packing and distribution. During this period, refered to as the ‘ageing’ period, consumer ratings of tenderness, flavour and overall acceptability increase (Monson et al. 2005). Miller et al. (1997) and Campo et al. (1999) also concluded that longer post-mortem ageing or conditioning times resulted in better tasting and more tender beef. The increase in tenderness is mainly due to the proteolysis of myofibrillar proteins (Koohmaraie and Geesink 2006) whilst changes in flavour are probably related to breakdown reactions yielding a range of flavour precursors which can then react together to give odour and flavour when meat is cooked. For example, enzymatic changes in post-mortem muscle include the breakdown of ribonucleotides to yield free ribose, hypoxanthine and phosphate; increases in free amino acids due to proteolysis and the depletion of glycogen to yield an assortment of sugar related metabolites (Koutsidis et al. 2008b). Increases in amino acids, nucleotides and sugars during the post mortem aging period (Koutsidis et al. 2008a) are important in relation to flavour development when the meat is cooked.
A single extraction procedure can be used for all the non-polar low molecular weight metabolites in beef muscle i.e. amino acids, nucleotides, aromatics and sugars; however different analytical procedures are required for the measurement of each type of metabolite. For instance Koutsidis et al. (2008a) used GC–MS to measure sugars, capillary electrophoresis to characterise nucleotide content and GC–MS coupled with the EZ-Faast amino acid derivatization technique to analyse the amino acids in beef tissue. 1H nuclear magnetic resonance (1H NMR) has been shown to be an ideal technique to systematically characterise and quantify low molecular weight metabolites in plants (Defernez and Colquhoun 2003; Graham et al. 2009); and has the potential to characterise a wide range of metabolites from other tissues thus removing the requirement for different analytical techniques. This not only reduces the cost but the time required for a comprehensive analysis of samples. High resolution 1H NMR has been used to investigate the relationship between stress, metabolite profiles and water-holding capacity in pigs (Bertram et al. 2010) but has not been applied to beef muscle. Previously the most widely explored area of NMR in meat science is proton-relaxometry using low-field NMR to determine water activity and content within muscle tissue (Damez and Clerjon 2008). However Lundberg and Vogel (1986) have reported on early post-mortem changes in beef using 1H and 13C NMR but did not quantify them. The aim of this study was to evaluate the ability of 1H NMR to characterise the changes in amino acids, nucleotides and sugars during the post mortem aging of beef.
2 Materials and methods
2.1 Cattle sampling
The cattle for the present study were obtained from an experimental program at the Agri-Food and Biosciences Institute (Hillsborough, Northern Ireland). On the day of slaughter the cattle (Liveweight = 541 kg) were transported a distance of 50 miles to an abattoir, held in lairage for 1 h and then stunned using a captive bolt procedure. Prior to chilling (45 min post slaughter) one side of each carcass was hung by the Achilles tendon (AT) the other side was hung using pelvic suspension (suspended using from the obturator foramen). Pelvic suspension (TS) physically stretches muscle on the outside of the hindquarter resulting in increased tenderness of these muscles (Ferguson et al. 2001). The carcasses were subjected to the chilling regime standard in the abattoir. This involved a two stage chilling, with an air temperature of 10°C for the first 10 h followed by a drop to an air temperature of 2°C for the remaining period up to 48 h post slaughter. Two days post slaughter the forerib joint from each side was removed and transported to the laboratory where the longissimus dorsi muscle was removed. Six of the animals were selected at random and from five of these animals a forerib joint from each side of the animal was obtained (one side AT the other TS, however form the sixth animal it was only possible to collect the forerib form one side. From each forerib joint four 2 cm thick slices were taken, placed in bags and vacuum packed. The samples were stored at 2°C for various periods and then blast frozen at 3, 7, 14 and 21 days post mortem and stored at −80°C for further analysis. This resulted in a total of 44 samples for analysis by NMR.
2.2 Perchloric acid extraction of polar metabolites
The beef samples (6 g) were homogenised in 12 ml of 0.6 M perchloric acid and 3 ml ddH2O using an Ultra-Turrax homogeniser (IKA, Werke, Germany) for approximately 1 min until a smooth homogenate was formed without heat generation. The homogeniser probe was cleaned after each sample. The samples were centrifuged at 3,500×g at 5°C for 20 min and the supernatant collected. The supernatants were neutralised to pH 7.0 using potassium hydroxide (5.0 and 0.5 M) and placed on ice for 30 min (lowers the solubility of chlorates) after which time the samples were centrifuged (3,500×g at 5°C) for 20 min and the supernatant collected. The pH of the supernatants was re-assessed to ensure that they remained at pH 7.0.
2.3 Preparation of samples for NMR analysis
Supernatants were frozen at −80°C and lyophilised using a Modulyod freeze dryer (Thermo Electron Company, USA) for 24 h. The samples were reconstituted in 650 μl of 0.1 M phosphate buffer (pH 7.0), in D2O, containing 1 mM of the internal standard sodium trimethylsilyl-2,2,3,3,-tetradeuteroproprionate (TSP, Sigma-Aldrich, UK). Insoluble material was removed by centrifugation (16,000×g for 15 min), and 600 μl of the remaining supernatant was transferred to a 5 mm diameter NMR tube.
2.4 NMR analysis
The spectra were recorded in D2O on a Bruker DPX 300 MHz spectrometer. The sample temperature was 303 K and the samples were spun at 20 Hz. Using a randomised running order one dimensional spectra were acquired across a spectral width of 7.5 kHz into 64 K data points: Relaxation Delay = 1 s, t1 = 6 ms. Thirty-two transients were acquired. Spectral processing was carried out using ACDlabs NMR Processor v9.0 (ACD labs, Toronto, Canada). The summed transients were multiplied by a 0.8 Hz apodisation factor prior to Fourier Transformation; chemical shifts (δ) are reported in parts per million (ppm) of the operating frequency and were referenced to the TSP resonance (δ = 0.00). Baseline correction was performed manually. Data reduction was carried out by manually binning the spectra and measuring the integral for each bin between 0.80 and 8.70 ppm (57 including TSP). The region from 4.75 to 5.00 ppm, which contained the signal for the water resonance, was excluded. 2D (1H/13C) heteronuclear multiple quantum coherence (HMQC) NMR experiments were recorded on a Bruker DRX 500 MHz spectrometer; one beef extract sample was used.
2.5 Metabolite identification
Metabolites were assigned based on chemical shift and identified from a library of in-house pure compounds, NMR databases (Madison Metabolomics Consortium Data base) and by using Chenomx NMR suite 5.1 (Chenomx Inc., Edmonton, Canada). After initial identification metabolites were confirmed through spiking with pure compounds (17 pure compounds used) and as a third line of evidence were identified using 2 dimensional proton-carbon HMQC.
2.6 Principal component analysis
Principal component analysis (PCA) was preformed using the statistical software package GenStat 11.1 (VSN International Ltd., Oxford) and the data were normalised to the total spectral intensity. Principal component analysis was carried out using the correlation method (Genstat GenStat 11.1) which involved mean centring of the variables followed by division by the standard deviation (autoscaling) on all the metabolites including the unidentified resonances to reduce the differences due to the magnitude of the lactate (approximately double the magnitude of any other metabolite) signal.
2.7 Metabolite quantification
Quantification of the metabolites was undertaken by using the following equation provided that the peaks were well defined with little or no overlap observed.
3 Results and discussion
3.1 NMR analysis
Figure 1 shows a 1H NMR spectrum of a beef sample extract (residual water resonance removed, δ4.75–5.00) used to identify 27 metabolites with prominent resonances at δ1.34 (d) and δ3.05 (s) corresponding to lactate and creatine, respectively, with lactate representing the single most dominant metabolite. This is not surprising in that following death, muscle attempts to retain its biochemical integrity and maintain the level of ATP (Pearson and Young 1989). This is partially achieved by post-mortem glycolysis producing large concentrations of lactic acid through anaerobic respiration (Lawrie 1992; Moss 1992) such that in the longiisimus dorsi muscle of normal ultimate pH (5.4–5.6) lactate levels are around 100 mmole kg−1 (Bendall 1979). High levels of creatine are also expected as published work has shown that red meat contains high concentrations of around 40 mmole kg−1 wet weight of muscle (Lawrie 1998; Polak et al. 2007) compared to total free amino acid content of around 15 mmole kg−1 wet weight of muscle (Polak et al. 2007). The amino acid region (δ0.70–3.50) consisted mainly of acetate, alanine, aspartate, carnosine, glutamine/glutamate, β-hydroxybutyrate, 2-hydroxybutyrate, lactate, isoleucine/leucine/valine, pyruvate and threonine. The crowded carbohydrate region (δ3.50–5.67) of the spectrum consisted of mainly overlapping peaks of glucose and ribose. However, from the HPLC analysis (data not presented) fructose was believed to be present but at such low levels that its peaks were masked by the larger peaks of glucose and ribose. The aromatic region (δ5.67–8.73) of the spectra consisted of peaks corresponding to adenine, adenosine, ATP, ADP, carnosine, hypoxanthine, inosine, phenylalanine and xanthine. A few peaks were unidentifiable in all three regions of the spectra.
300 MHz 1H NMR spectrum of an extract taken from a beef muscle (longissimus dorsi) matured for 14 days. All peaks are referenced to the resonance of TSP at 0.00 ppm. Major peak assignments: 1, Isoleucine/leucine/valine; 2, lactate; 3, alanine; 4, glutamine/glutamate; 5, aspartate; 6, creatine; 7, crowded carbohydrate region (adenosine, glucose, mannose and ribose); 8, anomeric proton of ATP/ADP/hypoxanthine/inosine; 9, carnosine; 10, adenine/hypoxanthine; 11, adenosine/inosine/carnosine; 12, ATP. The residual water peak has been removed
A list of all the metabolites and their chemical shifts identified in the NMR spectra from the beef extract samples are displayed in Table 1. A total of 27 metabolites were identified using 1H NMR. As outlined in the materials and methods section, assignments were achieved by comparison of spin systems with those found in some NMR databases (Madison Metabolomics Consortium Data base, Chenomx NMR Library Manager and an in house library). In specific cases where peaks lay close together or some overlap was observed between resonances, standard solutions of pure compounds were prepared at pH 7.0 and their spectra recorded in order to aid identification (i.e. ATP, ADP, adenosine and adenine). Due to the high rate of overlap between the metabolite resonances (particularly in the aromatic region of the spectra) beef extract samples had to be spiked with pure compounds to verify the presence of that particular metabolite (adenosine, ATP, ADP, hypoxanthine and inosine). In addition to this, a 2D (1H/13C) HMQC was carried out as another method used for positive identification of metabolites (Fig. 2). Table 2 displays a list of 20 unidentified integrals which demonstrates the potential for NMR to identify many more compounds and on the other hand, the need for complementary methods such as hyphenated NMR to improve spectral assignment (Graa et al. 2007).
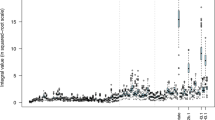
Apart from the unassigned peaks one resonance in particular was problematic due to the large variation in chemical shift between the samples. Figure 3 shows the bin (bin 54) corresponding to the downfield proton of carnosine, presented is the superposition of all the spectra containing this resonance (44 spectra). The bin width for this peak had to be increased due to large chemical shift changes of this resonance with minor pH and ionic differences between the samples.
3.2 PCA analysis
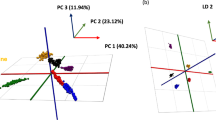
Principal component analysis (PCA) has been widely used throughout metabolomic studies comparing NMR spectral data obtained from various sources (biofluids and tissues) to determine whether spectral differences exist between groups (Weljie et al. 2006). Figure 4 shows the PCA scores plot for the beef extracts. Principal component one (PC1) explains 45% of the variation, PC2 explains 19% variation and PC3 explains 7.5% variation between the beef sample spectra. The arrow in Fig. 4 displays the progression between the aged samples across the diagonal of the axes; beginning with day 3, day 7, day 14 and progressing to day 21 across the components.
Figure 5a and b represent the loadings plots (PC1 vs. PC2 and PC1 vs. PC3 respectively) for the beef extract spectra. Principal component 1 and PC2 shows that carcasses matured for 3 and 7 day periods have lower levels of all the amino acids identified with exception of methionine. Days 3 and 7 also have lower levels of acetate, adenosine, carnosine, creatine, glucose, inosine, lactate, pyruvate, ribose, xanthine and higher levels of ATP and ADP than the beef samples matured for 14 and 21 day periods. Following slaughter ATP is depleted as the muscles attempt to retain functionality under anaerobic conditions as the carcasses go into rigor (Ferguson et al. 2001). The levels ot ATP reported in the current study at 3 days (2.31 ± 0.03 μmole g−1 FWt beef, Table 3) are higher than reported by Kima et al. (2000) and Kim et al. (2007) at 24 h post mortem. According to Kim et al. (2007) muscle ATP is depleted as the carcase goes into rigor from around 4.5 mmole g−1 prerigor (3 h post slaughter) to 0.8 μmole g−1 post rigor. Kondos and Taylor (1987), however, noted that ATP levels at 24 h post mortem in bovine M. sternocepahilicus were higher in muscles which went into rigor at lower temperatures. Levels of ATP during the post rigor aging period have not been reported for beef. Although in the current study the position of ATP (d, J = 6 Hz) was confirmed by spiking the samples with ATP, further work may be required on the ATP peak using NMR including comparison of NMR values with conventional methods for determination of ATP levels (e.g. HPLC or enzymatic methods).
Loadings plot a representative of PC1 and PC2; loadings plot b representative of the PC1 and PC3 for the scores plot in Fig. 4
The ATP had more of a bearing in PC1 than in PC2. It showed that muscle aged for 3 and 7 days contained more ATP than that of muscle matured for 14 and 21 days. In conjunction with ATP the levels of ATP/ADP/hypoxanthine/inosine also decreased over time. A single (Fig. 1) bin contained the anomeric proton for ATP/ADP/hypoxanthine/inosine and it was difficult to determine which metabolite was present due to the overlap. These metabolites are associated with shorter ageing periods, however, since both inosine and hypoxanthine increase as meat is aged (Lawrie 1998), it would appear appropriate to assume that this bin corresponded mainly to ATP and ADP; as these metabolites have been reported to be higher at earlier times post mortem (Lawrie 1998). Table 3 illustrates the increasing levels of adenine/hypoxanthine, adenosine/inosine and xanthine recorded over time. This was as expected as these metabolites are the downstream compounds which result from the breakdown of ATP in muscle (Lawrie 1998).
The loadings plot in Fig. 5a mirrors that of Fig. 5b. The levels of the amino acids, sugars and majority of the nucleosides (except ATP and ADP) are greater in the 14 and 21 days aged beef than in the 3 and 7 day beef samples. The pattern of changes in amino acid over the 21 day ageing period are similar to those of Feidt et al. (1996) who noted statistically significant increases in free amino acid content with time of aging from 3 to 14 days. The increased levels of the amino acids over the ageing period is as expected due to the increased endogenous proteolytic enzyme activity and myofibrillar protein degradation. The myofibrilliar proteins are broken down into polypeptide fragments by muscle proteinases (calpains, cathespins and multicatalytic proteinases), generating polypeptides, followed by the action of peptidyl peptidases (dipeptidyl peptidases, tripeptidyl peptidases, carboxipeptidases) which generate small peptides. The last step of this proteolysis chain observed in post-mortem muscle is the generation of free amino acids by muscle amino peptidases, di and tripeptidases (Mullen et al. 2000; Moya et al. 2001). Using NMR 12 amino acids were clearly identified (as outlined in Table 2) in the muscle compared to 15 and 17 identified by Feidt et al. (1996) and Mullen et al. (2000) using classical methods. Possible reasons for the lower number of amino acids identified by NMR is discussed later.
This study reflects data presented by Feidt et al. (1996) and Koutsidis et al. (2008a, b) who also found that as meat aged the levels of free amino acids increased. Similarly in the present study, levels of alanine, aspartate, glutamine, isoleucine, leucine, phenylalanine and threonine all increased in comparable levels to the published data. Phenylalanine, methionine, valine and isoleucine which had high loadings on PC1 (associated with longer aging periods) were also found by Koutsidis et al. (2008a, b) to show large increases from day 3 to day 21. In the current investigation the levels of leucine were at a much higher level than that recorded by Feidt et al. (1996) in comparison to the other amino acids. In addition the level of valine increased in our experimental analysis whereas Feidt et al. (1996) found it to decrease over time. From an analysis of the published data an assumption could be made that the levels of the amino acids that were not identified were so low that the surrounding peaks of the more abundant amino acids overlapped them keeping them from being identified. Another interesting discovery made in the present research was the identification of phenylalanine, not documented by Feidt et al. (1996) who analysed amino acid composition using gas chromatography but it was recorded by Mullen et al. (2000) using an amino acid analyser and Koutsidis et al. (2008a, b) who used GC–MS.
3.3 Quantification of metabolites
Table 3 displays the mean concentrations of the majority of the metabolites identified in the 1H NMR spectra across the four ageing periods. Listed within the table are the bins/integrals and the types of peaks used to calculate (singlet, doublet etc…) the concentration of each metabolite. Not all the concentrations of the identified metabolites were calculated using 1H NMR due to the large amount of overlap between the compounds. An example of which was the peak at 6.10 ppm (d, J = 6 Hz) which corresponded to the anomeric proton (–CH) of ADP, hypoxanthine and inosine. In addition, ribose was not quantified due to the large overlap of the HOD peak masking its anomeric proton. This adverse effect could be reduced using water suppression techniques, higher resolution NMR spectrometers (i.e. 500 or 600), using a different solvent (DMSO) or using a cryo-probe to increase the signal to noise ratio observed in the spectra. The bins used for the calculation of the concentrations had little or no overlap with other metabolites giving a representative value for that metabolite.
4 Concluding remarks
This experiment has shown that with minimal sample preparation, high resolution 1H NMR can be used to successfully identify and quantify metabolite changes that occur in the conversion of muscle to meat where previously a range of different quantification methods would have been required. Ageing of the samples did significantly affect metabolite concentrations, in particular, the concentration of the amino acids due to the increase in proteolysis whereby muscle proteins have been broken down by various peptidases causing the release of free amino acids.
Overall the novel method demonstrated herein to analyse beef samples across different ageing periods has produced significant results. This paper shows the potential of NMR to study post mortem muscle metabolism and the application of high resolution NMR metabolomics to identify relationships between muscle biochemistry and meat quality.
References
Bendall, J. R. (1979). Relations between muscle pH and important biochemical parameters during the post mortem changes in mammalian muscle. Meat Science, 3, 143–157.
Bertram, H. C., Oksbjerg, N., & Young, J. F. (2010). NMR-based metabonomics reveals relationship between pre-slaughter exercise stress, the plasma metabolite profile at time of slaughter, and water-holding capacity in pigs. Meat Science, 84, 108–113.
Campo, M. M., San˜udo, C., Panea, B., Alberti, P., & Santolaria, P. (1999). Breed type and ageing time effects on sensory characteristics of beef strip loin steaks. Meat Science, 51, 383–390.
Damez, J. -L., & Clerjon, S. (2008). Meat quality assessment using biophysical methods related to meat structure. Meat Science, 80, 132–149.
Defernez, M., & Colquhoun, I. J. (2003). Factors affecting the robustness of metabolite fingerprinting using 1H NMR spectra. Phytochemistry, 62(6), 1009–1017.
Feidt, C., Petit, A., Braus-Reignier, F., & Brun-Bellut, J. (1996). Release of free amino acids during ageing in bovine meat. Meat Science, 44, 19–25.
Ferguson, D. M., Bruce, H. L., Thompson, J. M., Egan, A. E., Perry, D., & Shortose, W. R. (2001). Factors affecting beef palatability-farmgate to chilled carcass Aus. Journal of Experimental Agriculture, 41, 879–891.
Graa, G., Duarte, I. F., Goodfellow, B. J., Barros, A. S., et al. (2007). Potential of NMR spectroscopy for the study of human Amniotic fluid. Analytical Chemistry, 79(21), 8367–8375.
Graham, S. F., Amigues, E., Migaud, M., & Browne, R. (2009). The application of NMR based metabolomics for mapping metabolite variation in European wheat. Metabolomics, 5(3), 302–306.
Kim, Y. S., Lee, C. N., DuPonte, M. W., & Fukomoto, G. K. (2007). Improving tenderness of forage-finished beef using a low-voltage stimulator. Food Safety and Technology, 22, 1–6.
Kima, K. H., Kim, Y. S., Leea, B. Y. K., & Baika, B. A. (2000). Postmortem muscle glycolysis and meat quality characteristics of intact male Korean native (Hanwoo) cattle. Meat Science, 55(1), 47–52.
Kondos, A. C., & Taylor, D. G. (1987). Effect of electrical stimulation and temperature changes in beef muscle. Meat Science, 19, 207–216.
Koohmaraie, M., & Geesink, G. H. (2006). Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the caplain system. Meat Science, 74, 34–43.
Koutsidis, G., Elsmore, J. S., Oruna-Concha, M. J., Campo, M. M., Wood, J. D., & Mottram, D. S. (2008a). Water soluble precursors of beef flavor: I. Effect of diet and breed. Meat Science, 79, 124–130.
Koutsidis, G., Elsmore, J. S., Oruna-Concha, M. J., Campo, M. M., Wood, J. D., & Mottram, D. S. (2008b). Water soluble precursors of beef flavor: Part II: Effect of post-mortem conditioning. Meat Science, 79, 270–277.
Lawrie, R. A. (1992). Conversion of muscle to meat. In D. E. Johnston, M. K. Knight, & D. A. Ledward (Eds.), The chemistry of muscle based foods. Cambridge: Royal Society of Chemistry.
Lawrie, R. A. (1998). Lawrie’s meat science (6th ed.). Cambridge, UK: Woodhead Publishing Company.
Lundberg, P., & Vogel, H. J. (1986). Carbon-13 and proton NMR studies of post mortem metabolism in bovine muscles. Meat Science, 18, 133–160.
Miller, M. F., Kerth, C. R., Wise, J. W., Lansdell, J. L., Stowell, J. E., & Ramsey, C. B. (1997). Slaughter plant location, USDA quality grade, external fat thickness and aging time effects on sensory characteristics of beef loin strip steak. Journal of Animal Science, 75, 662–667.
Monson, F., Sanudo, C., & Sierra, I. (2005). Influence of breed and ageing time on the sensory meat quality and consumer acceptability in intensively reared beef. Meat Science, 71, 471–479.
Moss, B. W. (1992). Lean meat, animal welfare and meat quality. In D. E. Johnsto, M. K. Knight, & D. A. Ledward (Eds.), The chemistry of muscle based foods. Cambridge: Royal Society of Chemistry.
Moya, V.-J., Flores, M., Aristroy, M.-C., & Toldra, F. (2001). Pork meat quality affects peptide and amino acid profiles during the ageing process. Meat Science, 58(2), 197–206.
Mullen, A. M., Stoeve, S., Laib, K., Gruebler, G., Voelter, W., & Troy, D. J. (2000). Preliminary analysis of amino acids at various locations along the M. longissimus dorsi in aged beef. Food Chemistry, 69, 461–465.
Pearson, A. M., & Young, R. B. (1989). Muscle and meat biochemistry (1st ed.). London, UK: Academic Press, Inc.
Polak, T., Gasperlin, L., & Zlender, B. (2007). Various instrumental and biochemical parameters as ageing indicators of Longissimus dorsi muscle and their relation to creatine and creatine content. European Food Research and Technology, 225(5–6), 849–855.
Weljie, A. M., Newton, J., Mercier, P., Carlson, E., & Slupsky, C. M. (2006). Targeted profiling: Quantitative analysis of 1H NMR metabolomics data. Analytical Chemistry, 78(13), 4430–4442.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graham, S.F., Kennedy, T., Chevallier, O. et al. The application of NMR to study changes in polar metabolite concentrations in beef longissimus dorsi stored for different periods post mortem. Metabolomics 6, 395–404 (2010). https://doi.org/10.1007/s11306-010-0206-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-010-0206-y