Abstract
The intracellular metabolic profile characterization of Saccharomyces cerevisiae throughout industrial ethanol fermentation was investigated using gas chromatography coupled to time-of-flight mass spectrometry. A total of 143 and 128 intracellular metabolites in S. cerevisiae were detected and quantified in continuous and batch fermentations, respectively. The two fermentation processes were both clearly distinguished into three main phases by principal components analysis. Furthermore, the levels of some metabolites involved in central carbon metabolism varied significantly throughout both processes. Glycerol and phosphoric acid were principally responsible for discriminating seed, main and final phases of continuous fermentation, while lactic acid and glycerol contributed mostly to telling different phases of batch fermentation. In addition, the levels of some amino acids such as glycine varied significantly during both processes. These findings provide new insights into the metabolomic characteristics during industrial ethanol fermentation processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Much attention has been focused on laboratory yeast during ethanol fermentation, which provided much information about the molecular responses of laboratory yeast cells to fermentation environments (Brejning et al. 2005; James et al. 2003; Kobi et al. 2004; Trabalzini et al. 2003). The fermentation environment around the cells in actual industrial process is significantly different from that in laboratory. The fermentation conditions (such as temperature, osmotic pressure, pH and aeration) are also different during both continuous and batch industrial processes. Most biochemical events, including cell stress sensing, accumulation of stress-protectants, signal transduction, transcription and translation, were involved in the responses of yeast cells to complex environmental changes (Devantier et al. 2005; Pham et al. 2006). So far, the laboratory yeast have been studied under various stresses, while for the industrial yeast strains, studies are on a very limited scale (Cavalieri et al. 2000; Backhus et al. 2001; Hansen et al. 2006; Cheng et al. 2008). Marks and colleagues also suggested that studies restricted to standard laboratory conditions were inadequate to understand the regulation of yeast metabolism in industrial fermentation (Marks et al. 2008). Usually, yeast cells undergo three phases including seed, main and final fermentation whether in continuous or batch ethanol industrial fermentation process. However, we have limited knowledge about yeast cells in response to industrial fermentation conditions. To efficiently manipulate the industrial fermentation process of yeast strains, it is necessary to give insights into the responses of the robust yeast throughout the industrial process at the system level, especially at the metabolome level.
Metabolites were the final downstream products of gene expression and cellular regulatory processes, and the changes in metabolic levels could be regarded as the ultimate response of biological systems to environmental variations (Fiehn 2002). In addition, changes in the metabolome are amplified relative to those in proteome and transcriptome (Kell and Westerhoff 1986). Meanwhile, analysis of cells at the metabolic level has a number of advantages over the more conventional transcriptome and proteome analysis (Fiehn 2002). Metabolomics mainly focuses on the comprehensive and quantitative study of metabolites in a biological system (Fiehn et al. 2000; Roessner et al. 2001). A number of high-throughput analytical methods have so far been applied for metabolite profiling (Lenz and Wilson 2007), such as gas chromatography–mass spectrometry, liquid chromatography–mass spectrometry, nuclear magnetic resonance and capillary electrophoresis–mass spectrometry. So, metabolic profile analysis should be a good choice to investigate the response of industrial yeast strains to the fermentation environment.
Considering the advantages of relatively high reproducibility, high resolution, high-throughput analysis and good sensitivity (Villas-Bôas et al. 2003; Wagner et al. 2003), gas chromatography coupled to time-of-flight mass spectrometry (GC-TOF-MS) was applied to detect the changes of intracellular metabolites throughout both continuous and batch processes. After that, principal components analysis (PCA) of intracellular metabolites in the industrial yeast was performed to distinguish three phases during continuous or batch fermentation process. Significant changes in metabolites related to amino acid biosynthetic pathway and central carbon metabolism were explored during both processes.
2 Materials and methods
2.1 Stains and fermentation conditions
The industrial strain S. cerevisiae, Angel thermal-tolerant alcohol active dry yeast (Angel Yeast Co., Ltd., China), was used throughout this work. In continuous fermentation, 11 fermenters with the same volume of 320 m3 were used successively for ethanol production. The feedstock was composed of 20% grain and 80% maize. The whole fermentation took about 60 h in all. There was no oxygen supply. The temperature was kept between 30 and 34°C. The seed incubation needed 10 to 12 h, while main fermentation phase lasted 30 to 40 h.
In batch fermentation, the fermenter volume was 480 m3. The feedstock was composed of 50% maize, 30% rice and 20% wheat. There was no oxygen supply either. The temperature was kept between 31 and 35°C. The whole fermentation process lasted about 60 h.
2.2 Analysis of cell number, residual glucose and ethanol in medium
Cell number was measured instantaneously after sampling with a blood cell counting chamber. Reducing sugar analysis was performed by HPLC (Waters Corp., USA) using an Aminex HPX-87H column (Bio-Rad) coupled to a refractive index (RI) detector. Ethanol was measured by direct sample injection into a SP 3420A Gas Chromatography instrument with a flame ionization detector using a DB-5MS column.
2.3 Sampling, quenching and extraction of intracellular metabolites
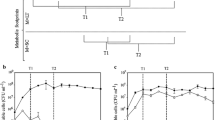
The S. cerevisiae cells during continuous fermentation were harvested from fermenters 1, 2, 3, 4, 5, 6 and 9 (Supplementary Fig. 1a). The S. cerevisiae cells during batch fermentation were harvested at seven time points (Supplementary Fig. 1b).
Cells were quenched and extracted according to Koning and Van Dam (1992) with slight modifications. Firstly, the samples were sprayed into cold (−40°C) 60% methanol (v/v) to arrest metabolism instantaneously. After centrifugation at 2,000g for 3 min, cells were ground to a fine powder in liquid nitrogen using a mortar and pestle. Fresh cells (100 mg) were firstly extracted with extraction buffer of methanol/chloroform/water (2:3.3:1.3, v/v/v, −20°C, 6 ml). To correct for minor variations occurring during sample preparation and analysis, the ribitol (0.2 mg ml−1 in water, 50 μl) was used as internal standard. The sample was thoroughly mixed to disassociate metabolites from cells. After centrifugation at 5,000g at 4°C for 10 min, the cell pellet was re-extracted with 2 ml methanol/water (1:1, v/v, −20°C). The extract was combined with the former one. The resulting water/methanol phase containing all hydrophilic metabolites was lyophilized under low temperature (−60°C) in lyophilizer. Five analytical replicates were performed for each sample.
2.4 Sample derivatization
For GC-TOF-MS analysis, two-stage chemical derivatization was performed on the extracted metabolites (Roessner et al. 2000). Firstly, oximation was carried out by dissolving the samples in methoxamine hydrochloride (20 mg ml−1 in pyridine, 50 μl) and incubating at 30°C for 90 min. Then, samples were derivatized further with the addition of N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA, 80 μl) at 37°C for 30 min to trimethylsilylate the polar functional groups. The derivate samples were equilibrated to room temperature before injection.
2.5 Detection of metabolites by GC-TOF-MS
The GC-TOF-MS system consisted of an Agilent 7683 autosampler, an Agilent 6890 gas chromatography (GC, Agilent Technologies, Palo Alto, CA, USA), and a TOF-MS (Waters Corp., USA). Sample (1 μl) was injected with a split ratio of 1:1 by the autosampler into GC which equipped with a fused-silica capillary column (30 m × 0.25 mm i.d., 0.25 μm DB-5MS stationary phase, J&W Scientific, Folsom, CA, USA). The injector temperature was 280°C (Mohler et al. 2008). It was operated on constant pressure mode at 91 kPa. Helium was used as carrier gas. The column temperature was held at 70°C for 2 min, and then increased to 290°C with the rate of 8°C min−1, holding for 3 min. The transfer line and ion source temperatures were 280 and 250°C, respectively. Ions were generated by a 70 eV electron beam at an ionization current of 40 μA. Two spectra were recorded per second in the mass range of 50-800 m/z with dynamic range extension function.
2.6 Data analysis
Masslynx software (Version 4.1, Waters Corp., USA) was applied for mass spectral peak identification and quantification. Automatic peak detection and deconvolution were performed using a peak width of 2.0 s. The signal-to-noise (S/N) values were based on the masses chosen by the software for quantification, and peaks with S/N values lower than 10 were rejected. Automatic assignments of unique fragment ions for each metabolite were taken as default, and manually corrected when necessary. Compound identification was performed by comparing the mass spectra with a commercially available standard library, National Institute of Standards and Technology mass spectral library (2005). After normalizing and mean centering, the large amounts of data derived from the metabolite profiling were analyzed by PCA, an unsupervised clustering method, which reduced the dimensionality of the raw data while preserved most of the variances in a 2- or 3-dimensional map, using the Markerlynx software (Version 4.1, Waters Corp., USA, Gika et al. 2008; Major et al. 2006). For relative quantification, all data were normalized to internal standard. Five replicates instead of mean value were used to perform PCA for each sample harvested from continuous or batch fermentation process.
3 Results and discussion
3.1 Metabolomic profiling by GC-TOF-MS
As illustrated in Fig. 1, the final ethanol content was approximate 11.1% (v/v) during continuous fermentation, while the final ethanol content was about 9.6% (v/v) during batch fermentation. Although there were difference in cell numbers, variation of reducing sugar in the continuous and batch fermentation was similar. There were three phases including seed growth, main and final fermentation phase according to sugar consumption and ethanol production during the industrial fermentation processes (Cheng et al. 2008). This finding indicated that there were the differences in cellular behaviors and living environments of the industrial S. cerevisiae in the two realistic industrial processes. Comparative metabolomic analysis can be useful for insights into the possible reasons for or consequences of these changes.
During continuous and batch fermentations of S. cerevisiae, a total of 143 and 128 unique intracellular metabolites were identified for continuous and batch fermentations, respectively. The main classes of these compounds included sugars, amino acids, organic acids and alcohols. Although similar kinds of metabolites were detected in the two fermentation modes, their expression levels differed significantly (Supplementary Fig. 2). As shown in Table 1, approximate 30 metabolites were related to amino acid biosynthetic pathway as well as central carbon metabolism in both fermentation processes. They played key roles in biological system including the substrate degradation, energy and cofactor regeneration, as well as biosynthetic precursor supply.
3.2 Multivariate data analysis
Multivariate data analysis was performed by PCA, which had been successfully applied to many kinds of data sets (e.g., Fiehn et al. 2000; Yang and Bernards 2007; Devantier et al. 2005; Ossipov et al. 2008). In the PCA scores plot, each data point represented a linear combination of all the metabolites from each individual sample. In the PCA loadings plot, each data point represented a mass fragment of a certain metabolite. The further a data point was from the origin, the greater the component it belonged to influenced the cluster formation.
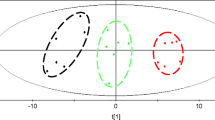
As shown in Fig. 2, the metabolite profiles of S. cerevisiae from continuous and batch fermentations were first analyzed together by PCA. The scores plot indicated that samples from continuous fermentation and batch fermentation could be clearly separated into group A and group B, respectively (Fig. 2a), indicating that the industrial S. cerevisiae displayed distinctly metabolic characteristics under different fermentation environments. It was also found out that glycerol and phosphoric acid contributed more significantly to distinguishing continuous and batch fermentations after loadings plot analysis (Fig. 2b).
Further analyses of the scores plot illustrated that three groups were observed in accordance with different fermentation phases of continuous fermentation (Fig. 3a). A similar result was also found out in batch fermentation (Fig. 4a). For continuous fermentation process, samples from fermenters 1 and 9 clearly separated from each other, forming distinct groups and representing the seed phase (group A) and the final fermentation phase (group C) respectively. Samples from fermenters 2, 3, 4, 5 and 6 formed another cluster (group B), representing the main fermentation phase. Particularly, group B was separated on PC 1 into two distinct subgroups, group D and group E, which represented the former fermentation phase and latter fermentation phase, respectively. For batch fermentation process, samples from 5 to 18 h formed group A (seed phase), while those from 30, 36 and 42 h in main fermentation phase formed group B. The last two samples from the final fermentation phase formed group C. As shown in the loadings plot, some metabolites (marked with ‘a’ in Table 1) were found to make great contribution for distinguishing different fermentation phases of samples from both continuous (Fig. 3b) and batch fermentations (Fig. 4b). Metabolites associated with glycolysis pathway (phosphoric acid, lactic acid and glycerol), intermediates of TCA cycle (citrate and malate) as well as amino acids (glycine and glutamine) contributed to the cluster formation most significantly.
Principal components analysis results of GC-TOF-MS analysis of S. cerevisiae from continuous fermentation. a PCA scores plot (PC 1 vs. PC 2). Group A: seed phase; group B: main fermentation phase; group C: final fermentation phase; groups D and E are the subgroups of group B which represent former fermentation and latter fermentation, respectively. b PCA loadings plot (PC 1 vs. PC 2)
3.3 Variations of intracellular metabolites
3.3.1 Glycerol and glycerol 3-phosphate
Throughout the industrial fermentation process, yeast was subjected to a variety of environmental stresses, including osmotic pressure, temperature change, ethanol concentration increase and gradual nutritional depletion (Cheng et al. 2008). Thus, yeast needed to rapidly adjust metabolism to the variable fermentation conditions. Glycerol, considered as the most important byproduct in yeast ethanol fermentation, helped cells adapt to the changing environmental conditions (Blomberg and Adler 1989). The precursors for glycerol, dihydroxyacetone phosphate (DHAP) and glycerol 3-phosphate (G3P), are derived from the upper part of glycolysis pathway. The major role of glycerol formation is to maintain the redox balance by reoxidizing the surplus of NADH during ethanol fermentation (Van Dijken and Scheffers 1986). Additionally, glycerol was considered as a compatible solute that can adjust the intracellular osmotic pressure by activating the high osmolarity glycerol (HOG) pathway during hyperosmotic stress (Blomberg and Adler 1992; Brewster et al. 1993; Hohmann 2002).
As shown in Fig. 5a, the level of glycerol in the fermenter 1 was relatively high during continuous fermentation, while it gradually decreased from fermenter 3 to fermenter 9. It was likely that the sugar concentration formed osmotic pressure at the beginning of fermentation, which would activate the HOG pathway. Moreover, we also found out that the variation of glycerol in batch fermentation was similar to that in continuous process except for the first time point (at 5 h) (Fig. 5b). It was possible that the dried industrial S. cerevisiae cells in batch fermentation were not completely activated at 5 h, at which time the yeast cells had no response to the high concentration of sugar. Afterwards, the HOG pathway was activated and glycerol accumulated gradually. The level of glycerol reached the highest level at 18 h, and then reduced. One possible reason is that intracellular glycerol reached high level rapidly to protect cells from shrinking under higher sugar concentration, while the osmolarity stress decreased with the consumption of sugar as the fermentation progressed. The levels of intracellular glycerol gradually decreased accordingly.
Variations of intracellular metabolites in S. cerevisiae related to glycolysis pathway during continuous (a) and batch (b) fermentation. The relative abundance was calculated by normalization of peak area of each metabolite to internal standard ribitol, and each value represents the mean of five independent replicates ± SD
Glycerol 3-phosphate was the direct precursor of glycerol and it can be dephosphorylated by G3Pase to form glycerol (Gancedo et al. 1968). In this study, G3P was found to represent a similar though less significant trend to glycerol in both fermentation processes. It was likely for the sake of maintaining proper concentration of glycerol to adapt to the variable conditions throughout fermentation process.
3.3.2 Phosphoric acid
Phosphoric acid is an important intermediate metabolite and participates in the regulation of signal transduction pathway. As shown in Fig. 5a, the level of intracellular phosphoric acid decreased as the continuous fermentation progressed. It was possible that a large amount of phosphoric acid took part in phosphorylating ADP to ATP and oxidizing citrate in glycolysis pathway and TCA cycle at the same time. Another reason is that phosphoric acid activated some protein kinases to regulate the signal transduction pathways in response to the environmental variations during fermentation. It was reported that protein kinase C 1 (PKC 1) pathway, a second osmosensing signal transduction pathway, was found to respond proportionally to the decrease of osmolarity and increase of temperature during yeast fermentation (Davenport et al. 1995; Kamada et al. 1995).
Figure 5b shows that the intracellular phosphoric acid accumulated during the former 18 h during batch fermentation, which was similar to the variations of glycerol, G3P and TCA cycle intermediates. This might be related to the activation of dried industrial S. cerevisiae cells. After 18 h, phosphoric acid level had a similar variation trend to that in continuous process. Taken together, these results indicated that yeast cells needed longer time to accumulate metabolites related to glycolysis pathway in batch fermentation than that in continuous fermentation.
3.3.3 Lactic acid
Lactic acid was another important byproduct in yeast ethanol fermentation (Hohl and Joslyn 1941). While there is not enough oxygen to oxidize pyruvic acid into ethanol, the pyruvic acid is partly transformed into lactic acid to generate energy (ATP). During batch fermentation, the lactic acid was identified as one of the metabolites that significantly influenced the cluster formation of samples belonging to different fermentation phases. The lactic acid increased gradually for the lacking of oxygen (Fig. 5b). However, the lactic acid level was relatively stable during the continuous fermentation (Fig. 5a). It was possible that there was still a little oxygen dissolving in the saccharfication feedstock during continuous fermentation, although no additional oxygen was supplied. Taken together, lactic acid was identified as potential biomarker to distinguish S. cerevisiae samples derived from two industrial fermentation modes.
3.3.4 Tricarboxylic acid cycle intermediates
As shown in Fig. 6, among the intermediates involved in TCA cycle, the change of citrate level was the most significant in both continuous and batch fermentations. Moreover, similar changes in the levels of succinate, fumarate and malate were also investigated during the both processes. It was known that the formation of citrate was the rate-limiting step in TCA cycle (Krebs et al. 1960). The significant changes in citrate level might reveal its key role in TCA cycle for regulating adaptation of yeast cells to these fermentation environments.
Variations of intracellular metabolites in S. cerevisiae involved in TCA cycle during continuous (a) and batch (b) fermentation. The relative abundance was calculated by normalization of peak area of each metabolite to internal standard ribitol, and each value represents the mean of five independent replicates ± SD
As shown in Fig. 6, most of the TCA cycle intermediates gradually increased at first, then maintained a higher level for a period, and finally decreased whether during continuous or batch process. During the seed phase, the lower level of these intermediates might be caused by glucose repression on respiratory pathways under high glucose concentration. During the main fermentation phase, although there was no additional oxygen supply, the dissolved oxygen in the saccharfication feedstock seemed to be enough for yeast respiration. This might explain why the increasing levels of TCA cycle intermediates were explored during this period. The concentrations of TCA cycle intermediates were significantly higher in anaerobic condition (main fermentation phase) than in aerobic condition (seed phase) (Fig. 6). The similar results were reported by Villas-Bôas through high-throughput metabolic state analysis (Villas-Bôas et al. 2005). It was also reported that high concentrations of TCA cycle intermediates were maintained under anaerobic conditions (Nissen et al. 1997; Franzen 2003). Wiebe further confirmed the levels of TCA cycle intermediates in S. cerevisiae were higher during anaerobic condition than aerobic condition (Wiebe et al. 2008).
During continuous fermentation, the level of citrate increased from fermenter 2, and then maintained a higher level at fermenters 4, 5 and 6, afterwards decreased rapidly at final phase. It is may be related to transition of respiration into fermentation of yeast cells after fermenter 2 (Fig. 6a). As illustrated in Fig. 6b, similar findings were investigated during batch fermentation. The levels of citrate and malate reached a peak value at 30 and 36 h, respectively during batch fermentation. Wiebe and coworkers also reported that S. cerevisiae cells needed approximate 30 h to fully adapt to anaerobic conditions during ethanol fermentation, although cells had been growing under low amounts of air (Wiebe et al. 2008).
3.3.5 Amino acids
Some intracellular amino acids varied significantly during both continuous and batch fermentations (Fig. 7). All of these amino acids displayed a declining trend. It was possibly caused by nitrogen depletion as the fermentation progressed. This might also due to the high osmotic stress in both processes. Pham had reported that high osmotic stress could cause decreases in the expression levels of most proteins involved in amino acid metabolism (Pham et al. 2006).
Intracellular amino acids in S. cerevisiae significantly varied during continuous (a) and batch (b) fermentation. The relative abundance was calculated by normalization of peak area of each metabolite to internal standard ribitol, and each value represents the mean of five independent replicates ± SD
It was reported that glycine participated in the biosynthesis of purines in yeast (Abrams et al. 1948). Our present results showed that the change of glycine was significant throughout both the continuous and batch fermentation processes (Fig. 7). However, there were different variation trends of glycine during the two fermentation modes. During batch fermentation, the glycine level gradually declined, whereas it reached the highest level in fermenter 4 during continuous fermentation. As shown in Fig. 7, glutamine displayed a similar variation trend with some intermediates involved in TCA cycle during continuous and batch fermentation. Glutamine, which played a main role in nitrogen catabolism and anabolism, was also considered as a major source of cellular nitrogen (Dickinson and Scheweizer 1999). It was a precursor for the biosynthesis of other amino acids and nucleotides. Decreases in level of glutamine were likely related to nitrogen depletion during the final fermentation phase.
The strategy of GC-TOF-MS-based metabolomics in this study provides general metabolic profiles of S. cerevisiae during the continuous and batch processes. Moreover, it is a starting point for further investigation into the metabolism network of S. cerevisiae under complex industrial fermentation conditions. The observed variations of some metabolite might reflect the cellular processes involved in responses to the stresses during continuous and batch fermentations. Further investigations are needed to figure out how to efficiently regulate metabolism network of yeast under complex industrial fermentation conditions with lipidomic, proteomic and transcriptomic analysis, so as to improve the ethanol productivity of the industrial yeast strain.
4 Concluding remarks
The metabolome variations in the industrial S. cerevisiae throughout the continuous and batch fermentations were investigated. More than 100 intracellular metabolites were detected by GC-TOF-MS in both processes. Samples from different fermentation modes (continuous and batch) and phases (seed, main and final fermentation) were clearly separated by performing PCA on the detected intracellular metabolites in the scores plot. Furthermore, it was found out that the levels of metabolites involved in central carbon metabolism and amino acid biosynthetic pathway significantly changed under the different fermentation phases and modes. Glycerol and phosphoric acid in continuous fermentation and lactic acid and glycerol in batch fermentation contributed most for distinguishing the different fermentation phases, respectively. These findings will help us understand the actual industrial fermentation process.
References
Abrams, R., Hammarsten, E., & Shemin, D. (1948). Glycine as a precursor of purines in yeast. The Journal of Biological Chemistry, 173, 429.
Backhus, L. E., DeRisi, J. P. O., & Bisson, L. F. (2001). Functional genomic analysis of a commercial wine strain of Saccharomyces cerevisiae under differing nitrogen conditions. FEMS Yeast Research, 1, 111–125. doi:10.1111/j.1567-1364.2001.tb00022.x.
Blomberg, A., & Adler, L. (1989). Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. Journal of Bacteriology, 171, 1087–1092.
Blomberg, A., & Adler, L. (1992). Physiology of osmotolerance in fungi. Advances in Microbial Physiology, 33, 145–212. doi:10.1016/S0065-2911(08)60217-9.
Brejning, J., Arneborg, N., & Jespersen, L. (2005). Identification of genes and proteins induced during the lag and early exponential phase of lager brewing yeasts. Journal of Applied Microbiology, 98, 261–271. doi:10.1111/j.1365-2672.2004.02472.x.
Brewster, J. L., De Valoir, T., Dwyer, N. D., Winter, E., & Gustin, M. C. (1993). An osmosensing signal transduction pathway in yeast. Science, 259, 1760–1763. doi:10.1126/science.7681220.
Cavalieri, D., Townsend, J. P., & Hartl, D. L. (2000). Manifold anomalies in gene expression in a vineyard isolate of Saccharomyces cerevisiae revealed by DNA microarray analysis. Proceedings of the National Academy of Sciences of the United States of America, 97, 12369–12374. doi:10.1073/pnas.210395297.
Cheng, J. S., Qiao, B., & Yuan, Y. J. (2008). Comparative proteome analysis of robust Saccharomyces cerevisiae insights into industrial continuous and batch fermentation. Applied Microbiology and Biotechnology, 81, 327–338. doi:10.1007/s00253-008-1733-6.
Davenport, K. R., Sohaskey, M., Kamada, Y., Levin, D. E., & Gustin, M. C. (1995). A second osmosensing signal transduction pathway in yeast: Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. The Journal of Biological Chemistry, 270, 30157–30161. doi:10.1074/jbc.270.50.30157.
Devantier, R., Scheithauer, B., Villas-Bôas, S. G., Pedersen, S., & Olsson, L. (2005). Metabolite profiling for analysis of yeast stress response during very high gravity ethanol fermentations. Biotechnology and Bioengineering, 90, 703–714. doi:10.1002/bit.20457.
Dickinson, J. R., & Scheweizer, M. (1999). Stress responses in the metabolism and molecular physiology of Saccharomyces cerevisiae (p. 343). New York: Taylor and Francis.
Fiehn, O. (2002). Metabolomics—the link between genotypes and phenotypes. Plant Molecular Biology, 48, 155–171. doi:10.1023/A:1013713905833.
Fiehn, O., Kopka, J., Dormann, P., Altmann, T., Trethewey, R. N., & Willmitzer, L. (2000). Metabolite profiling for plant functional genomics. Nature Biotechnology, 18, 1157–1161. doi:10.1038/81137.
Franzen, C. J. (2003). Metabolic flux analysis of RQ-controlled microaerobic ethanol production by Saccharomyces cerevisiae. Yeast (Chichester, England), 20, 117–132. doi:10.1002/yea.956.
Gancedo, C., Gancedo, J. M., & Sols, A. (1968). Glycerol metabolism in yeasts Pathways of utilization and production. European Journal of Biochemistry, 5, 165–172. doi:10.1111/j.1432-1033.1968.tb00353.x.
Gika, H. G., Theodoridis, G. A., & Wilson, I. D. (2008). Liquid chromatography and ultra-performance liquid chromatography–mass spectrometry fingerprinting of human urine: Sample stability under different handling and storage conditions for metabonomics studies. Journal of Chromatography A, 1189, 314–322. doi:10.1016/j.chroma.2007.10.066.
Hansen, R., Pearson, S. Y., Brosnan, J. M., Meaden, P. G., & Jamieson, D. J. (2006). Proteomic analysis of a distilling strain of Saccharomyces cerevisiae during industrial grain fermentation. Applied Microbiology and Biotechnology, 72, 116–125. doi:10.1007/s00253-006-0508-1.
Hohl, L. A., & Joslyn, M. A. (1941). Lactic acid formation in alcoholic fermentation by yeast. Plant Physiology, 16, 343–360.
Hohmann, S. (2002). Osmotic stress signaling and osmoadaptation in yeasts. Microbiology and Molecular Biology Reviews, 66, 300–372. doi:10.1128/MMBR.66.2.300-372.2002.
James, T. C., Campbell, S., Donnelly, D., & Bond, U. (2003). Transcription profile of brewery yeast under fermentation conditions. Journal of Applied Microbiology, 94, 432–448. doi:10.1046/j.1365-2672.2003.01849.x.
Kamada, Y., Jung, U. S., Piotrowski, J., & Levin, D. E. (1995). The protein kinase C-activated MAP kinase pathway in Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes & Development, 9, 1559–1571. doi:10.1101/gad.9.13.1559.
Kell, B., & Westerhoff, H. V. (1986). Metabolic control theory: Its role in microbiology and biotechnology. FEMS Microbiology Letters, 39, 305–320. doi:10.1111/j.1574-6968.1986.tb01863.x.
Kobi, D., Zugmeyer, S., Potier, S., & Jaquet-Gutfreund, L. (2004). Two dimensional protein map of an “ale”-brewing yeast strain: Proteome dynamics during fermentation. FEMS Yeast Research, 5, 213–230. doi:10.1016/j.femsyr.2004.07.004.
Koning, W., & Van Dam, K. (1992). A method for the determination of changes of glycolytic metabolites in yeast on a subsecond time scale using extraction at neutral pH. Analytical Biochemistry, 204, 118–123. doi:10.1016/0003-2697(92)90149-2.
Krebs, H. A., Lowenstein, J. M., & Greenberg, D. M. (1960). Metabolic pathways (Vol. 1, p. 129). New York/London: Academic Press.
Lenz, E., & Wilson, I. D. (2007). Analytical strategies in metabonomics. Journal of Proteome Research, 6, 443. doi:10.1021/pr0605217.
Major, H. J., Williams, R., Wilson, A. J., & Wilson, I. D. (2006). A metabonomic analysis of plasma from Zucker rat strains using gas chromatography/mass spectrometry and pattern recognition. Rapid Communications in Mass Spectrometry, 20, 3295–3302. doi:10.1002/rcm.2732.
Marks, V. D., Ho Sui, S. J., Erasmus, D., Van der Merwe, G. K., et al. (2008). Dynamics of the yeast transcriptome during wine fermentation reveals a novel fermentation stress response. FEMS Yeast Research, 8, 35–52.
Mohler, R. E., Tu, B. P., Dombek, K. M., Hoggard, J. C., Young, E. T., & Synovec, R. E. (2008). Identification and evaluation of cycling yeast metabolites in two-dimensional comprehensive gas chromatography-time-of-flight-mass spectrometry data. Journal of Chromatography A, 1186, 401–411. doi:10.1016/j.chroma.2007.10.063.
Nissen, T. L., Schulze, U., Nielsen, J., & Villadsen, J. (1997). Flux distributions in anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae. Microbiology, 143, 203–218.
Ossipov, V., Ossipova, S., Bykov, V., Oksanen, E., Koricheva, J., & Haukioja, E. (2008). Application of metabolomics to genotype and phenotype discrimination of birch trees grown in a long-term open-field experiment. Metabolomics, 4, 39–51. doi:10.1007/s11306-007-0097-8.
Pham, T. K., Chong, P. K., Gan, C. S., & Wright, P. C. (2006). Proteomic analysis of Saccharomyces cerevisiae under high gravity fermentation conditions. Journal of Proteome Research, 5, 3411–3419. doi:10.1021/pr060377p.
Roessner, U., Luedemann, A., Brust, D., et al. (2001). Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. The Plant Cell, 13, 11–29.
Roessner, U., Wagner, C., Kopka, J., Trethewey, R. N., & Willmitzer, L. (2000). Simultaneous analysis of metabolites in potato tuber by gas chromatography–mass spectrometry. The Plant Journal, 23, 131–142. doi:10.1046/j.1365-313x.2000.00774.x.
Trabalzini, L., Paffetti, A., Scaloni, A., et al. (2003). Proteomic response to physiological fermentation stresses in a wild-type wine strain of Saccharomyces cerevisiae. The Biochemical Journal, 370, 35–46. doi:10.1042/BJ20020140.
Van Dijken, J. P., & Scheffers, W. A. (1986). Redox balances in the metabolism of sugars by yeast. FEMS Microbiology Reviews, 32, 199–224.
Villas-Bôas, S. G., Delicado, D. G., Akesson, M., & Nielsen, J. (2003). Simultaneous analysis of amino and nonamino organic acids as methyl chloroformate derivatives using gas chromatography–mass spectrometry. Analytical Biochemistry, 322, 134–138. doi:10.1016/j.ab.2003.07.018.
Villas-Bôas, S. G., Moxley, J. F., Akesson, M., Stephanopoulos, G., & Nielsen, J. (2005). High-throughput metabolic state analysis: The missing link in integrated functional genomics of yeasts. The Biochemical Journal, 388, 669–677. doi:10.1042/BJ20041162.
Wagner, C., Sefkow, M., & Kopka, J. (2003). Construction and application of a mass spectral and retention time index database generated from GC/EI-TOF-MS metabolite profiles. Phytochemistry, 62, 887–900. doi:10.1016/S0031-9422(02)00703-3.
Wiebe, M. G., Rintala, E., Tamminen, A., et al. (2008). Central carbon metabolism of Saccharomyces cerevisiae in anaerobic, oxygen-limited and fully aerobic steady-state conditions and following a shift to anaerobic conditions. FEMS Yeast Research, 8, 140–154.
Yang, W. L., & Bernards, M. A. (2007). Metabolite profiling of potato (Solanum tuberosum L.) tubers during wound-induced suberization. Metabolomics, 3, 147–159. doi:10.1007/s11306-007-0053-7.
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (Key Program Grant No.20736006), the National Basic Research Program of China (Program: 2007CB714301), and Key Projects in the National Science & Technology Pillar Program (No.2007BAD42B02), and the National Natural Science Foundation of China (No. 20706044).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ding, MZ., Cheng, JS., Xiao, WH. et al. Comparative metabolomic analysis on industrial continuous and batch ethanol fermentation processes by GC-TOF-MS. Metabolomics 5, 229–238 (2009). https://doi.org/10.1007/s11306-008-0145-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-008-0145-z