Abstract
With unmatched mass resolution, mass accuracy, and exceptional detection sensitivity, Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR-MS) has the potential to be a powerful new technique for high-throughput metabolomic analysis. In this study, we examine the properties of an ultrahigh-field 12-Tesla (12T) FTICR-MS for the identification and absolute quantitation of human plasma metabolites, and for the untargeted metabolic fingerprinting of inbred-strain mouse serum by direct infusion (DI). Using internal mass calibration (mass error ≤1 ppm), we determined the rational elemental compositions (incorporating unlimited C, H, N and O, and a maximum of two S, three P, two Na, and one K per formula) of approximately 250 out of 570 metabolite features detected in a 3-min infusion analysis of aqueous extract of human plasma, and were able to identify more than 100 metabolites. Using isotopically-labeled internal standards, we were able to obtain excellent calibration curves for the absolute quantitation of choline with sub-pmol sensitivity, using 500 times less sample than previous LC/MS analyses. Under optimized serum dilution conditions, chemical compounds spiked into mouse serum as metabolite mimics showed a linear response over a 600-fold concentration range. DI/FTICR-MS analysis of serum from 26 mice from 2 inbred strains, with and without acute trichloroethylene (TCE) treatment, gave a relative standard deviation (RSD) of 4.5%. Finally, we extended this method to the metabolomic fingerprinting of serum samples from 49 mice from 5 inbred strains involved in an acute alcohol toxicity study, using both positive and negative electrospray ionization (ESI). Using these samples, we demonstrated the utility of this method for high-throughput metabolomics, with more than 400 metabolites profiled in only 24 h. Our experiments demonstrate that DI/FTICR-MS is well-suited for high-throughput metabolomic analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabolomics is the study of the metabolome, i.e., the complete set of metabolites in a cell, biofluid, tissue, or organism, in the same way that genomics looks at the complete set of genes, and proteomics examines the entire complement of proteins. While genomics and proteomics involve the study of molecules that are chemically similar (DNA for genomics, proteins for proteomics), metabolomics deals with structurally heterogeneous and physico-chemically diverse small-molecule metabolites (typically <1000 Da). Metabolomics seeks to identify and quantify the metabolites produced under a given set of conditions (Fiehn et al. 2001; Goodacre et al. 2004). These metabolites are the end-products of the combined effects of gene expression and protein function, and include a diverse array of biomolecules, including amino acids, antioxidants, cofactors, hormones, enzyme inhibitors, neurotransmitters, etc. (Gamache et al. 2004). Quantifying the perturbations of these metabolites in response to specific diseases, drugs, environmental or genetic modifications (Denkert et al. 2006; Goodacre et al. 2004; Nicholson et al. 1999) can be important for understanding the biochemical pathways involved in these processes. It has been estimated that the mammalian metabolome consists of at least 3,000 distinct chemical entities (Brown et al. 2005).
The large potential dynamic range of metabolite concentrations––spanning up to nine orders of magnitude (Dunn et al. 2005)––and their short half-life (Brown et al. 2005) poses additional technical obstacles for metabolomics. Quantitation is also challenging because of the limited number of available isotopically-labeled metabolite standards and the difficulty of generating standard curves for metabolites which have been analyzed simultaneously. A truly effective analytical technique must be capable not only of sensitive and selective detection of diverse molecules, but also of accurate quantitation over a wide range of concentrations, in a high-throughput fashion (Gamache et al. 2004). Despite technical challenges, interest in metabolomics is growing rapidly because multiple metabolic pathways and networks are known to be involved in the development of diseases, including cancer (Fan et al. 2004; Kolch et al. 2005) and various disorders caused by inborn errors of metabolism (Halket et al. 1999).
Mass spectrometric techniques, including liquid chromatography––mass spectrometry (LC/MS) (Allen et al. 2003; Buchholz et al. 2001; De Vos et al. 2007; Wikoff et al. 2007), gas chromatography––mass spectrometry (GC/MS) (Denkert et al. 2006; Jones et al. 2007; Lee et al. 2007), and capillary electrophoresis––mass spectrometry (CE/MS) (Sato et al. 2004; Soga et al. 2006) are among the analytical techniques currently used in metabolomics research. These mass spectrometric techniques commonly use up-front separation steps to reduce the sample complexity and reduce suppression effects, thereby enhancing the detection sensitivity. This increases the coverage of the metabolome, but at the same time adds more challenges in data processing due to the run-to-run variability of the chromatographic or electrophoretic separations, and increases the analysis time. These time-consuming separation steps are also detrimental to the overall speed of the analysis, although this situation has somewhat improved in recent years due to the introduction of ultrahigh performance liquid chromatography (Plumb et al. 2004).
Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FTICR-MS) has several unique advantages for the complex sample analysis compared to other mass spectrometric techniques. The ultrahigh resolving power of FTICR analyzers [>100,000 Full Width Half Maximum (FWHM)] has been shown to be capable of resolving more than 10,000 distinct species in a single spectrum of crude oil (Hughey et al. 2002), implying that this technique could potentially be adapted for high-throughput metabolomic analysis, with reduced or eliminated chromatography. The extremely high mass accuracy of FTICR-MS enables mass determination to within 1 ppm (Brown et al. 2005; Dunn et al. 2005), and metabolite candidates can often be assigned based on accurate mass alone (Aharoni et al. 2002; Tohge et al. 2005), by querying specific metabolomic databases. Over the past few years, several applications have been published on the use of DI/FTICR-MS for plant metabolomics (Aharoni et al. 2002; Hirai et al. 2004; Nakamura et al. 2007; Ohta et al. 2007). However, the suitability of this technique has not yet been evaluated for quantitative metabolic analyses. To address this issue, and to test the suitability of DI/FTICR-MS for the analysis of endogenous metabolites in blood, we utilized a 12T FTICR-MS instrument to identify and quantify human plasma metabolites, and for the untargeted metabolic fingerprinting of inbred-strain mouse serum.
2 Materials and methods
Methanol, chloroform, water, formic acid, and ammonium hydroxide were LC/MS grade and were obtained from Fluka (Buchs & Steinheim, Germany). Standard chemical compounds, including haloperidol, diclofenac, verapamil hydrochloride, and reserpine, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deuterated choline (choline-[N,N,N-trimethyl-d 9] bromide) was obtained from CNS Isotopes (Quebec, Canada).
2.1 Human plasma metabolite extraction
Human plasma was obtained from the same healthy adults subjected to a choline-adequate and then to a choline-deficient diet. Extraction of polar metabolites from the plasma was done following a protocol established at the University of North Carolina at Chapel Hill School of Public Health (Koc et al. 2002). Briefly, 200 μl aliquots of plasma samples were extracted overnight at −20°C in safe-lock Eppendorf tubes with 750 μl of mixed methanol/chloroform (2:1, v/v) followed by vortex mixing and centrifugation at 1,500×g for 5 min. The supernatant was transferred to a 5-ml glass test tube and the residue was extracted one more time with 500 μl of mixed methanol/chloroform/water (2:1:0.8, v/v/v), vortexed, and centrifuged as before. The supernatant extracts were then combined, and 200 μl of chloroform, followed by 200 μl of water, was added. The combined extract was vortexed and centrifuged as before. The upper (aqueous) phase was carefully pipetted into a fresh tube and lyophilized in a SPD1010 speed-vac concentrator (Thermo-Fisher, Milford, MA, USA). The residue was re-suspended in 20 μl of water, followed by 280 μl of methanol, and then centrifuged to remove any precipitated protein. For FTICR-MS analysis, an aliquot of the supernatant was diluted 100-fold with 50% methanol/0.1% formic acid.
2.2 Mouse acute treatment and serum metabolite extraction
For the trichloroethylene (TCE) acute toxicity experiment, twenty-six 7-week-old male mice from 2 inbred strains (thirteen animals per strain) were used. Six animals from each strain were fed a high-fat standard diet and dosed once with 1 g/kg TCE dissolved in corn oil via intra-gastric infusion to serve as the treatment group; six animals were fed the identical diet and dosed with corn oil alone to serve as the control group; the one remaining animal in each inbred strain was fed the identical diet as a naïve control. At 24 h after dosing, all animals were sacrificed and serum samples were collected using micro-serum gel tubes (Sarstedt, Aktiengesellschaft and Co., Numbrecht, Germany) and stored at −80°C.
For the alcohol toxicity experiment, four to five 8–9-week-old male mice from each of the 5 different inbred strains (A/J, AKR/J, C3H/HeJ, C57BL/6J, DBA/2J) were used. The mice were fed the same high-fat diet as above, and were dosed once with 20% ethanol in saline at 5 g/kg via intra-gastric infusion. Control animals were fed the identical diet and were dosed with saline via intra-gastric infusion. Six hours after dosing, all animals were sacrificed and serum samples were collected using micro-serum gel tubes and stored at −80°C.
For metabolite extraction, the frozen mouse serum samples were thawed on ice. A 10 μl-aliquot from each animal was pipetted into a self-lock 1.5-ml Eppendorf tube, followed by 190 μl water. For positive ion electrospray ionization (ESI) MS, 100 μl methanol containing 1 μg/ml haloperidol, 0.4 μg/ml verapamil, and 0.4 μg/ml reserpine was spiked as internal standards for mass calibration, while haloperidol was also used as the internal standard for MS intensity normalization. After sonicating for 1 min in an ice water bath, followed by 30 s vortex mixing, a total of 700 μl methanol was added stepwise to the tube. Samples were placed at −20°C for 2 h and then centrifuged at 12,000 rpm in a microcentrifuge at 4°C for 15 min. 5 μl of 10% formic acid was added to 500 μl of the supernatant for positive ion ESI FTICR-MS. For negative ion ESI MS, another 10-μl portion of each serum was extracted as described above, except it was spiked with 100 μl of methanol containing 30 μg/ml diclofenac, 25 μg/ml haloperidol, and 10 μg/ml reserpine for internal mass calibration. Diclofenac was also used for MS intensity normalization. Twenty μl of 25% ammonium hydroxide was added to 500-μl aliquots of the supernatant immediately before FTICR-MS analysis.
2.3 Mass spectrometry
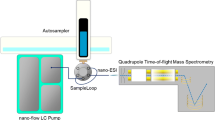
The extracted metabolites were analyzed on an actively shielded 12 T apex-Qe® Qq-FTICR mass spectrometer equipped with an Apollo® II ion source, a quadruple mass filter, and a hexapole collision cell external to the ICR cell (Bruker Daltonics Inc., Billerica, MA). Samples were introduced by DI into the ion source, using a syringe pump at a flow rate of 2.4 μl/min. Each spectrum was acquired with a total of 0.3–0.5-s external ion accumulation times, and up to 100 scans were accumulated over the mass range of m/z 100–900 in 1–3 min. To generate the calibration curves using ESI-LC/MS, an Advance LCQ ion trap mass spectrometer (ThermoFisher, San Jose, CA) equipped with an API2 ESI source, a Surveyor HPLC system with an Alltech Solvent Miser Silica column, and a Surveyor autosampler, was used. HPLC and MS parameters used for ESI-LC/MS were as described previously (Koc et al. 2002).
2.4 Quantitative analysis
To evaluate DI/FTICR-MS for quantitative analysis of metabolites using isotopically-labeled internal standards, we compared FTICR-MS and LC/MS choline calibration curves using isotopic-dilution MS. Calibration curves were generated by mixing 20 nmol of d 9-labeled choline with varying amounts of unlabeled choline. Because of the ultrahigh resolution and reproducible peak shapes obtained with FTICR-MS analyses, calibration curves for FTMS were generated by determining the ratio of the relative intensity of deuterated versus unlabeled choline, over the concentration range tested. Calibration curves for choline using LC/MS were constructed by determining the ratio of the peak area of unlabeled versus deuterated standard, according to the previously-described method (Koc et al. 2002).
For the serum dilution experiments, a 100-μl aliquot of mouse serum was diluted with 100 μl of water and extracted with 800 μl methanol as above. The supernatant (equivalent to a dilution ratio 1:10) and a series of dilutions with 80% methanol (1:25, 1:50, 1:100, 1:200 and 1:500) were acidified with 0.1% formic acid. For the dynamic range test, varying amounts of standard chemical compounds including diphendramine, haloperidol, verapamil, terfinadine, and reserpine (with the concentrations of each compound spanning at least 3 orders of magnitude) were spiked into 10 μl aliquots of mouse serum, followed by extraction and 1:100 dilution into 80% methanol/0.1% formic acid before DI/FTICR-MS analysis.
2.5 Data processing
To identify human plasma metabolites with m/z between 90 and 570 Da, rational chemical formulas (incorporating unlimited C, H, N and O, and a maximum of two S, three P, two Na, and one K per formula) were generated based on internally-calibrated monoisotopic masses within ≤1 ppm mass error, using the Generate Molecular Formula tool within the DataAnalysis™ (DA) software package (Bruker Daltonics). To generate chemical formulas, only even-electron ions were considered and the nitrogen rule was applied (Kind and Fiehn 2007). The chemically-reasonable formulas generated with the above constraints were then submitted to three metabolome databases––the Kyoto Encyclopedia of Genes and Genomes (KEGG) ligand database (Ogata et al. 1999), the Human Metabolome Database (HMDB) (Wishart et al. 2007), and the LIPID MAPS database (Fahy et al. 2007; Sud et al. 2007) in order to determine potentially-matching human metabolites consistent with the measured masses. The isotopic abundance distribution patterns of the matched metabolite candidates were simulated with the Generate Molecular Formula software tool and compared with those observed in the mass spectra to further reduce the number of potential elemental compositions (Kind and Fiehn 2006, 2007).
For mouse serum metabolic fingerprinting, raw mass spectra were initially batch-processed with a custom VBA script we developed within DA. The first step of this script is an automated internal mass calibration with the reference masses of the spiked calibration standards (for positive ESI mode: haloperidol, verapamil, and reserpine; for negative ion mode: diclofenac, haloperidol, and reserpine) or known metabolites. Monoisotopic peaks corresponding to the metabolite isotopic distributions were then automatically determined. Na- and K-adduct ions were then filtered out of the positive ESI spectra based on the expected mass differences for these ions (within 2 ppm) to yield a list of unique chemical component masses together with their peak intensities. For negative-ion MS data, no filtering was applied because no apparent adduct ions, such as (M+Cl)−, (M+NH2COO)− or (M+HCOO)−, were observed in this study, although these adducts were often observed in the negative ion mode for simple sample mixtures.
The resulting mass lists from all the mass spectra in both positive and negative ion modes were further processed with another customized integrated software program developed with LabView® (National Instruments, Austin, TX). This software converts the monoisotopic masses to neutral masses by subtracting 1.007276 (the mass of proton 1.007825 Da minus an electron 0.00054858 Da) for positive ESI mode, or by adding 1.007276 Da for the negative ion mode. The peak intensities of all the monoisotopic neutral masses are then normalized to the internal standard peak intensity (haloperidol and diclofenac for positive and negative ion modes, respectively). Triplicate measurements of each sample were compared to filter out any masses that are detected in only one analysis and to ensure that no instrumental problems affecting signal acquisition or instrument calibration occurred during that particular run. Masses observed in at least two of the triplicate measurements were combined into a single biological replicate by averaging peak intensities from masses that matched within a mass accuracy of 2 ppm.
Biological replicates from multiple animals in each control or treated group were then aligned within 2 ppm to yield a list of masses observed in most of the biological replicates (two out of three animals, and three out of four and five animals per inbred strain). The mass lists from all the sets of control and treated animals were then combined with the same mass accuracy, keeping all mass values regardless of occurrence across the sets. All metabolite masses were combined without any filtering. The combining of the datasets was based on the consistency of metabolite masses from different groups. If the metabolite masses from two groups were within 2 ppm, then these were considered to be the same metabolite, otherwise, they were considered to be a different metabolites. If a mass was only found in a single group, and not in another group, this mass might be especially interesting because it might indicate a biomarker which could be used to differentiate between the two groups. These neutral masses, together with their normalized intensities, were then hierarchically clustered using Cluster software available at http://rana.lbl.gov/EisenSoftware.htm (Eisen et al. 1998), and expressed as two-dimensional heat maps of metabolite features using the Java TreeView software available at http://sourceforge.net/projects/jtreeview (Saldanha 2004).
3 Results and discussion
3.1 Detection of human plasma metabolites
To evaluate the utility of FTICR-MS for metabolomic analysis, it is important to determine how the mass resolution, mass accuracy, and measurement precision offered by FTICR mass analyzers affects metabolite detection and identification. We therefore first evaluated the feasibility of the ultrahigh-field 12T FTICR instrument for the detection and identification of human plasma metabolites by DI. Mass spectra in the mass range m/z 90–570 were obtained by direct-infusion analysis of aqueous extracts of human plasma taken from same person subjected to a choline-adequate and then a choline-deficient diet, as described elsewhere (Koc et al. 2002).
The resolving power of the ultrahigh-field FTICR-MS enabled detection of ca. 570 distinct metabolite features represented by monoisotopic masses above S/N 3 within the mass range m/z 90–570 (a 480-Da mass range). All of these metabolites were detected in a single DI experiment which took only a few minutes (Fig. 1a and b). The resolving power of the FTICR-MS allowed the detection of numerous metabolites that cluster around nominal masses, thus indicating that the requirement for liquid chromatographic separation might be reduced or eliminated. Minimizing the requirement for a chromatographic step would allow high-throughput FTICR-based metabolomic analysis.
More than 570 metabolite features from an aqueous extract of human plasma were detected in a single (+) ESI/FTICR mass spectrum. (a) The overlaid mass spectra acquired from a 3-min infusion of plasma metabolites in aqueous extract taken from each of 4 subjects. (b) Expansion of a 50 Da m/z window (red box) demonstrating the mass resolution of the 12T FTICR-MS. (c) Demonstration of FTICR-MS mass accuracy with internal calibration. Even relatively low abundance ions can be sufficiently resolved to permit identification based on the calibrated mass. Inset shows the identification of phosphocholine (calculated mass = 184.07332) based on accurate mass alone. The observed mass of 184.07329 differed from the calculated mass by less than 0.2 ppm.
The mass resolution of ultrahigh-field FTICR-MS is not achieved at the expense of mass accuracy, as shown in Fig. 1c. Internal mass calibration, based on standards or known metabolites, has led to mass accuracies within 0.2 ppm for most metabolites and within 0.65 ppm for all of the identified metabolites (see supplementary information), when the instrument has been appropriately calibrated, and allows the possibility of metabolite identification based on mass alone, even for metabolites present at low abundance. Although high mass accuracy—even at less 1 ppm––is sometimes insufficient for metabolite identification due to the high number of potential elemental compositions [especially for higher masses (Kind and Fiehn 2006)], the built-in constraints of the metabolite queries within the metabolome databases (KEGG, HMDB and LIPID MAPS), the use of the molecular formulas, and the comparison of the measured and the simulated isotopic abundance distribution patterns, effectively reduced the number of metabolite candidates. For example, using these constraints and a mass accuracy of within 1 ppm, the ion signal observed at m/z 184.07329 (Fig. 1c inset) only matched the elemental composition of C5H15N1O4P1, which corresponds to phosphocholine (calculated m/z 184.07332; error −0.163 ppm). Thus, in most cases, the ultrahigh mass accuracy possible with the FTICR-MS enabled direct identification of metabolite candidates through determination of their elemental compositions based on accurate mass alone.
FTICR-MS also provides exceptional mass measurement precision, as demonstrated in Fig. 2. For the ion at m/z 203.05, we obtained a single molecular formula with a calculated mass of 203.05261 corresponding to sodiated hexose using the DA Generate Molecular Formula tool. The internal mass calibration led to a mass error of less than 0.2 ppm for this ion (Fig. 2, red spectrum), while errors in externally-calibrated spectra (Fig. 2, blue, gray, green spectra) were consistently less than 0.5 ppm. It should be noted that sub-ppm mass errors were achievable even when extrapolating beyond the mass calibration points [m/z 104 (choline) and m/z 140 (betaine)].
Demonstration of ultrahigh mass precision possible with the 12T FTICR-MS using either internal or external mass calibration. Mass measurement accuracy (MMA) errors of less than 0.2 ppm for sodiated hexose were achieved with internal calibration of the spectra, while the average MMA error for external calibration was less than 0.5 ppm.
3.2 Identification of human plasma metabolites
To assess the efficacy of the ultrahigh-field FTICR-MS for metabolite identification in complex samples without prior separation, we attempted to determine the identities of all 570 metabolite features detected in the 3-min DI analysis. Approximately 250 of the 570 metabolite features detected gave reasonable elemental compositions based on mass alone (assuming 1 ppm mass accuracy) when allowing for incorporation of the designated numbers of C, H, N, O, S, P, Na and K in the formulas. Approximately 40% of these 250 metabolite features (104) matched known metabolites in the metabolome databases (see supplementary information for details). Figure 3 shows the results of selected identifications based on accurate mass alone. Two ions detected in all four plasma sample extracts were found within a 0.025 Da window (167.06000–167.08250 m/z). The ion at m/z 167.06788 was assigned to the elemental composition C7H12O3Na, which corresponds to trans-4-hydroxycyclohexanecarboxylate (sodiated) and/or (5-L-glutamyl)-L-glutamine (sodiated). The second ion (at m/z 167.07920) could be uniquely assigned to C6H12O2N2Na, which corresponds to sodiated ectoine, an osmoprotective aspartate derivative (Galinski 1995).
(a) Expansion of overlaid (+) ESI/FTICR mass spectra of four aqueous plasma extracts showing a 0.02 Da window from m/z 167.06 to 167.0825. The identity of the metabolite on the left (m/z 167.06788) could be narrowed down to either trans-4-hydroxycyclohexanecarboxylate (sodiated) or (5-L-glutamyl)-L-glutamine (sodiated) by querying the KEGG ligand database for elemental formulas within 1 ppm mass accuracy. The metabolite on the right (m/z 167.07920) could be uniquely identified as sodiated ectoine. (b) Expansion of the m/z range 169.04 to 169.13 showing the overlaid (+) ESI/FTICR mass spectra of four aqueous plasma extracts. Four metabolites were detected within this 0.09 Da window. Glutamine and lysine could be identified on the basis of accurate mass alone. In addition, the molecular formulas of two unknown metabolites could be determined based on mass.
Similarly, of the 4 ions in the 0.09 Da window from m/z 169.0400 to 169.1300 (Fig. 3b), two ions (m/z 169.0583 and m/z 169.0947) could be uniquely assigned to sodiated glutamine (C5H10N2O3Na) and sodiated lysine (C6H14N2O2Na), respectively. In contrast, the ion at m/z 169.1198, present in one sample extract only, could not be uniquely identified based only on mass. While the databases produced no unequivocal matches corresponding to m/z 169.1198, the ion at m/z 169.0471 was suggested to be a unique chemical formula C6H10O4Na, but this elemental composition corresponds to 9 possible isomers in the KEGG database. Thus, even though FT-ICR cannot resolve isomers, we were able to obtain reasonable identification information for approximately 100 metabolites from a DI analysis of a complex plasma sample, in only 3 min. Our results therefore suggest that ultrahigh field FTICR-MS is a promising method for high-throughput metabolite identification.
3.3 Dynamic range
The high mass accuracy [±0.00020 amu (n = 3) of the signal apex of choline (m/z 104.10699)], and the highly-consistent peak shapes characteristic of FTICR-MS allow easy detection of metabolites present at different concentrations in different samples. The direct infusion FTICR-MS experiment showed a dynamic range (i.e., the ratio of the highest to lowest concentration metabolites detected) comparable to that of the previously-reported LC/MS method (Koc et al. 2002). Ion signals 750 times lower in intensity than the most abundant ion signals could be detected in aqueous plasma extracts by direct infusion, using “broadband detection” as described in this study. Differential abundances of a number of metabolites can be clearly discerned when spectra from identically prepared and analyzed samples are overlaid (Figs. 1 and 3).
Unfortunately, however, even this factor of 750 is insufficient when compared to the large dynamic range of expected metabolite concentrations––up to nine orders of magnitude (Dunn et al. 2005). This enormous potential dynamic range of metabolite concentrations in biological fluids such as plasma poses a formidable challenge for mass spectrometry-based metabolomics. We recognize that this FTICR-MS based approach described here is not comprehensive, and is subject to known shortcomings––for example, the inability of differentiation of metabolite isomers, and severe ionization suppression effects compared to LC/MS. However, it should be pointed out that we were able to detect 570 metabolite features within the m/z range 90-570, in a single 3-min experiment.
Several improvements could probably be made to improve the dynamic range of DI/FTICR. Suppression of ion signals from low-abundance metabolites by ion signals from higher-abundance metabolites could be partially overcome by sample fractionation to reduce sample complexity and enhance detection of the low abundance metabolites prior to sample introduction into the mass spectrometer. Differential extraction techniques to fractionate the plasma samples prior to mass spectrometry represents an important area of research (Want et al. 2006). The method used in this manuscript involves analysis of only the aqueous phase, but could easily be modified to include analysis of the organic phase, or to include extractions involving other organic solvents or procedures.
Another method for enhancing the dynamic range of FT-MS instruments equipped with quadruple mass filters or ion guides, is the dynamic range expansion applied to mass spectrometry (DREAMS) technique developed at the Pacific Northwest National Laboratory (Belov et al. 2001). This technique involves applying a dipolar excitation signal containing specific frequencies to opposing rods of the quadrupole. The frequencies correspond to the secular frequencies of oscillation of particularly abundant ions within the spectrum. By exciting these ions they are ejected from the quadrupole, and are effectively removed from the ion beam, thus eliminating their contribution to the total charge capacity of the storage cell and/or ICR cell. The extra capacity can then be filled with the remaining low-abundance ions, resulting in enhanced trapping and higher detection sensitivity.
Automation of the analysis process is another improvement that could be incorporated into our current method. This could be done by using the highly-sensitive chip-based infusion technique (Southam et al. 2007). Nanospray chip infusion is characterized by low flow rates that reduce ion suppression and allow signal averaging to give improved signal-to-noise ratios and to enhance the sensitivity and dynamic range of detection (Staack et al. 2005).
3.4 Absolute quantitation of human plasma metabolites
To test the potential of FTICR-MS for quantitative determination of endogenous metabolites in complex human plasma mixture, we used isotopically-labeled internal standards for absolute quantitation of low–molecular-weight metabolites. These results were compared with GC/MS and LC/MS, the most commonly-used techniques in metabolomics research. Figure 4a shows a spectrum from a DI experiment used for analysis of two metabolites, choline and betaine (a derivative of choline). Deuterated forms of the metabolites were added to human plasma samples to serve as internal standards. In addition to the endogenous forms of choline and betaine, their deuterated analogues as well as sodiated forms of betaine and d 9-betaine can be easily identified in the spectrum.
(a) Quantitation of choline using (+) ESI/MS. FTICR mass spectrum from m/z 100 to 150 obtained by mixing 20 nmol of d 9-choline with a single concentration of unlabeled analyte, followed by DI/12T FTICR-MS for generation of a calibration curve from the peak intensity ratios for unlabeled choline versus deuterated choline (d9-Choline). (b and c) (+) ESI DI/FTICR-MS metabolite quantitation compared to metabolite quantitation by LC/MS. Calibration curves for choline obtained by DI on a 12T FTICR-MS (b) versus LC/MS on an LCQ mass spectrometer (c). For FTICR-MS calibration, the results show the ratio of peak intensities for unlabeled choline (Cho) to deuterated choline (d-Cho) versus concentration. For LC/MS analysis, the results show the unlabeled choline to deuterated choline peak area ratio versus concentration. FTICR-MS produces correlation coefficients >0.99, comparable to LC/MS.
Figure 4b shows the regression lines obtained for the quantitation of choline based on DI/FTICR-MS, compared to a previously-reported isotope dilution ESI-LC/MS method (Koc et al. 2002). Excellent R2 values >0.99 (0.9923 for FTICR and 0.9995 for LC/MS as shown in Fig. 4b) were achieved in both cases, indicating that reliable metabolite quantitation is possible with DI/FTICR-MS. The advantage of FTICR analysis compared to LC/MS for high-throughput metabolomics experiments however, is the dramatic reduction in analysis time. Analysis of 25 samples (3 replicates/sample) required several days using a GC/MS method (Pomfret et al. 1989); and approximately one full day was required for analysis of the same number of samples using an LC/MS method (Koc et al. 2002). In contrast, the time required for analysis of the same number of samples by the DI/FTICR-MS method described here required only ca. 4 h (3 min per acquisition).
Our DI/FTICR-MS approach is also considerably more sensitive than previously-reported LC/MS methods for the analysis of these two metabolites. A 10 μl-aliquot of the original 80 μl extract was used for Koc et al.’s LC/ESI-MS analyses, while only 2 μl of a 100-fold diluted identically-prepared sample was needed for the DI/FTICR-MS experiments which yielded comparable quantitation accuracy (data not shown). Thus, 500 times less sample was required for FTICR-MS plasma metabolite experiments than was required for previously-reported LC/MS analyses using identically-prepared samples. This experiment demonstrates the potential of DI/FTICR-MS for absolute quantitation of targeted metabolites in human plasma samples (an extremely complicated matrix) using isotopically-labeled internal standards.
3.5 Metabolic fingerprinting of mouse serum
DI/FTICR-MS is particularly attractive for untargeted metabolomics because of its potential to generate profiles for quantitative comparison of a large number of metabolites, across multiple samples, in a short time. However, there are two major difficulties to be overcome for the DI/FTICR-MS quantitation of metabolites in complex samples without isotopically-labeled internal standards (and these may not be available for all the metabolites): ion suppression/enhancement in ESI, and space charging in the ICR cell. In contrast to lower-magnetic-field instruments, high-field FTICR-MS instruments provide not only the higher resolving power, but also a much reduced space-charging effects and increased ion-trapping capability (Marshall et al. 2002), thus allowing the tolerance of a larger ion population in the ICR cell. This increased ion-trapping capability of higher-field FTICR-MS improves the quantitative relationship between metabolite concentrations and their MS intensities.
Due to ionization suppression/enhancement effects, our prior experiments (data not shown) showed that DI/FTICR-MS was not a good choice for direct quantitative comparison of the endogenous metabolites in human plasma without the use of isotope-labeled internal standards. This is because there is very high degree of biological variability among individual humans, and the resulting highly-variable matrices in human plasma samples can cause inconsistent ion suppression/enhancement effects.
In contrast to humans, inbred mouse strains (because of their common genetics) have much less biological variability. We therefore tested the suitability of DI/FTICR-MS for quantitative fingerprinting of serum metabolites derived from inbred strain mice. First, we investigated how the total metabolite concentrations of mouse serum affected MS intensities of individual metabolites. Figure 5a shows the MS intensities of the 36 most abundant metabolite features (represented by their neutral monoisotopic masses) detected in positive-ion ESI, with a series of sample dilutions. It shows that with a dilution of less than 1:50 (10 μl serum extracted and diluted into 500 μl spray media), there was no quantitative relationship between the metabolite concentration and MS intensity, indicating severe ion suppression and/or the inability of efficient ICR cell ion trapping. However, when more dilute serum samples were analyzed by DI, the metabolite MS intensities were proportional to their metabolite concentrations. A dilution of 1:100 was therefore used in this study.
The suitability of DI/FTICR-MS for metabolic fingerprinting of serum samples from inbred mouse strains. (a) Effect of mouse serum dilution on MS intensities in positive ESI, indicating an optimal dilution of greater than 1:50 (v/v). A dilution of 1:100 was chosen for this metabolic fingerprinting study. (b) Linearity of standard chemicals (diphenhydramine, haloperidol, verapamil, terfenadine and reserpine) spiked as metabolite mimics into a mouse serum extract at a dilution of 1:100 (v/v), showing a linear response over a 600-fold concentration range. (c) The measurement variation of a metabolite mimic, verapamil, spiked equally into 26 serum samples from mice from 2 inbred strains involved in a TCE acute toxicity experiment, showing a relative standard deviation (RSD) of 4.5%.
Second, we investigated the linearity of metabolite quantitation under the appropriate sample dilution conditions. Figure 5b shows the linear response of 5 standard chemicals spiked as metabolite mimics into serum extracts at 1:100 dilution. All five compounds showed excellent linearity over a 600-fold range of concentration, indicating that quantitative comparison of these metabolites could be achieved by DI in mouse serum samples from inbred strains. Third, we further examined the suitability of DI/FTICR-MS for determining serum metabolites from 26 mice from 2 inbred strains involved in an acute TCE exposure experiment. Figure 5c shows the measured relative concentrations of verapamil, spiked at equal amounts into 26 serum samples as a metabolite mimic, using haloperidol as an internal standard for intensity normalization. The result shows a relative standard deviation (RSD) of 4.5%, which we believe to be equivalent to that of a typical LC/MS analysis.
Next, we prepared methanol extracts from serum samples collected from 49 mice from 5 inbred strains that had been involved in a preliminary 6-h alcohol treatment, in order to profile up- and down-regulated metabolites. The analysis of the 49 samples (including 3 technical replicates of each sample) required only 24 h of instrument time for both positive and negative ionization modes. After data processing as outlined in the data processing section, we were able to profile a total of 298 metabolites from the 49 samples in the positive ion mode over the mass range 100–900 Da (Fig. 6a), using a 3-min infusion time per sample. In the negative ion mode, we were able to profile a total of 133 metabolites in the same mass range (Fig. 6b). Altogether, by combining both positive and negative ion modes, more than 400 metabolites were detected. Among the metabolites detected in the negative ion mode, ethyl glucuronide (C8H14O7) was detected at m/z 221.06667 in most of the treated animals. This is one of the minor in vivo metabolites of ethanol resulting from glucuronidation (Neubauer 1901).
Two-dimensional metabolite features of 49 mouse serum methanol extracts analyzed by DI/FTICR-MS without prior chromatography in both (+) (a) and (−) (b) ESI modes. Blue bars represent control samples, Red bars represent treated samples. Numbers indicate individual animals (biological replicates). Three technical replicates for each biological replicate are shown. Metabolites: Red = up-regulated; Green = down-regulated; Black = median of individual relative intensities for a given metabolite across all spectra. After data processing, a total of 298 metabolites were detected in the positive ion mode across the mass range 100–900 Da, and a total of 133 metabolites were detected in the negative ion mode.
It is important to note that all of the data collection (in both positive and negative ion modes) from the mouse sera was completed within 24 h. In contrast, high performance LC/MS analysis of the same number of samples could reasonably be expected to take several days. The speed with which we were able to generate a differential metabolite profile for these samples indicates that DI/FTICR-MS is a promising tool for high-throughput toxicologically-related metabolomics studies in inbred mouse strains. The reproducibility of this technique makes it possible to use DI/FTICR-MS for relative quantitation of blood metabolites without the need of isotopically-labeled internal standards.
4 Concluding remarks
Our experiments demonstrate that ultrahigh-field FTICR-MS holds great promise in the area of qualitative metabolomics. Its unmatched mass resolution facilitates the detection of hundreds of metabolites in complex samples in the DI mode. The mass accuracy (sub-ppm) often allows the identification of low-mass metabolite candidates based on mass alone, by generating elemental compositions followed by searching the available metabolome databases.
Ultrahigh-field FTICR-MS also shows great promise for quantitative metabolomic analysis. We have demonstrated in this study that, after addition of isotopically-labeled internal standards, DI/FTICR-MS can be used for absolute metabolite quantitation, even in complicated matrices like human plasma. In cases where the elemental composition of a targeted analyte is unique, the analysis time for a single sample can be as short as 1 min by DI/FTICR-MS, thus opening up the possibility of high-throughput metabolomics. Comparable quantitation accuracies for the same choline-related metabolites were achieved by both DI and LC/MS analysis; however, the DI/FTICR-MS analysis consumed much less sample and was significantly faster.
We also demonstrated the utility of DI/FTICR-MS for rapidly generating differential metabolite profiles in samples from large studies. In a study on mouse serum samples from 49 experimental animals, we were able to detect more than 400 metabolites in approximately one day’s analysis time, several days faster than typical LC/MS methods. It should be mentioned that, though DI/FTICR-MS is an outstanding technique for rapid, high-throughput assessment of overall differences in the metabolome, it is not comprehensive and is therefore not likely to eliminate the need for chromatographic separations to achieve increased metabolome coverage and to differentiate metabolite isomers. In the case of isomers, for example, LC/MS or LC/FTICR-MS would still need to be employed in a complementary fashion.
To enhance the potential of FTICR-MS for high-throughput metabolomics, we are planning to develop a software package which will facilitate this analysis by automatic detection of differentially-abundant metabolites, followed by their automated identification via elemental composition determination and metabolome database searching. If the identification is ambiguous, MS/MS analysis by collision cell CID outside the ICR cell will also be initiated automatically.
References
Aharoni, A., Ric de Vos, C. H., Verhoeven, H. A., Maliepaard, C. A., Kruppa, G., Bino, R., Goodenowe, D. B. (2002). Nontargeted metabolome analysis by use of Fourier transform ion cyclotron mass spectrometry, Omics, 6(3), 217–234.
Allen, J., Davey, H. M., Broadhurst, D., Heald, J. K., Rowland, J. J., Oliver, S. G., Kell, D. B. (2003). High-throughput classification of yeast mutants for functional genomics using metabolic footprinting, Nature Biotechnology, 21(6), 692–696.
Belov, M. E., Anderson, G. A., Angell, N. H., Shen, Y., Tolic, N., Udseth, H. R., Smith, R. D. (2001). Dynamic range expansion applied to mass spectrometry based on data-dependent selective ion ejection in capillary liquid chromatography Fourier transform ion cyclotron resonance for enhanced proteome characterization, Analytical Chemistry, 73(21), 5052–5060.
Brown, S. C., Kruppa, G., Dasseux, J. L. (2005). Metabolomics applications of FT-ICR mass spectrometry, Mass Spectrometry Reviews, 24(2), 223–231.
Buchholz, A., Takors, R., Wandrey, C., (2001). Quantification of intracellular metabolites in Escherichia coli K12 using liquid chromatographic-electrospray ionization tandem mass spectrometric techniques, Analytical Biochemistry, 295(2), 129–137.
De Vos, R. C., Moco, S., Lommen, A., Keurentjes, J. J., Bino, R. J., Hall, R. D. (2007). Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry, Nature Protocols, 2(4), 778–791.
Denkert, C., Budczies, J., Kind, T., Weichert, W., Tablack, P., Sehouli, J., Niesporek, S., Konsgen, D., Dietel, M., Fiehn, O., (2006). Mass spectrometry-based metabolic profiling reveals different metabolite patterns in invasive ovarian carcinomas and ovarian borderline tumors, Cancer Research, 66(22), 10795–10804.
Dunn, W. B., Bailey, N. J., Johnson, H. E. (2005). Measuring the metabolome: Current analytical technologies, Analyst, 130(5), 606–625.
Eisen, M. B., Spellman, P. T., Brown, P. O., Botstein, D., (1998). Cluster analysis and display of genome-wide expression patterns, Proceedings of the National Academy of Sciences, 95(25), 14863–14868.
Fahy, E., Sud, M., Cotter, D., Subramaniam, S., (2007). LIPID MAPS online tools for lipid research, Nucleic Acids Research, 35(Web Server issue), W606–W612.
Fan, T. W., Lane, A. N., Higashi, R. M. (2004). The promise of metabolomics in cancer molecular therapeutics, Current Opinion in Molecular Therapeutics, 6(6), 584–592.
Fiehn, O., Kloska, S., Altmann, T., (2001). Integrated studies on plant biology using multiparallel techniques, Current Opinion in Biotechnology, 12(1), 82–86.
Galinski, E. A. (1995). Osmoadaptation in bacteria, Advances in Microbial Physiology, 37, 272–328.
Gamache, P. H., Meyer, D. F., Granger, M. C., Acworth, I. N. (2004). Metabolomic applications of electrochemistry/mass spectrometry, American Society for Mass Spectrometry, 15(12), 1717–1726.
Goodacre, R., Vaidyanathan, S., Dunn, W. B., Harrigan, G. G., Kell, D. B. (2004). Metabolomics by numbers: Acquiring and understanding global metabolite data, Trends in Biotechnology, 22(5), 245–252.
Halket, J. M., Przyborowska, A., Stein, S. E., Mallard, W. G., Down, S., Chalmers, R. A. (1999). Deconvolution gas chromatography/mass spectrometry of urinary organic acids–potential for pattern recognition and automated identification of metabolic disorders, Rapid Communications in Mass Spectrometry, 13(4), 279–284.
Hirai, M. Y., Yano, M., Goodenowe, D. B., Kanaya, S., Kimura, T., Awazuhara, M., Arita, M., Fujiwara, T., Saito, K., (2004). Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana, Proceedings of the National Academy of Sciences, 101(27), 10205–10210.
Hughey, C. A., Rodgers, R. P., Marshall, A. G. (2002). Resolution of 11,000 compositionally distinct components in a single electrospray ionization Fourier transform ion cyclotron resonance mass spectrum of crude oil, Analytical Chemistry, 74(16), 4145–4149.
Jones, O. A., Spurgeon, D. J., Svendsen, C., Griffin, J. L. (2007). A metabolomics based approach to assessing the toxicity of the polyaromatic hydrocarbon pyrene to the earthworm Lumbricus rubellus. Chemosphere [Epub ahead of print].
Kind, T., Fiehn, O., (2006). Metabolomic database annotations via query of elemental compositions: Mass accuracy is insufficient even at less than 1 ppm, BMC Bioinformatics, 7, 234.
Kind, T., Fiehn, O., (2007). Seven Golden Rules for heuristic filtering of molecular formulas obtained by accurate mass spectrometry, BMC Bioinformatics, 8, 105.
Koc, H., Mar, M. H., Ranasinghe, A., Swenberg, J. A., Zeisel, S. H. (2002). Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry, Analytical Chemistry, 74(18), 4734–4740.
Kolch, W., Mischak, H., Pitt, A. R. (2005). The molecular make-up of a tumour: Proteomics in cancer research, Clinical Science (London), 108(5), 369–383.
Lee, S. H., Woo, H. M., Jung, B. H., Lee, J., Kwon, O. S., Pyo, H. S., Choi, M. H., Chung, B. C. (2007). Metabolomic approach to evaluate the toxicological effects of nonylphenol with rat urine, Analytical Chemistry, 79(16), 6102–6110.
Marshall, A. G., Hendrickson, C. L., Shi, S. D. (2002). Scaling MS plateaus with high-resolution FT-ICRMS, Analytical Chemistry, 74(9), 252A–259A.
Nakamura, Y., Kimura, A., Saga, H., Oikawa, A., Shinbo, Y., Kai, K., Sakurai, N., Suzuki, H., Kitayama, M., Shibata, D., Kanaya, S., & Ohta, D., (2007). Differential metabolomics unraveling light/dark regulation of metabolic activities in Arabidopsis cell culture. Planta, 227(1), 57–66.
Neubauer, O., (1901). Archiv, Fur Experimentelle Pathologie und Pharmakologie, 46, 133–154.
Nicholson, J. K., Lindon, J. C., Holmes, E., (1999). ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data, Xenobiotica, 29(11), 1181–1189.
Ogata, H., Goto, S., Sato, K., Fujibuchi, W., Bono, H., Kanehisa, M., (1999). KEGG: Kyoto Encyclopedia of Genes and Genomes, Nucleic Acids Research, 27(1), 29–34.
Ohta, D., Shibata, D., Kanaya, S., (2007). Metabolic profiling using Fourier-transform ion-cyclotron-resonance mass spectrometry, Analytical and Bioanalytical Chemistry, 389(5), 1469–1475.
Plumb, R., Castro-Perez, J., Granger, J., Beattie, I., Joncour, K., Wright, A., (2004). Ultra-performance liquid chromatography coupled to quadrupole-orthogonal time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 18(19), 2331–2337.
Pomfret, E. A., daCosta, K. A., Schurman, L. L., Zeisel, S. H. (1989). Measurement of choline and choline metabolite concentrations using high-pressure liquid chromatography and gas chromatography-mass spectrometry. Analytical Biochemistry, 180(1), 85–90.
Saldanha, A. J. (2004). Java Treeview–extensible visualization of microarray data, Bioinformatics, 20(17), 3246–3248.
Sato, S., Soga, T., Nishioka, T., Tomita, M., (2004). Simultaneous determination of the main metabolites in rice leaves using capillary electrophoresis mass spectrometry and capillary electrophoresis diode array detection, Plant Journal, 40(1), 151–163.
Soga, T., Baran, R., Suematsu, M., Ueno, Y., Ikeda, S., Sakurakawa, T., Kakazu, Y., Ishikawa, T., Robert, M., Nishioka, T., Tomita, M., (2006). Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption, Journal of Biological Chemistry, 281(24), 16768–16776.
Southam, A. D., Payne, T. G., Cooper, H. J., Arvanitis, T. N., Viant, M. R. (2007). Dynamic range and mass accuracy of wide-scan direct infusion nanoelectrospray Fourier transform ion cyclotron resonance mass spectrometry-based metabolomics increased by the spectral stitching method, Analytical Chemistry, 79(12), 4595–4602.
Staack, R. F., Varesio, E., Hopfgartner, G., (2005). The combination of liquid chromatography/tandem mass spectrometry and chip-based infusion for improved screening and characterization of drug metabolites, Rapid Communications in Mass Spectrometry, 19(5), 618–626.
Sud, M., Fahy, E., Cotter, D., Brown, A., Dennis, E. A., Glass, C. K., Merrill, A. H., Jr., Murphy, R. C., Raetz, C. R., Russell, D. W., Subramaniam, S., (2007). LMSD: LIPID MAPS structure database. Nucleic Acids Research, 35(Database issue), D527–D532.
Tohge, T., Nishiyama, Y., Hirai, M. Y., Yano, M., Nakajima, J., Awazuhara, M., Inoue, E., Takahashi, H., Goodenowe, D. B., Kitayama, M., Noji, M., Yamazaki, M., Saito, K., (2005). Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor, Plant Journal, 42(2), 218–235.
Want, E. J., O’Maille, G., Smith, C. A., Brandon, T. R., Uritboonthai, W., Qin, C., Trauger, S. A., Siuzdak, G., (2006). Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry, Analytical Chemistry, 78(3), 743–752.
Wikoff, W. R., Gangoiti, J. A., Barshop, B. A., Siuzdak, G., (2007). Metabolomics identifies perturbations in human disorders of propionate metabolism. Clinical Chemistry. [Epub ahead of print].
Wishart, D. S., Tzur, D., Knox, C., Eisner, R., Guo, A. C., Young, N., Cheng, D., Jewell, K., Arndt, D., Sawhney, S., Fung, C., Nikolai, L., Lewis, M., Coutouly, M. A., Forsythe, I., Tang, P., Shrivastava, S., Jeroncic, K., Stothard, P., Amegbey, G., Block, D., Hau, D. D., Wagner, J., Miniaci, J., Clements, M., Gebremedhin, M., Guo, N., Zhang, Y., Duggan, G. E., Macinnis, G. D., Weljie, A. M., Dowlatabadi, R., Bamforth, F., Clive, D., Greiner, R., Li, L., Marrie, T., Sykes, B. D., Vogel, H. J., Querengesser, L., (2007). HMDB: The human metabolome database. Nucleic Acids Research, 35 (Database issue), D521–D526.
Acknowledgements
The authors would like to thank Dr. David Robinette for his assistance with the preparation of the manuscript. This study was partially funded by a gift from an anonymous donor to support research in proteomics at UNC, by NIH grants P30 CA0-16086, 1S10RR16776, caBIG-ICR-07-20-01, R01 AA016258, and P30 Es010126, by collaboration with Bruker Daltonics, and by start-up funds from the University of Victoria. We also acknowledge the use of the 12-Tesla FTICR mass spectrometer at the UNC-Duke Michael Hooker Proteomics Center, NC (NIH 1-S10-RR019889-01 and NCBC 2005-IDG-1015) for some of these experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, J., Danell, R.M., Patel, J.R. et al. Towards high-throughput metabolomics using ultrahigh-field Fourier transform ion cyclotron resonance mass spectrometry. Metabolomics 4, 128–140 (2008). https://doi.org/10.1007/s11306-008-0104-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11306-008-0104-8