Abstract
Ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) are cell surface-located transmembrane ecto-enzymes of the CD39 superfamily which regulate inflammation and tissue repair by catalyzing the phosphohydrolysis of extracellular nucleotides and modulating purinergic signaling. In the liver, NTPDase2 is reportedly expressed on portal fibroblasts, but its functional role in regulating tissue regeneration and fibrosis is incompletely understood. Here, we studied the role of NTPDase2 in several models of liver injury using global knockout mice. Liver regeneration and severity of fibrosis were analyzed at different time points after exposure to carbon tetrachloride (CCl4) or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) or partial hepatectomy in C57BL/6 wild-type and globally NTPDase2-deficient (Entpd2 null) mice. After chronic CCl4 intoxication, Entpd2 null mice exhibit significantly more severe liver fibrosis, as assessed by collagen content and histology. In contrast, deletion of NTPDase2 does not have a substantial effect on biliary-type fibrosis in the setting of DDC feeding. In injured livers, NTPDase2 expression extends from the portal areas to fibrotic septae in pan-lobular (CCl4-induced) liver fibrosis; the same pattern was observed, albeit to a lesser extent in biliary-type (DDC-induced) fibrosis. Liver regeneration after partial hepatectomy is not substantively impaired in global Entpd2 null mice. NTPDase2 protects from liver fibrosis resulting from hepatocellular injury induced by CCl4. In contrast, Entpd2 deletion does not significantly impact fibrosis secondary to DDC injury or liver regeneration after partial hepatectomy. Our observations highlight mechanisms relating to purinergic signaling in the liver and indicate possible therapeutic avenues and new cellular targets to test in the management of hepatic fibrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver fibrosis is the common pathological consequence of various chronic liver diseases that ultimately leads to cirrhosis and liver failure. This disease state is characterized by the excessive production of extracellular matrix predominantly generated by myofibroblastic cells that stem from hepatic stellate cells (HSCs) and possibly also by portal fibroblasts [1]. Irrespective of their cellular origin, these myofibroblasts are activated and regulated via a multitude of signaling pathways, inclusive of those involving intracellular (cyclic adenosine monophosphate, cAMP) and extracellular purinergic mediators, such as adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine [2].
The concentrations of extracellular nucleotides and nucleosides are tightly regulated by ecto-nucleotide triphosphate diphosphohydrolases (E-NTPDases), a family of transmembrane proteins expressed on the cell surface. NTPDases catalyze the phosphohydrolysis of extracellular ATP to ADP and that of ADP to AMP. AMP is then converted to adenosine by CD73.
Also known as CD39, NTPDase1 is the best characterized NTPDase. It is expressed on endothelium and immune cells and plays important roles in immunoregulation and thromboregulation [3,4,5]. Mice globally null for CD39 exhibit impaired liver regeneration after partial hepatectomy [6].
In contrast, NTPDase2 is expressed predominantly on perivascular cells, glial cells, and periportal fibroblasts in the liver [7,8,9]. This ecto-enzyme is primarily an ATPase with minimal ADPase activity, which results in the accumulation of ADP in the extracellular milieu [10].
In vitro co-incubation studies have linked the expression of NTPDase2 on periportal fibroblasts to the regulation of cholangiocyte proliferation, a process possibly implicated in liver regeneration and fibrosis [11]. In this context, it has been hypothesized that NTPDase2 may inhibit excessive bile duct proliferation by scavenging extracellular ATP, which is an important ligand of basolateral P2Y receptors of cholangiocytes that promotes their proliferation [11].
The contribution of these portal fibroblasts to the myofibroblast population that produces extracellular matrix in fibrosis remains controversial [12, 13]. A recent study describes a population of portal myofibroblasts that promote liver cirrhosis via release of pro-angiogenic microvesicles [14]. These observations raise the question whether NTPDase2 is involved in regulating liver fibrosis via its expression on portal myofibroblasts.
Current insights into NTPDase2 function in liver pathophysiology have been largely limited to descriptive immunohistochemistry analysis and in vitro studies. To better define the functional role of NTPDase2 in liver regeneration and fibrosis, we subjected mice with global NTPDase2 deletion (Entpd2 null) to well-characterized models of liver injury and regeneration.
Methods
Animals
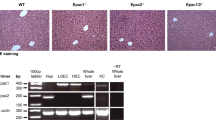
The generation of global NTPDase2-deficient (Entpd2 null) mice on a C57BL/6 background has been described previously [15]. For all experiments, we used 8- to 12-week-old male mice with age-, sex-, and strain-matched wild-type mice as controls (Taconic, Hudson, NY).
Partial hepatectomy
Animals were anesthetized with ketamine and xylazine. Approximately two thirds of the liver was resected as described by Mitchell and Willenbring [16]. Briefly, the liver was exposed by a median laparotomy, and the left lobe and the median lobes were individually ligated and resected with a 6-0 silk ligature. Meloxicam was used for post-surgical analgesia. Mice were euthanized for tissue harvest at 24 h, 48 h, 72 h, and 5 days after surgery (n ≥ 5 per genotype and time point). For sham hepatectomy (n = 3 per genotype and time point), mice received the same treatment, including anesthesia, laparotomy, manipulation of the liver, and surgical closure of the abdomen, without ligation and resection of any liver lobes.
Carbon tetrachloride treatment
Liver fibrosis was induced in wild-type and Entdp2 null mice, according to an optimized escalating dose regimen of carbon tetrachloride (CCl4) (Sigma-Aldrich, St. Louis, MO) in corn oil by oral gavage three times per week for 2 and 6 weeks, as previously established [17]. Control mice of each genotype received corn oil by gavage for the same amount of time (n = 2 per genotype and time point). Mice were euthanized at 2 weeks (n = 9 wild-type and n = 6 Entpd2 null mice) and 6 weeks (n = 14 wild-type and n = 6 Entpd2 null mice) for tissue harvest and fibrosis assessment.
3,5-Diethoxycarbonyl-1,4-dihydrocollidine feeding
For induction of progressive cholangitis with subsequent biliary fibrosis, Entpd2 null (n = 6) and wild-type control (n = 5) mice were fed with a diet supplemented with 0.1% 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) (Sigma-Aldrich, Research Diets) for 3 weeks [18]. Mice on standard chow were used as controls.
Measurement of hepatic collagen content
Hepatic collagen deposition was determined biochemically by measuring the hydroxyproline content. In short, 250–300 mg liver samples underwent complete acid hydrolysis in 5 ml of 6 N hydrochloric acid (HCl) at 110 °C for 16 h, then hydroxyproline was quantitated photometrically as described [19].
RNA isolation and real-time quantitative polymerase chain reaction
Liver tissue was snap frozen in liquid nitrogen after harvest and stored at − 80 °C until further processing. The tissue was homogenized with TRIzol® Reagent (Life Technologies, Carlsbad, CA) to isolate RNA according to the manufacturer’s protocol and transcribed to cDNA using the iScript™ cDNA Synthesis kit (Bio-Rad, Hercules, CA). Real-time quantitative polymerase chain reaction (qPCR) was performed on a Stratagene Mx3005P (Agilent Technologies) using standard protocols. The following Entpd2 primers were used as published previously [6]: Fw: TGACTGCCAACTACCTGCTG and Rev.: CCGCAAATGGACCTCATTAT.
Immunohistochemistry
Formalin-fixed, paraffin-embedded, or frozen murine liver tissues were cut into 6-μm sections. One slide from each block was stained by hematoxylin and eosin for morphological analysis. For immunohistochemistry, sections were fixed in acetone and blocked with 7% horse serum (Vector Laboratories, Burlingame, CA) for 0.5 h. The tissues were first incubated with primary antibodies overnight at 4 °C. Primary antibodies were rabbit polyclonal antimouse NTPDase2 (http://ectonucleotidases-ab.com, as characterized previously [20]), Thy-1 (Abcam, Cambridge, UK), Ki67 (Dako, Santa Clara, CA), and A6 (kind gift from V. Factor, NIH). After peroxidase and biotin activity blocking, sections were incubated with the biotinylated secondary antibody for 1 h (goat polyclonal antirabbit IgG, Vector Laboratories), continued with avidin-biotin complex HRP, and visualized with ImmPACT DAB (Vector Laboratories). All slides were mounted on Cytoseal and examined and recorded on a Nikon microscope. For fluorescent double staining, we used the respective fluorescent secondary antibodies. Sections were co-stained with Hoechst and covered with polyvinyl alcohol mounting medium (Sigma-Aldrich) and examined on a Nikon multiphoton fluorescent microscope.
Statistical analysis
All statistical analyses were performed with GraphPad Prism 5. Two-tailed Student’s t test was used to test for the significant difference between groups.
Results
Hepatic expression of NTPDase2 increases after liver injury and induction of liver fibrosis
NTPDase2 expression in the normal liver is limited to periportal areas, where a high level of expression is found on perivascular cells and periductular cells, resembling periportal myofibroblasts (Fig. 1a), in agreement with prior studies [7, 9].
NTPDase2 expression in normal liver and liver fibrosis. a NTPDase2 in control liver. b NTPDase2 after 2 weeks of CCl4 treatment. c NTPDase2 after 6 weeks of CCl4 treatment. d NTPDase2 after 3 weeks of DDC feeding. Scale bars show 100 μm. Entpd2 null samples are shown in a, b, and d as controls for antibody specificity
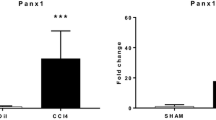
Following partial hepatectomy, the expression patterns of NTPDase2 do not substantively change at the protein level (data not shown), but NTPDase2 expression levels as measured by real-time qPCR are significantly upregulated, reaching median 3.5-fold increments over those of sham-operated control on day 5 (p < 0.005) (Fig. 2a). After induction of fibrosis, by both CCl4 treatment and DDC feeding, the hepatic expression of NTPDase2 as measured by real-time qPCR is also significantly higher when compared to that of untreated controls (CCl4, 3 ± 0.6 vs. 1.2 ± 0.3, p < 0.001; DDC, 11.9 ± 6.6 vs. ± 1.2 ± 0.8, p = 0.015) (Fig. 2b, c).
Entpd2 is upregulated in liver tissue in different models of hepatic injury. Entpd2 expression by real-time qPCR in wild-type whole liver tissue, relative to one sham-operated control liver. a After partial hepatectomy. b After 6 weeks of CCl4 treatment. c After 3 weeks of DDC feeding. Error bars represent standard deviation. *p < 0.05; **p < 0.01; ***p < 0.001. n ≥ 5 per treatment group and time point
In the healthy wild-type liver, NTPDase2 expression is limited to a thin line around the portal vein and bile duct area (Fig. 1a). However, after 2 weeks of treatment with CCl4, evolving fibrotic areas start expressing NTPDase2 beyond the portal triads, in a more diffuse pattern (Fig. 1b). After 6 weeks, there is a distinct expression of NTPDase2 within the fibrotic areas between portal triads, on or around smaller groups of cells that appear to be surrounding proliferating bile duct cells or newly forming pseudo-ducts (Fig. 1c).
After 3 weeks of DDC feeding, there is a strong expression of NTPDase2 within the thickened portal areas, but we do not see a broader expansion of NTPDase2 expression into the areas between portal fields as noted in the CCl4-treated livers (Fig. 1d).
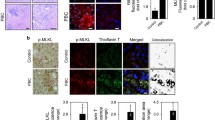
To better understand the altered expression pattern of NTPDase2 in CCl4-induced fibrosis, we performed co-immunostaining of NTPDase2 and Thy-1, a marker of portal fibroblasts in a healthy liver (Fig. 3a) and CCl4-induced fibrosis (Fig. 3b). The staining for both NTPDase2 and Thy-1 demonstrates a similar pattern with a substantial overlap around the portal structures, consistent with portal fibroblasts.
NTPDase2 expression extends into scar tissue in fibrosis but is still predominant on portal fibroblasts. a Co-immunostaining of healthy wild-type liver tissue of NTPDase2 (red) with Thy-1 (green), a marker of portal fibroblasts. b After 6 weeks of CCl4 treatment. c Co-immunostaining of healthy wild-type liver tissue of NTPDase2 (red) with A6 (green), a marker of bile duct epithelia and hepatic progenitor cells. d After 6 weeks of CCl4 treatment
In fibrotic livers, both NTPDase2- and Thy-1-positive cells extend further into the parenchyma and fibrous tissue around the portal fields, with a sustained overlap of both markers. Selected single cells and groups of comparable-type cells also display staining for Thy-1, without NTPDase2 (Fig. 3b, asterisk). Thy-1 is known to be also expressed by oval cells. To confirm that the co-expression of NTPDase2 and Thy-1 is limited to portal fibroblasts, we added co-immunostaining of NTPDase2 with A6, an oval cell marker that is also expressed on bile duct epithelia. NTPDase2-positive cells surround these A6-positive cells and are in close proximity. However, in both healthy liver (Fig. 3c) and fibrotic livers after 6 weeks of induction with CCl4 (Fig. 3d), there is no overlap between NTPDase2 and A6, suggesting a lack of NTPDase2 expression on both bile duct epithelium and hepatic progenitor cells.
Deletion of NTPDase2 does not impair normal liver function or liver regeneration after partial hepatectomy
Entpd2 null mice show normal development and normal liver morphology and function, when compared to wild-type mice. In a model of two-thirds hepatectomy, liver weight was restored to 86 ± 11% of the original weight by day 5, which was comparable to that seen in wild-type mice (94 ± 6%, not significant) (Fig. 4a). Proliferation rates as determined by the percentage of Ki67-positive cells do not show a significant difference at the examined time points (Fig. 4b, c).
NTPDase2 deletion does not impair liver regeneration after partial hepatectomy. a Regeneration of liver weight as the percentage of original weight. b Proliferation rate as the percentage of Ki67-positive cells out of all nucleated cells. c Ki67 staining in wild-type vs. Entpd2 null livers. Error bars represent standard deviation. n ≥ 5 per genotype and time point
NTPDase2 is protective in CCl4-induced liver fibrosis
After experimental fibrosis induction with CCl4, histological staining of collagen with Sirius Red exhibits a pattern compatible with more advanced liver fibrosis in Entpd2 null mice when compared to wild-type mice (Fig. 5a). In keeping with histological observations, Entpd2 null mice show more advanced fibrosis when compared to wild-type mice, as determined by biochemical analysis of hydroxyproline content (Fig. 5b).
NTPDase2 is protective in CCl4-induced liver fibrosis. a Sirius Red staining of hepatic collagen in wild-type vs. Entpd2 null livers after treatment with vehicle or CCl4. Scale bars show 100 μm. b Hydroxyproline content relative to liver weight. Error bars represent standard deviation. *p < 0.05; **p < 0.01. n ≥ 6 per genotype and time point for CCl4 treatment; n ≥ 2 per genotype and time point for vehicle treatment
This difference was significant after 2 weeks of CCl4 treatment with a hepatic hydroxyproline concentration of 35.7 ± 2.9 μg/100 mg liver in Entpd2 null mice compared to that of 29.3 ± 4.8 μg/100 mg liver in wild-type mice (p = 0.01) and even more pronounced after 6 weeks (45.5 ± 4.1 μg/100 mg liver vs. 32.6 ± 8.2 μg/100 mg liver, p = 0.002).
NTPDase2 does not significantly impact biliary fibrosis induced by DDC.
After 3 weeks of DDC feeding, both Entpd2 null and wild-type mice developed cholestasis and liver fibrosis. Histological assessment of connective tissue staining showed a similar degree of cholangitis and fibrosis (Fig. 6a). There were no significant differences in hydroxyproline content as a measurement of hepatic collagen production between Entpd2 null and wild-type mice (46.8 ± 5.7 vs. 42.6 ± 7.6 μg/100 mg liver, ns) (Fig. 6b).
NTPDase2 deletion has a minimal impact on DDC-induced biliary fibrosis. a Sirius Red staining of hepatic collagen in wild-type vs. Entpd2 null livers after 3 weeks of DDC feeding. Scale bars show 100 μm. b Hydroxyproline content relative to liver weight. No significant difference between wild-type and Entpd2 null mice after 3 weeks of DDC feeding. Error bars represent standard deviation. n = 5 per genotype and time point
We did not observe a significant difference in the rate of bile duct proliferation between wild-type and Entpd2 null livers, using cytokeratin 19 (Ck19) or Ki67 staining as markers. There was a trend toward more bile duct proliferation in Entpd2 null mice, when using both markers (Supplementary Fig. 1).
The overall number of myofibroblasts and portal fibroblasts is similar between wild-type and Entpd2 null livers in fibrosis
To test if the composition of myofibroblast subpopulations might be altered by different levels of NTPDase2 expression, we determined the expression of α-smooth muscle actin (α-SMA) as a general marker for myofibroblasts.
There was no significant difference in the overall expression of α-SMA in fibrotic livers with or without deletion of NTPDase2 (Supplementary Figure 2a). The pattern of Thy-1 expression as a marker of the portal fibroblast-derived subpopulation of myofibroblasts is also comparable between fibrotic wild-type and Entpd2 null liver tissues (Supplementary Figure 2b).
Discussion
Purinergic signaling mediates diverse physiological and pathological processes in the liver, including inflammation, repair, and regeneration [21]. In basal states, extracellular concentrations of ATP are in the nanomolar range, but levels can rise through release from cells or platelets in response to stress [3]. Within 2 h after toxic liver injury, a marked increase in extracellular ATP can be observed in the liver [22]. These pro-inflammatory signals are rapidly dampened by NTPDases and other ecto-nucleotidases expressed on the plasma membrane.
In the liver, CD39 (NTPDase1) is expressed on Kupffer cells, other sinusoidal immune cells, and vascular endothelium and is known to have immunoregulatory functions in inflammatory diseases, such as autoimmune hepatitis [23]. In contrast to CD39, NTPDase2 expression in the healthy liver is substantially less abundant and limited to periductal and perivascular fibrocytic cells in portal areas. The pattern of localization infers a possible link to bile duct pathophysiology. This supposition would be in keeping with observations of an antiproliferative effect of NTPDase2-positive portal fibroblasts on cholangiocytes in vitro, which can be abolished after pharmacological inhibition of P2Y receptors or knockdown of NTPDase2 [11].
We have previously shown that CD39 expression on endothelial and myeloid cells facilitates liver regeneration by modulating VEGF and P2Y receptor signaling in sinusoidal endothelial cells [6] and by increasing hematopoietic stem cell recruitment to the liver [24]. We expected to observe a similar pro-regenerative effect of the portal fibroblast expression of NTPDase2.
However, our study demonstrates that the role of NTPDase2 in liver injury is more complex. After partial hepatectomy, we did not observe an impaired liver regeneration, nor was there endothelial cell damage in Entpd2 null mice as noted in Cd39 null mice. Furthermore, the percentage of proliferating cells was not altered in Entpd2 null mice. Two-thirds hepatectomy, an experimental model commonly used to induce liver regeneration, does not trigger a considerable ductular reaction in the early days, as regeneration here is largely driven by hepatocyte proliferation [25]. The regulation of extracellular nucleotides by NTPDase2 in its specific, spatially confined periportal/periductal compartment seems to play a dispensable role for the stimulation of hepatocyte proliferation in this classic model of liver regeneration.
Intriguingly, we observed a rather significant protective effect of NTPDase2 in a model of hepatocellular injury induced by CCl4. Mice deficient in NTPDase2 show significantly faster progression of liver fibrosis, when compared to wild-type mice. NTPDase2-mediated protection in CCl4-induced fibrosis may have several explanations. Extracellular ATP has been shown to have pro-fibrotic effects via activation of P2Y receptors on hepatic stellate cells [26]. Hence, both CD39 and NTPDase2 can, in theory, be protective, as these ecto-enzymes catabolize ATP in the extracellular space. However, NTPDase2 has a lower ADPase activity compared to CD39, leading to a relative accumulation of ADP, slower conversion to AMP, and ultimately lower generation of adenosine from ATP [10]. Adenosine can activate stellate cells through A2A receptors to induce collagen production [27, 28]. Therefore, unlike CD39 and its potentially dualistic impact on liver fibrosis, NTPDase2 may exert an antifibrotic effect—despite the more limited expression—as the specific ecto-enzymatic activity does not readily lead to the generation of adenosine.
Deletion of NTPDase2 and subsequent decreases in local extracellular ADP concentrations over boosted ATP levels might involve changes in the rate of platelet sequestration and/or alterations in the desensitization of sinusoidal, biliary, or hepatocyte P2Y receptors. Decreases in platelet recruitment would be expected to limit fibrogenesis [29], but this is not what is observed with Entpd2 deletion. Alternatively, others and we have demonstrated extracellular nucleotide-mediated P2Y receptor desensitization in the Cd39 null mouse, e.g., in the context of platelet activation or smooth muscle contraction of the vas deferens [3, 30, 31]. In an analogous manner to aberrant desensitization responses in the Entpd2 null mouse, inhibition of P2Y receptors by pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS) has been shown to prevent liver fibrosis induced by CCl4 [27].
Furthermore, although NTPDase2 expression in normal state is limited to the portal areas, its biological activity via paracrine signaling can extend further into the liver parenchyma. This is particularly so with liver injury, for example after CCl4 treatment, as we could show that NTPDase2-positive cells spread into fibrotic areas and may have a more significant impact on levels of ATP in the extracellular milieu.
Such a mechanism may explain the pronounced effect of NTPDase2 deletion in CCl4-mediated fibrosis whereas after partial hepatectomy, NTPDase2 expression is limited to periportal areas, and the effect is negligible. Indeed, after induction of liver fibrosis, we observed a much broader NTPDase2 expression pattern on cells penetrating the area of fibrosis. These cells co-express Thy-1, suggesting these are at least, in part, comprised of portal fibroblasts. These NTPDase2-expressing cells are, however, distinct from cells expressing A6, a marker of oval cells and bile duct epithelium.
When testing whole liver tissue, we found significant upregulation of Entpd2 mRNA after partial hepatectomy and in both hepatotoxic (CCl4) and biliary (DDC) models of fibrosis. Interestingly, others have reported changes after CCl4-induced liver injury, albeit with downregulation of Entpd2 in the setting of late bile duct ligation (BDL) [32, 33]. It has to be noted that the latter were studies performed in rats and that portal fibroblasts were isolated first and RNA was extracted selectively from this cell population, while we used whole liver tissue to encompass other cell types, including stellate cells, which have been described to express NTPDase2 depending on their activation state [26].
A recent study by Lua et al. investigated isolated portal fibroblasts from healthy mouse livers and different models of liver fibrosis including CCl4 and DDC. These authors noted that portal fibroblasts significantly downregulated Entpd2 expression compared to sham controls in all types of liver fibrosis [13]. Since we have here determined Entpd2 expression in whole liver tissue, as opposed to isolated portal fibroblasts, this discrepancy could be explained by either upregulation of Entpd2 on different cell types after fibrosis induction or a significant increase in the number of portal fibroblasts with lower expression levels in individual cells.
As NTPDase2 was previously shown to be downregulated in bile duct ligated liver tissue, and given the higher mortality and inconsistency of this experimental model [34], we chose DDC feeding as an additional model for liver fibrosis induced by cholestasis and biliary inflammation [35]. We demonstrate that Entpd2 is upregulated in DDC-induced liver injury. Interestingly, distinct from CCl4-induced fibrosis, NTPDase2 deficiency does not seem to significantly impact biliary-type fibrosis induced by DDC feeding. Our data do not rule out smaller effects that would require a larger number of experimental animals to discern. In fact, we see a consistent trend toward more bile duct proliferation in Entpd2 null mice after DDC feeding using both Ck19 and Ki67 as markers, as well as a trend toward more fibrosis in Entpd2 null mice. Nonetheless, these trends do not reach statistical significance, suggesting the impact of NTPDase2 in the repair and regeneration from this type of biliary injury is less relevant than that in hepatocellular injury induced by CCl4. This is consistent with the observation that, in a manner different from the CCl4 model, we do not see the spreading of NTPDase2 expression into the fibrotic parenchyma after DDC injury.
Our observations highlight the plasticity and the complexity of E-NTPDase-mediated purinergic signaling in liver injury. While NTPDase2 shows a strong protective effect in the hepatotoxic model of CCl4, most likely due to heightened expression into the liver parenchyma encompassing myofibroblasts in the sinusoids, the effect in biliary inflammation and fibrosis, as induced by DDC, is less pronounced. One possible explanation lies in the different pathological pathways of both models with massive hepatocyte death occurring after CCl4 treatment, leading to a substantial release of ATP into the extracellular space when compared to a relatively low rate of cell death in biliary fibrosis secondary to DDC feeding. The effect of NTPDase2 as an ATP-scavenging enzyme can be assumed to be more relevant in the first context.
Furthermore, our results suggest a redundancy of function between different E-NTPDases: in liver regeneration following hepatectomy, a loss of NTPDase2 may be possibly compensated for by CD39 that is more abundantly expressed in the liver. The latter observation is particularly interesting for potential pharmacological treatment options for inflammatory and fibrotic liver disease targeting mediators of purinergic signaling. It appears that bolstering both CD39 and NTPDase2 in a combinational manner would have a strong protective effect in liver inflammation and fibrosis.
Taken together, we evaluated the role of NTPDase2 in liver regeneration and fibrosis in various mouse models. Our results demonstrate that despite high-level expression in the periportal area in a normal liver, NTPDase2 does not significantly impact liver regeneration or fibrosis secondary to biliary injury. Rather, NTPDase2 is protective in fibrosis caused by hepatocellular injury likely due to its unique substrate preference and altered patterns of expression following liver injury.
References
Dranoff JA, Wells RG (2010) Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology 51:1438–1444. https://doi.org/10.1002/hep.23405
Lu D, Insel PA (2014) Cellular mechanisms of tissue fibrosis. 6. Purinergic signaling and response in fibroblasts and tissue fibrosis. Am J Physiol Cell Physiol 306:88. https://doi.org/10.1152/ajpcell.00381.2013
Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS et al (1999) Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5:1010–1017. https://doi.org/10.1038/12447.
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A et al (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265. https://doi.org/10.1084/jem.20062512
Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I et al (2013) IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 14:1054–1063. https://doi.org/10.1038/ni.2695
Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E et al (2008) Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology 135:1751–1760. https://doi.org/10.1053/j.gastro.2008.07.025
Dranoff JA, Kruglov EA, Robson SC, Braun N, Zimmermann H, Sévigny J (2002) The ecto-nucleoside triphosphate diphosphohydrolase NTPDase2/CD39L1 is expressed in a novel functional compartment within the liver. Hepatology 36:1135–1144. https://doi.org/10.1053/jhep.2002.36823
Wink MR, Braganhol E, Tamajusuku ASK, Lenz G, Zerbini LF, Libermann TA et al (2006) Nucleoside triphosphate diphosphohydrolase-2 (NTPDase2/CD39L1) is the dominant ectonucleotidase expressed by rat astrocytes. Neuroscience 138:421–432. https://doi.org/10.1016/j.neuroscience.2005.11.039
Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I et al (2002) Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood 99:2801–2809
Heine P, Braun N, Heilbronn A, Zimmermann H (1999) Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem 262:102–107
Jhandier MN, Kruglov EA, Lavoie EG, Sévigny J, Dranoff JA (2005) Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J Biol Chem 280:22986–22992. https://doi.org/10.1074/jbc.M412371200
Iwaisako K, Jiang C, Zhang M, Cong M, Moore-Morris TJ, Park TJ et al (2014) Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci U S A 111:E3297–E3305. https://doi.org/10.1073/pnas.1400062111.
Lua I, Li Y, Zagory JA, Wang KS, French SW, Sévigny J et al (2016) Characterization of hepatic stellate cells, portal fibroblasts, and mesothelial cells in normal and fibrotic livers. J Hepatol. https://doi.org/10.1016/j.jhep.2016.01.010
Lemoinne S, Cadoret A, Rautou P-E, El Mourabit H, Ratziu V, Corpechot C et al (2015) Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 61:1041–1055. https://doi.org/10.1002/hep.27318
Gampe K, Stefani J, Hammer K, Brendel P, Pötzsch A, Enikolopov G et al (2014) NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells. https://doi.org/10.1002/stem.1846
Mitchell C, Willenbring H (2008) A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 3:1167–1170. https://doi.org/10.1038/nprot.2008.80
Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL et al (2011) Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology 140:1642–1652. https://doi.org/10.1053/j.gastro.2011.01.040
Fickert P, Stöger U, Fuchsbichler A, Moustafa T, Marschall H-U, Weiglein AH et al (2007) A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol 171:525–536. https://doi.org/10.2353/ajpath.2007.061133
Popov Y, Patsenker E, Bauer M, Niedobitek E, Schulze-Krebs A, Schuppan D (2006) Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFkappaB. J Biol Chem 281:15090–15098. https://doi.org/10.1074/jbc.M600030200.
Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE (2006) Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol 497:1–12. https://doi.org/10.1002/cne.20954.
Burnstock G, Vaughn B, Robson SC (2014) Purinergic signalling in the liver in health and disease. Purinergic Signal 10:51–70. https://doi.org/10.1007/s11302-013-9398-8
Ayata CK, Ganal SC, Hockenjos B, Willim K, Vieira RP, Grimm M et al (2012) Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 143:1620. https://doi.org/10.1053/j.gastro.2012.08.049
Grant CR, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC et al (2013) Dysfunctional CD39 regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology. https://doi.org/10.1002/hep.26583
Schmelzle M, Duhme C, Junger W, Salhanick SD, Chen Y, Wu Y et al (2013) CD39 modulates hematopoietic stem cell recruitment and promotes liver regeneration in mice and humans after partial hepatectomy. Ann Surg 257:693–701. https://doi.org/10.1097/SLA.0b013e31826c3ec2
Fausto N, Campbell JS, Riehle KJ (2006) Liver regeneration. Hepatology 43:S45–S53. https://doi.org/10.1002/hep.20969
Dranoff JA, Ogawa M, Kruglov EA, Gaça MDA, Sévigny J, Robson SC et al (2004) Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 287:24. https://doi.org/10.1152/ajpgi.00294.2003
Dranoff JA, Kruglov EA, Abreu-Lanfranco O, Nguyen T, Arora G, Aurora G et al (2007) Prevention of liver fibrosis by the purinoceptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS). In Vivo 21:957–965
Chiang DJ, Roychowdhury S, Bush K, McMullen MR, Pisano S, Niese K et al (2013) Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS One 8:e69114. https://doi.org/10.1371/journal.pone.0069114
Yoshida S, Ikenaga N, Liu SB, Peng Z-W, Chung J, Sverdlov DY et al (2014) Extra-hepatic PDGFB, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. https://doi.org/10.1053/j.gastro.2014.08.038
Flores RV, Hernández-Pérez MG, Aquino E, Garrad RC, Weisman GA, Gonzalez FA (2005) Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor. Mol Cell Biochem 280:35–45. https://doi.org/10.1007/s11010-005-8050-5
Kauffenstein G, Pelletier J, Lavoie EG, Kukulski F, Martin-Satue M, Dufresne SS et al (2014) Nucleoside triphosphate diphosphohydrolase-1 ectonucleotidase is required for normal vas deferens contraction and male fertility through maintaining P2X1 receptor function. J. Biol. Chem. https://doi.org/10.1074/jbc.M114.604082
Dranoff JA, Kruglov EA, Toure J, Braun N, Zimmermann H, Jain D et al (2004) Ectonucleotidase NTPDase2 is selectively down-regulated in biliary cirrhosis. J Investig Med 52:475–482
Yu J, Lavoie EG, Sheung N, Tremblay JJ, Sévigny J, Dranoff JA (2008) IL-6 downregulates transcription of NTPDase2 via specific promoter elements. Am J Physiol Gastrointest Liver Physiol 294:56. https://doi.org/10.1152/ajpgi.00208.2007
Aoki H, Aoki M, Yang J, Katsuta E, Mukhopadhyay P, Ramanathan R et al (2016) Murine model of long-term obstructive jaundice. J Surg Res 206:118–125. https://doi.org/10.1016/j.jss.2016.07.020
Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L et al (2010) Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 182:774–783. https://doi.org/10.1164/rccm.201003-0359OC
Funding
LF received a research fellowship from the Deutsche Forschungsgemeinschaft (DFG; FE1434/1-1), ZGJ was granted a clinical research award from ACG and a clinical translational research award from AASLD, JS received a “Chercheur National” scholarship award from the Fonds de recherche du Québec-Santé (FRQS). This work was also, in part, supported by the National Institutes of Health grants to SCR (NIH; P01HL107152, R21CA164970) and Canadian Institute of Health Research to JS (CIHR; MOP-102472).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Linda Feldbrügge declares that she has no conflict of interest.
Z. Gordon Jiang declares that he has no conflict of interest.
Eva Csizmadia declares that she has no conflict of interest.
Shuji Mitsuhashi declares that he has no conflict of interest.
Stephanie Tran declares that she has no conflict of interest.
Eric U. Yee declares that he has no conflict of interest.
Sonja Rothweiler declares that she has no conflict of interest.
Kahini A. Vaid declares that she has no conflict of interest.
Jean Sévigny declares that he has no conflict of interest.
Moritz Schmelzle declares that he has no conflict of interest.
Yury V. Popov declares that he has no conflict of interest.
Simon C. Robson declares that he has no conflict of interest.
Ethical approval
The animal experiment protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Beth Israel Deaconess Medical Center.
Electronic supplementary material
ESM 1
(PDF 883 kb)
Rights and permissions
About this article
Cite this article
Feldbrügge, L., Jiang, Z.G., Csizmadia, E. et al. Distinct roles of ecto-nucleoside triphosphate diphosphohydrolase-2 (NTPDase2) in liver regeneration and fibrosis. Purinergic Signalling 14, 37–46 (2018). https://doi.org/10.1007/s11302-017-9590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-017-9590-3