Abstract
Extracellular ATP, signalling through P2 receptors, exerts well-documented effects on bone cells, inhibiting mineral deposition by osteoblasts and stimulating the formation and resorptive activity of osteoclasts. The aims of this study were to determine the potential osteotropic effects of adenosine, the hydrolysis product of ATP, on primary bone cells in vitro. We determined the effect of exogenous adenosine on (1) the growth, alkaline phosphatase (TNAP) activity and bone-forming ability of osteoblasts derived from the calvariae of neonatal rats and mice and the marrow of juvenile rats and (2) the formation and resorptive activity of osteoclasts from juvenile mouse marrow. Reverse transcription polymerase chain reaction (RT-PCR) analysis showed marked differences in the expression of P1 receptors in osteoblasts from different sources. Whilst mRNA for the A1 and A2B receptors was expressed by all primary osteoblasts, A2A receptor expression was limited to rat bone marrow and mouse calvarial osteoblasts and the A3 receptor to rat bone marrow osteoblasts. We found that adenosine had no detectable effects on cell growth, TNAP activity or bone formation by rodent osteoblasts in vitro. The analogue 2-chloroadenosine, which is hydrolysed more slowly than adenosine, had no effects on rat or mouse calvarial osteoblasts but increased TNAP activity and bone formation by rat bone marrow osteoblasts by 30–50 % at a concentration of 1 μM. Osteoclasts were found to express the A2A, A2B and A3 receptors; however, neither adenosine (≤100 μM) nor 2-chloroadenosine (≤10 μM) had any effect on the formation or resorptive activity of mouse osteoclasts in vitro. These results suggest that adenosine, unlike ATP, is not a major signalling molecule in the bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of extracellular purines and pyrimidines on cell surface receptors have been extensively studied for over 40 years [1]. ATP and related compounds exert their physiological affects via seven P2X ligand-gated ion channel receptors and eight P2Y G-protein-coupled receptors that are expressed in most tissues [2, 3].
The roles played by P2X and P2Y receptors in regulating the function of bone cells have received considerable attention in recent years [4]. Osteoblasts, the bone-forming cells, express multiple P2 receptors [5, 6], in a differentiation-dependent manner [7, 8], and respond to extracellular nucleotides with a prompt increase in intracellular calcium [7–10]. Importantly, exogenous ATP, UTP and other nucleotide analogues also potently inhibit mineralisation of bone formed by osteoblasts in culture [8, 11, 12]. Moreover, endogenous ATP released by osteoblasts also appears to act as a significant local inhibitor of mineralisation [13]. The action of UTP, together with data from pharmacological studies using other selective P2 receptor agonists and antagonists indicated that P2Y2, P2X1 and P2X7 receptors could be involved in mediating the inhibition of mineralisation by ATP [8, 12, 13].
Osteoclasts, the bone-resorbing cells also express most P2 receptors [14] and respond to extracellular nucleotides with elevation of intracellular calcium [15]. Moreover, extracellular ATP, ADP and UDP have been shown to increase the formation and resorptive activity of primary rodent osteoclasts in vitro [14, 16–18]. Pharmacological evidence now suggests that the pro-resorptive action of ATP and related molecules are mediated by the P2Y1, P2Y6 and P2Y12 receptors [14, 17–19].
Adenosine is a hydrolysis product of ATP and is formed extracellularly by the actions of ecto-nucleotidases. There are four major families of these ecto-enzymes: (1) ecto-nucleoside triphosphate diphosphohydrolases (NTPdases), which hydrolyse ATP to ADP and finally AMP; (2) ecto-nucleotide pyrophosphatase/phosphodiesterases (NPPs) that hydrolyse ATP to AMP, with the release of pyrophosphate; (3) alkaline phosphatases, which sequentially remove single phosphate groups and can hydrolyse ATP through to adenosine; and (4) ecto-5′nucleotidase that hydrolyses AMP to adenosine. Extracellular adenosine concentrations are usually <300 nM [20] but can rise to approximately 1 μM under conditions of physiological stress [21].
The physiological actions of adenosine have been studied for over 90 years. Adenosine acts via the G-protein coupled P1-receptors, found on the surface of many cell types. The P1 receptor family can be subdivided into the A1, A2A, A2B and A3 receptors [22]. The A2A and A2B adenosine receptors are predominantly stimulatory and are coupled to Gs to stimulate cAMP signalling; the A1 and A3 receptors are mainly Gi coupled and act to inhibit cAMP signalling [22].
Both osteoblasts and osteoclasts have been reported to express all four P1 receptor subtypes [23–27]. However, the actions of extracellular adenosine on bone cells appear to be somewhat less clear-cut than those of ATP. Synthetic adenosine analogues caused a receptor-mediated rise in cAMP levels in calvarial osteoblast-like cells [28], but adenosine had no effect on intracellular calcium levels in these cells [7]. Two independent groups failed to find an effect of adenosine on the formation of mineralised bone nodules by rat calvarial osteoblasts [11, 29]. However, a more recent study indicated that adenosine, acting via the A2B receptor, may increase the osteogenic differentiation of rat long bone mesenchymal stem cells [24]. In addition, a synthetic A2B receptor agonist has been shown to increase bone formation, and bone marrow osteoblasts from A2B receptor knockout mice display reduced levels of bone formation [30]. Recently, it has also been reported that stimulation of the A2A receptors can enhance bone regeneration [31].
The study of Lerner et al. (1987) found that adenosine analogues had no effect on the resorption of cultured mouse calvarial bones. Adenosine was later shown to be without effect on the formation or resorptive activity of primary rodent osteoclasts in vitro [16, 17]. Adenosine was also reported to have no effect on intracellular calcium levels in rabbit osteoclasts in vitro [32]. However, more recent work has indicated that adenosine, acting through the A2A receptor, may stimulate the formation of osteoclasts from human peripheral blood cells [25]. In contrast, Mediero et al. (2012) [33] found that A2A receptor agonists inhibited mouse osteoclast formation in vitro. Blockade or deletion of the A1 receptor has additionally been reported to reduce the formation of mouse osteoclasts in culture [23]; however, the same group also found that stimulation of the A1 receptor had no effect on mouse osteoclasts [34].
The aim of the present study was to determine the direct actions of adenosine on normal osteoblasts and osteoclasts, using well-characterised assays that measure the accepted physiological functions (i.e. bone formation and bone resorption) of these cells.
Methods
Reagents
All tissue culture and molecular biology reagents were purchased from Life Technologies (Paisley, UK) unless stated otherwise. Chemical reagents were purchased from Sigma-Aldrich (Poole, UK). 2-chloroadenosine, GR79236, BAY606583, CGS15943 and pentostatin were purchased from Tocris (Bristol, UK). P1 receptor antibodies were obtained from Alomone (Jerusalem, Israel), the β-actin antibody from Abcam (Cambridge, UK) and HRP-conjugated secondary antibodies from Jackson Immunoresearch Laboratories (Philadelphia, USA).
Primary bone cell culture
This study used osteoblasts from several sources namely rat/mouse calvaria and rat bone marrow. Osteoclasts were obtained from mouse bone marrow. These methods represent the most widely used and well-validated methods for obtaining primary bone cells for in vitro research.
Rat/mouse calvarial osteoblasts
Primary cells were derived from the calvarial bones of 2–4-day-old rats (Sprague-Dawley) and mice (C57BL/6 or 129/SvTerJ). Osteoblasts were obtained using methods similar to those previously described [35–37]. Briefly, calvariae were digested using 0.25 % trypsin for 10 min, 0.2 % collagenase in Hank’s buffered salt solution (HBSS) for 30 min and finally 0.2 % collagenase in HBSS for 60 min, all at 37 °C. The first two digests were discarded, and cells from the final digest were resuspended in Dulbecco’s modified essential medium supplemented with 10 % foetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin (mixture abbreviated to ‘DMEM’). Due to increased nutritional requirements [36], mouse cells were resuspended in α-modified essential medium supplemented with 10 % foetal calf serum, 70 μg/ml gentamicin, 50 U/ml penicillin, 50 μg/ml streptomycin, and 0.125 μg/ml amphotericin (mixture abbreviated to ‘α-MEM’).
Osteoblasts were cultured for 4 days in 75 cm2 flasks in a 5 % CO2 atmosphere at 37 °C until confluent. Upon confluence, rat cells were then plated into six-well trays in DMEM further supplemented with 2 mM β-glycerophosphate, 50 μg/ml ascorbate and 10 nM dexamethasone (mixture abbreviated to ‘supplemented DMEM’) [35–37]. Mouse cells were plated into six-well trays in α-MEM further supplemented with 2 mM β-glycerophosphate and 50 μg/ml ascorbate (mixture abbreviated to ‘supplemented α-MEM’). Osteoblasts were treated with 1nM–100 μM adenosine, 2-chloroadenosine, GR79235 (selective A1 receptor agonist), BAY606583 (a selective A2B receptor agonist), CGS15943 (a non-selective P1 receptor antagonist), pentostatin (an adenosine deaminase inhibitor), ATP or phosphate-buffered saline (PBS) (vehicle) for the duration of the culture. Half-medium changes were performed every third day of culture. Experiments were terminated by fixing the cells in 2.5 % glutaraldehyde for 5 min. Cell culture plates were imaged at 800 dpi using a flat-bed scanner (Epson Perfection 4990 Photo), and the total area of bone nodules formed was quantified by image analysis, as described previously [35–37].
Rat bone marrow osteoblasts
Primary rat osteoblasts of bone marrow/stromal cell origin were obtained from the long bones of 6-week-old Sprague-Dawley rats. The epiphyses were cut across and the marrow was flushed out of the bones using PBS. The collected cells were suspended in α-MEM and pre-cultured in a 75 cm2 flask in 5 % CO2 at 37 °C. After 24 h, all the α-MEM was replaced in order to eliminate non-adherent cells; adherent stromal cells were cultured for a further 2 days until confluent. Upon confluence, cells were plated into six-well trays and cultured as described above.
Mouse osteoclasts
Osteoclasts were formed from precursors flushed from the bone marrow of 8-week-old mice using previously described methods [38]. Cells were pre-incubated in a 75 cm2 flask containing modified essential medium supplemented with 10 % FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin and 100 nM prostaglandin E2 (abbreviated as ‘MEM’), supplemented with 2.5 ng/ml macrophage colony stimulating factor (M-CSF; R&D Abingdon, UK) in 5 % CO2 at 37 °C. After 24 h, the non-adherent mononuclear cells remaining in the culture medium were collected. The cells were resuspended in MEM supplemented with 10 ng/ml M-CSF and 3 ng/ml receptor activator of NF-κB (RANKL) (R&D Abingdon, UK) and seeded onto 5-mm-diameter ivory discs in a 96-well tray (106 cells/disc). After a further 24 h, the ivory discs were transferred into six-well trays and cultured for 6 days at pH 7.30. Discs were cultured for the final 2 days in a medium acidified to pH 6.90 to activate osteoclastic resorption [38] before fixation in 2.5 % glutaraldehyde and staining to demonstrate tartrate-resistant acid phosphatase (TRAP). Osteoclasts were identified as TRAP-positive cells with ≥2 nuclei. The numbers of osteoclasts and area resorbed per disc were evaluated ‘blind’ using transmitted and reflected light microscopy, as described previously [38].
Alkaline phosphatase activity
Osteoblast tissue non-specific alkaline phosphatase (TNAP) activity was measured in cell lysates taken at defined stages of osteoblast differentiation (proliferating, differentiating, mature, mature bone-forming) using a colorimetric kit (Anaspec, CA, USA), as previously described [35]. TNAP activity was normalised to cell protein using the Bradford reagent (Sigma-Aldrich, Poole, UK).
Cell number and viability assays
Osteoblast cell number was measured at regular intervals throughout the culture period using a commercially available kit (CytoTox 96, Promega UK, Southampton, UK), as previously described [13]. This assay measures the activity of lactate dehydrogenase (LDH), a cytosolic enzyme which is released on cell lysis.
RNA extraction and RT-PCR
Osteoblasts were cultured in six-well trays for up to 28 days, and total RNA was extracted using TRIzol reagent, according to the manufacturer’s instructions. Osteoclasts were cultured on 1-cm-diameter dentine discs for up to 10 days before RNA extraction. RNA was treated with RNase-free DNase I (Promega UK, Southampton, UK) for 30 min at 37 °C to remove contaminating genomic DNA. The reaction was terminated by heat inactivation at 65 °C for 10 min. Total RNA was quantified spectrophotometrically by measuring absorbance at 260 nm. Complementary DNA (cDNA) was synthesised from approximately 1 μg of RNA using Superscript III reverse transcriptase, oligo dT, RNasin and a deoxyribo-nucleotide mix.
The cDNA produced from osteoblast and osteoclast RNA was amplified by PCR using 1 U GoTaq DNA polymerase, 1.5 mM MgCl2, 0.8 μM nucleotide mix (Promega UK, Southampton, UK) and 0.5 μM primers (MWG Biotech, Ebersberg, Germany). The primer sequences used for rat and mouse reverse transcription polymerase chain reaction (RT-PCR) are shown in Table 1.
Western blot
Protein was extracted from mature rat calvarial osteoblasts and osteoclasts. Cell layers were lysed in ice-cold radio immunoprecipitation (RIPA) lysis buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.1 % SDS 1 mM phenyl methyl sulfonyl fluoride (PMSF), 1 mg/ml aprotinin, 1 mM Na3VO4 and 2.5 mg/ml deoxycholic acid). Cell homogenates were sonicated for 5 min and stored at −80 °C for at least half an hour before use. Protein concentrations from lysates were determined using the Bradford assay (Sigma-Aldrich, Gillingham, Dorset, UK). Prior to loading, total protein samples were denatured by incubating at 95 °C for 5 min in the presence of 5× reducing sample buffer (60 mM Tris-HCl pH 6.8, 25 % glycerol, 2 % SDS, 10 % β-mercaptoethanol and 0.1 % bromophenol blue). Protein samples (20 μg/lane) were loaded into SDS-PAGE (10 %) gels and transferred onto a polyvinylidenifluoride (PVDF) membrane (Amersham, Buckinghamshire, UK) by the use of a wet tank blotter (Bio-Rad, Hercules, CA, USA) at 150 V for 1 h. Membranes were then blocked with 5 % non-fat milk and incubated with one of the P1 receptor antibodies (1:200) or β-actin (1:1000) overnight at room temperature. After washing, blots were incubated in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature (1:10,000). A peroxidase detection system (Immobilon™ Western, Millipore UK, Watford, UK) was used for the visualisation of the immunoreactivity.
Statistics
Statistical comparisons were made using one-way analysis of variance (ANOVA) and adjusted for multiple comparisons using the Bonferroni method. Calculations were performed using In Stat 3 (GraphPad, San Diego, CA). All data are presented as means ± SEM for 6–12 biological replicates. Results are representative of experiments performed at least three times, using cells from different animals.
Results
Rodent osteoblasts and osteoclasts express P1 receptor mRNA in vitro
Total RNA was extracted from mature, bone-forming osteoblasts derived from rat calvaria (day 14), rat bone marrow (day 17) and mouse calvaria (day 28). RT-PCR showed messenger RNA (mRNA) expression of the A1 and A2B receptors in rat calvarial osteoblasts and all P1 receptors in rat bone marrow osteoblasts (Fig. 1a). Mouse calvarial osteoblasts expressed mRNA for A1, A2A and A2B receptors but not the A3 receptor (Fig. 1a).
Expression of P1 receptors by rodent bone cells. a Rat calvarial osteoblasts expressed A1 and A2B receptor mRNA whilst rat bone marrow osteoblasts showed expression of all four adenosine receptors. Mouse calvarial osteoblasts expressed the A1, A2A and A2B receptors. Mouse osteoclasts expressed mRNA for the A2A, A2B and A3 receptors. Positive control: rat/mouse brain. b Western blot analysis showed that rat calvarial osteoblasts express low levels of A2A and A2B receptor proteins. Mouse osteoclasts expressed protein for all four of the adenosine receptors. Images are representative of experiments performed using mRNA and protein from three separate cell populations
RNA was extracted from mature, resorbing osteoclasts (day 10 of culture) for investigation of P1 receptor expression. Osteoclasts were found to express mRNA for the A2A, A2B and A3 receptors (Fig. 1a)
Total protein was extracted from mature rat calvarial osteoblasts and mouse osteoclasts. Western blot analysis revealed expression of A2A and A2B, receptor protein in osteoblasts; A1 and A3 receptor protein was not detected (Fig. 1b). Osteoclasts were found to express protein for all four adenosine receptors (Fig. 1b).
The effects of P1 receptor agonists on bone formation
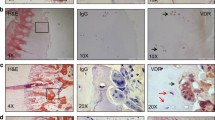
Rodent calvarial osteoblasts and rat bone marrow osteoblasts were cultured for up to 28 days with adenosine, 2-chloroadenosine and ATP. Rat calvarial osteoblasts were also additionally treated with the more selective agonists, GR79236 (A1) and BAY606583 (A2B). Representative light microscopy images of adenosine and 2-chloroadenosine-treated cell layers are shown in Fig. 2. In cultures of calvarial and bone marrow osteoblasts, adenosine had no effect on the level of bone formation (Fig. 3a–c). 2-chloroadenosine was without effect in calvarial osteoblasts (Fig. 3d, e) but caused a small stimulatory effect at 1 μM in bone marrow osteoblasts (Fig. 3f). Concentrations of ≥10 μM 2-chloroadenosine appeared to have toxic effects, resulting in a complete abolition of bone formation. ATP (≥10 μM) inhibited mineralisation by ≤90 and ≤85 % in calvarial and bone marrow osteoblasts, respectively (Fig. 3g–i). Treatment with GR79236 and BAY606583 also had no effect on bone formation by osteoblasts (Fig. 4a, b).
Effects of adenosine and 2-chloroadenosine on mineralised bone nodule formation by rodent osteoblasts. Representative images (n = 5) showing alizarin red-stained mineralised bone nodules, viewed by phase contrast microscopy (left) and low power reflected light scans (right). Adenosine (≤100 μM) had no effect on bone formation by rat calvarial, mouse calvarial or rat bone marrow osteoblasts (cultured on plastic for 14, 28 and 17 days, respectively). 2-chloroadenosine (1 μM) caused a modest increase in bone formation by rat bone marrow osteoblasts only. Scale bars: left, 1 mm; right, 1 cm
Effects of adenosine, 2-chloroadenosine and ATP on mineralised bone nodule formation by cultured rodent osteoblasts. Adenosine had no effect on mineralised nodule formation in cultures of a rat calvarial, b mouse calvarial or c rat bone marrow osteoblasts. 2-chloroadenosine was also without effect in d rat calvarial or e mouse calvarial osteoblasts but caused f a ~50 % increase in nodule formation by rat bone marrow osteoblasts (1 μM only). The complete abolition of bone formation at concentrations of e, f 10 μM and d 100 μM 2-chloroadenosine suggests toxicity at these levels. ATP inhibited bone formation by g rat calvarial, h mouse calvarial and i rat bone marrow osteoblasts by up to 90 %. Data are means ± SEM for six replicate determinations, n = 5; *p < 0.05; **p < 0.01; ***p < 0.001
The effects of selective adenosine receptor agonists and endogenous adenosine on bone formation by osteoblasts. At concentrations up to 10 μM the selective a A1 agonist, GR79236, and b A2B agonist, BAY606583 did not affect mineralised bone nodule formation by rat calvarial osteoblasts. c The non-selective adenosine receptor antagonist, CG15943, and d the adenosine deaminase inhibitor, pentostatin, also had no effect on the level of bone formation. Data are means ± SEM for six replicate determinations (n = 3)
Endogenous adenosine does not affect bone formation
Rat osteoblasts were cultured with CGS15943, a non-selective P1 receptor antagonist, and pentostatin, an adenosine deaminase inhibitor, to determine whether endogenous adenosine influences bone formation. Both CGS15943 and pentostatin (≤1 μM) had no effect on the level of bone formation (Fig. 4c, d).
Increased TNAP activity in bone marrow osteoblasts treated with 2-chloroadenosine
The effect of adenosine and 2-chloroadenosine on TNAP activity was measured in calvarial and long bone osteoblasts at different stages of differentiation (proliferating, differentiating, mature, mature bone-forming). Adenosine had no effect on TNAP activity (Fig. 5a–c). 2-chloroadenosine (1 μM) was without effect in calvarial osteoblasts (Fig. 5d, e) but increased TNAP activity by ≤48 % in rat bone marrow osteoblasts (Fig. 5f). This effect was evident in differentiating (day 11), mature (day 14) and mature, bone-forming (day 17) osteoblasts.
Effects of adenosine and 2-chloroadenosine on alkaline phosphatase (TNAP) activity of rodent osteoblasts. Culture with adenosine had no effect on TNAP activity in a rat calvarial, b mouse calvarial and c rat bone marrow osteoblasts at any stage of culture. 2-chloroadenosine had no effect on TNAP activity in d rat and e mouse calvarial osteoblasts but f increased TNAP activity by up to 48 % in rat bone marrow osteoblasts. Data are means ± SEM for six replicate determinations (n = 3–5): *p < 0.05, **p < 0.01
Osteoblast numbers are unaffected by adenosine or 2-chloroadenosine
Calvarial and long bone marrow osteoblasts were cultured for up to 28 days with adenosine or 2-chloroadenosine; cell numbers were estimated at the different stages of osteoblast differentiation using a lactate dehydrogenase assay. Adenosine had no effect on calvarial or long bone osteoblast numbers at any time point at concentrations up to 100 μM (Fig. 6a–c). 2-chloroadenosine did not influence cell number in cultures of rat calvarial osteoblasts at concentrations up to 10 μM (Fig. 6d). In mouse calvarial and rat long bone osteoblasts, ≤1 μM 2-chloroadenosine had no effect on cell number but 10 μM 2-chloroadenosine was toxic, resulting in widespread cell death (Fig. 6e, f).
Osteoblast numbers are unaffected by adenosine and 2-chloroadenosine. Treatment with adenosine had no effect on cell number in cultures of a rat calvarial, b mouse calvarial and c rat bone marrow osteoblasts at any stage. 2-chloroadenosine (≤1 μM) was also without effect in d rat calvarial, e mouse calvarial and f bone marrow osteoblasts. 0 indicates that there were no viable cells present suggesting toxicity at concentrations of ≥10 μM 2-chloroadenosine. Data are means ± SEM for six replicate determinations (n = 3–5)
Lack of effect of adenosine or 2-chloroadenosine on osteoclast formation and resorptive activity
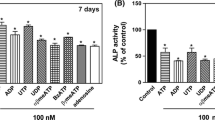
The effect of adenosine, 2-chloroadenosine and ATP was examined in cultures of mouse osteoclasts cultured on ivory discs. Representative light microscopy images of treated osteoclasts are shown in Fig. 7a. At all the concentrations tested, adenosine (Fig. 7b, e) and 2-chloroadenosine (Fig. 7c, f) had no effect on osteoclast number or the amount of resorption per osteoclast. In contrast, ATP increased osteoclast formation up ≤75 % and bone resorption by up to twofold (Fig. 7d, g).
Lack of effect of adenosine and 2-chloroadenosine on the formation and resorptive activity of mouse osteoclasts. Osteoclasts were generated in 10-day cultures of mouse marrow cells on ivory discs, in the presence or absence of adenosine, 2-chloroadenosine or ATP. Cells were acidified to pH 6.90 on day 8 of culture to activate resorption. a Representative transmitted light images of cultures, showing tartrate-resistant acid phosphatase-positive multinucleated osteoclasts (large red cells) and resorption pits (tan areas); scale bar = 50 μm. b, e Adenosine and c, f 2-chloroadenosine were without effect on osteoclast formation and resorptive activity. d, g ATP increased osteoclast formation by ≤75 % and resorption by twofold. Data are means ± SEM for eight replicate determinations (n = 3); *p < 0.05; **p < 0.01; **p < 0.001
Discussion
The role of adenosine in the regulation of bone cell function has been a significant area of study (see review [39]) yet published data present conflicting results. The aim of this investigation was to clarify the functional effects of adenosine on osteoblasts and osteoclasts. We found that adenosine and the selective P1 receptor agonists, GR79236 (A1) and BAY606583 (A2B), had no effect on osteoblast number and/or bone formation. However, 2-chloroadenosine (a synthetic, universal P1 receptor agonist) modestly increased TNAP activity and bone formation by rat bone marrow osteoblasts but did not affect rat and mouse calvarial osteoblasts. Osteoclast formation and activity was also unaffected by adenosine or 2-chloroadenosine. In contrast, the established osteogenic inhibitory effects [12] and osteoclastic stimulatory effects [17] of ATP were readily observed in all cells.
The work presented here showed no effects of adenosine, 2-chloroadenosine, GR79236 or BAY606583 on calvarial-derived osteoblasts; this is in broad agreement with previous studies which showed exogenous adenosine had no effect on cultured rat osteoblasts [11, 29]. However, our results are at variance with several studies which found that adenosine or adenosine analogues, acting via the A2A or A2B receptors, stimulate the differentiation and function of human and rodent bone marrow osteoblasts and promote bone regeneration [24, 30, 31, 40]. Our data also do not concur with the reported inhibitory effects of adenosine analogues, acting via A1 or A2A receptors, on the differentiation of rodent osteoblast-like cells [41] or human osteoblasts [40]. We did observe small stimulatory effects of 2-chloroadenosine on rat bone marrow osteoblasts. This synthetic agonist is more potent than adenosine and is hydrolysed more slowly [42]. Differences in agonist pharmacology may therefore explain why we observed small effects with this analogue, but adenosine was inactive in bone marrow osteoblasts.
It is possible that significant differences in osteoblast culture methodologies also contributed to the divergent results between studies. Bone formation in vitro can be influenced by a number of variables including use of glucocorticoids (which are strongly osteogenic for rat but not mouse-derived cells in vitro [36, 43]) in the culture medium, culture duration, cell seeding densities, tissue culture medium, β-glycerophosphate concentration and the age of the animals from which the cells were isolated.
The breakdown of ATP released by cells represents a key source of extracellular adenosine. Osteoblasts release ATP constitutively [44, 45] and can generate low micromolar concentrations of adenosine in vitro [21, 27]. Therefore, the possibility remains that endogenous adenosine exerts effects on osteoblasts that are not enhanced further by the addition of exogenous adenosine. To investigate this possibility further, rat osteoblasts were cultured with a non-selective P1 receptor antagonist, CGS15943, to block all adenosine-mediated signalling. Cells were also cultured with pentostatin which inhibits adenosine deaminase and prevents adenosine breakdown to inosine. Both CGS15943 and pentostatin had no effect on the level of bone formation in vitro. Taken together, this suggests that signalling mediated by endogenous adenosine does not exert a significant effect on calvarial osteoblast function.
Earlier work has shown that osteoblasts express all the adenosine receptors [24, 27]. The present study examined the expression profile of P1 receptors in mature, bone-forming osteoblasts from different sources. In agreement with the previous studies, rat bone marrow expressed low levels of mRNA for all four receptors. However, calvarial osteoblasts displayed more restricted expression at both the protein and mRNA level. Osteoblasts expressed mRNA for the A1 receptor yet no protein was detected, suggesting this receptor is not translated. In contrast, we found that osteoblasts expressed A2A receptor protein but not mRNA. This discrepancy suggests that expression of A2A receptor mRNA is below the threshold that can be detected by conventional PCR. The limited adenosine receptor expression on calvarial osteoblasts may also contribute to the lack of functional effects seen in these cells.
Previous work reported that P1 receptor expression by human and rat bone marrow mesenchymal stem cells and osteoblasts is strongly dependent on differentiation [24, 40], as is P2 receptor expression by rat calvarial osteoblasts [7]. Although not investigated here, it is possible that P1 receptor expression in osteoblasts is also affected by differentiation.
Several studies have shown that A1 receptors and A2A receptors can form homomers [46, 47] or A1-A2A heteromers [48]. A1-P2Y1 and A1-P2Y2 adenosine-receptor-ATP-receptor G-protein heteromers have also been reported [49, 50]. This receptor dimerisation may lead to alterations in downstream signalling and cellular responses to P1 receptor agonists, potentially contributing to the different effects observed between cell types.
Available data regarding the effects of adenosine and P1 receptors on osteoclasts are also conflicting. Consistent with earlier studies, we showed that osteoclasts express all four adenosine receptors, albeit at a low level [23, 25]. However, we demonstrated that adenosine had no effect on the formation or resorptive activity of mouse osteoclasts grown on dentine. These results are in agreement with some previous investigations [16, 17] but differ from others. For example, A2A receptor agonists have been shown to both inhibit [33] and stimulate [25] the formation and activity of osteoclasts. Kara and colleagues [51] found that A1 receptor antagonists decreased osteoclast formation and resorption. However, we found that mouse osteoclasts only express low levels of A1 receptor protein. Pellegatti et al. [25] also reported that the A1 receptor was only weakly expressed by osteoclasts formed from human peripheral blood. Taken together, this suggests that the A1 receptor is unlikely to play a role in regulating osteoclast function. It should be noted that the culture conditions used by the above studies varied considerably. Osteoclast formation and activity is strongly influenced by factors including RANKL/M-CSF concentration, substrate (dentine, bone or plastic), pH, source of cells and culture duration [38]. Thus, different experimental conditions combined with variations in P1 receptor expression may account for the disparity between studies.
The lack of effect of adenosine on osteoblasts and osteoclasts in this study suggests that the P1 receptors are not critical in regulating bone cell function directly. However, A1 and A2B receptor knockout mice are reported to display increases in the trabecular and/or cortical bone [23, 30], whilst A2A receptor knockout mice have decreased cortical and trabecular bone [33]. At present, the A3 receptor knockout has not been investigated for specific changes in the bone; however, no overt changes in phenotype have been noted [52]. Adenosine receptors display widespread expression and are involved in many biological processes including coronary vasodilation [53], VEGF production, angiogenesis [54, 55] and nerve transmission [56]. Thus, it is possible that the changes in bone mass seen in the knockout mouse models are occurring indirectly via actions on other tissues.
In summary, this study used established and well-validated assays for measuring accepted bone cell function. We clearly show that supraphysiological concentrations of adenosine did not affect rodent osteoblasts or mouse osteoclasts. In rat bone marrow osteoblasts, 2-chloroadenosine exerted small effects on TNAP activity and bone formation but only when added to cell cultures in extremely high concentrations. 2-chloroadenosine was without effect on the other cell types used. We also provide evidence to suggest endogenous adenosine does not influence bone formation. Taken together these data suggests that adenosine has very little direct effect on osteoblast and osteoclast function.
References
Burnstock G (1972) Purinergic nerves. Pharmacol Rev 24:509–581
Abbracchio MP, Burnstock G (1994) Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64:445–475
Burnstock G (2007) Purine and pyrimidine receptors. Cell Mol Life Sci 64:1471–1483
Burnstock G, Arnett TR, Orriss IR (2013) Purinergic signalling in the musculoskeletal system. Purinergic Signal 9:541–572
Hoebertz A, Townsend-Nicholson A, Glass R, Burnstock G, Arnett TR (2000) Expression of P2 receptors in bone and cultured bone cells. Bone 27:503–510
Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA (2001) Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone 28:507–512
Orriss IR, Knight GE, Ranasinghe S, Burnstock G, Arnett TR (2006) Osteoblast responses to nucleotides increase during differentiation. Bone 39:300–309
Orriss IR, Key ML, Brandao-Burch A, Patel JJ, Burnstock G, Arnett TR (2012) The regulation of osteoblast function and bone mineralisation by extracellular nucleotides: the role of P2X receptors. Bone 51:389–400
Kumagai H, Sakamoto H, Guggino S, Filburn CR, Sacktor B (1989) Neurotransmitter regulation of cytosolic calcium in osteoblast-like bone cells. Calcif Tissue Int 45:251–254
Schofl C, Cuthbertson KS, Walsh CA, Mayne C, Cobbold P, von zur MA, Hesch RD, Gallagher JA (1992) Evidence for P2-purinoceptors on human osteoblast-like cells. J Bone Miner Res 7:485–491
Hoebertz A, Mahendran S, Burnstock G, Arnett TR (2002) ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: a novel role for the P2Y2 receptor in bone remodeling. J Cell Biochem 86:413–419
Orriss IR, Utting JC, Brandao-Burch A, Colston K, Grubb BR, Burnstock G, Arnett TR (2007) Extracellular nucleotides block bone mineralization in vitro: evidence for dual inhibitory mechanisms involving both P2Y2 receptors and pyrophosphate. Endocrinology 148:4208–4216
Orriss IR, Key ML, Hajjawi MO, Arnett TR (2013) Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralisation. PLoS One 8:e69057
Orriss IR, Wang N, Burnstock G, Arnett TR, Gartland A, Robaye B, Boeynaems JM (2011) The P2Y6 receptor stimulates bone resorption by osteoclasts. Endocrinology 152:3706–3716
Reyes JP, Sims SM, Dixon SJ (2011) P2 receptor expression, signaling and function in osteoclasts. Front Biosci (Schol Ed) 3:1101–1118
Morrison MS, Turin L, King BF, Burnstock G, Arnett TR (1998) ATP is a potent stimulator of the activation and formation of rodent osteoclasts. J Physiol 511:495–500
Hoebertz A, Meghji S, Burnstock G, Arnett TR (2001) Extracellular ADP is a powerful osteolytic agent: evidence for signaling through the P2Y1 receptor on bone cells. FASEB J 15:1139–1148
Su X, Floyd DH, Hughes A, Xiang J, Schneider JG, Uluckan O, Heller E, Deng H, Zou W, Craft CS, Wu K, Hirbe AC, Grabowska D, Eagleton MC, Townsley S, Collins L, Piwnica-Worms D, Steinberg TH, Novack DV, Conley PB, Hurchla MA, Rogers M, Weilbaecher KN (2012) The ADP receptor P2RY12 regulates osteoclast function and pathologic bone remodeling. J Clin Invest 122:3579–3592
Syberg S, Brandao-Burch A, Patel JJ, Hajjawi M, Arnett TR, Schwarz P, Jorgensen NR, Orriss IR (2012) Clopidogrel (Plavix(R)), a P2Y12 receptor antagonist, inhibits bone cell function in vitro and decreases trabecular bone in vivo. J Bone Miner Res 27:2373–2386
Martin C, Leone M, Viviand X, Ayem ML, Guieu R (2000) High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med 28:3198–3202
Fredholm BB, Sollevi A (1981) The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol 313:351–367
Fredholm BB APIJ, Jacobson KA, Linden J, Muller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63:1–34
Kara FM, Doty SB, Boskey A, Goldring S, Zaidi M, Fredholm BB, Cronstein BN (2010) Adenosine A(1) receptors regulate bone resorption in mice: adenosine A(1) receptor blockade or deletion increases bone density and prevents ovariectomy-induced bone loss in adenosine A(1) receptor-knockout mice. Arthritis Rheum 62:534–541
Gharibi B, Abraham AA, Ham J, Evans BA (2011) Adenosine receptor subtype expression and activation influence the differentiation of mesenchymal stem cells to osteoblasts and adipocytes. J Bone Miner Res 26:2112–2124
Pellegatti P, Falzoni S, Donvito G, Lemaire I, Di Virgilio F (2011) P2X7 receptor drives osteoclast fusion by increasing the extracellular adenosine concentration. FASEB J 25:1264–1274
Vincenzi F, Targa M, Corciulo C, Gessi S, Merighi S, Setti S, Cadossi R, Goldring MB, Borea PA, Varani K (2013) Pulsed electromagnetic fields increased the anti-inflammatory effect of A(2)A and A(3) adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One 8:e65561
Evans BA, Elford C, Pexa A, Francis K, Hughes AC, Deussen A, Ham J (2006) Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. J Bone Miner Res 21:228–236
Lerner UH, Sahlberg K, Fredholm BB (1987) Characterization of adenosine receptors in bone. Studies on the effect of adenosine analogues on cyclic AMP formation and bone resorption in cultured mouse calvaria. Acta Physiol Scand 131:287–296
Jones SJ, Gray C, Boyde A, Burnstock G (1997) Purinergic transmitters inhibit bone formation by cultured osteoblasts. Bone 21:393–399
Carroll SH, Wigner NA, Kulkarni N, Johnston-Cox H, Gerstenfeld LC, Ravid K (2012) A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J Biol Chem 287:15718–15727
Mediero A, Wilder T, Perez-Aso M, Cronstein BN (2015) Direct or indirect stimulation of adenosine A2A receptors enhances bone regeneration as well as bone morphogenetic protein-2. FASEB J 29:1577–1590
Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ (2004) Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J Bone Miner Res 19:642–651
Mediero A, Kara FM, Wilder T, Cronstein BN (2012) Adenosine A(2A) receptor ligation inhibits osteoclast formation. Am J Pathol 180:775–786
He W, Cronstein BN (2012) Adenosine A1 receptor regulates osteoclast formation by altering TRAF6/TAK1 signaling. Purinergic Signal 8:327–337
Orriss IR, Taylor SE, Arnett TR (2012) Rat osteoblast cultures. Methods Mol Biol 816:31–41
Orriss IR, Hajjawi MO, Huesa C, MacRae VE, Arnett TR (2014) Optimisation of the differing conditions required for bone formation in vitro by primary osteoblasts from mice and rats. Int J Mol Med 3:1201–1208
Taylor SE, Shah M, Orriss IR (2014) Generation of rodent and human osteoblasts. BoneKEy Rep 3:585
Orriss IR, Arnett TR (2012) Rodent osteoclast cultures. Methods Mol Biol 816:103–117
Evans BA (2012) Does adenosine play a role in bone formation, resorption and repair? Purinergic Signal 8:177–180
Costa MA, Barbosa A, Neto E, Sa-e-Sousa A, Freitas R, Neves JM, Magalhaes-Cardoso T, Ferreirinha F, Correia-de-Sa P (2011) On the role of subtype selective adenosine receptor agonists during proliferation and osteogenic differentiation of human primary bone marrow stromal cells. J Cell Physiol 226:1353–1366
Gharibi B, Abraham AA, Ham J, Evans BA (2012) Contrasting effects of A1 and A2b adenosine receptors on adipogenesis. Int J Obes 36:397–406
Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA (1993) Structure-activity relationships for 2-substituted adenosines at A1 and A2 adenosine receptors. Pharmacology 46:91–100
Bellows CG, Wang YH, Heersche JN, Aubin JE (1994) 1,25-dihydroxyvitamin D3 stimulates adipocyte differentiation in cultures of fetal rat calvaria cells: comparison with the effects of dexamethasone. Endocrinology 134:2221–2229
Orriss IR, Knight GE, Utting JC, Taylor SE, Burnstock G, Arnett TR (2009) Hypoxia stimulates vesicular ATP release from rat osteoblasts. J Cell Physiol 220:155–162. doi:10.1002/jcp.21745
Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL (2005) Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res 20(1):41–49
Ciruela F, Casado V, Mallol J, Canela EI, Lluis C, Franco R (1995) Immunological identification of A1 adenosine receptors in brain cortex. J Neurosci Res 42:818–828
Canals M, Burgueno J, Marcellino D, Cabello N, Canela EI, Mallol J, Agnati L, Ferre S, Bouvier M, Fuxe K, Ciruela F, Lluis C, Franco R (2004) Homodimerization of adenosine A2A receptors: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Neurochemi 88:726–734
Ciruela F, Ferre S, Casado V, Cortes A, Cunha RA, Lluis C, Franco R (2006) Heterodimeric adenosine receptors: a device to regulate neurotransmitter release. Cell Mol Life Sci 63:2427–2431
Yoshioka K, Saitoh O, Nakata H (2001) Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci U S A 98:7617–7622
Suzuki T, Namba K, Tsuga H, Nakata H (2006) Regulation of pharmacology by hetero-oligomerization between A1 adenosine receptor and P2Y2 receptor. Biochem Biophys Res Commun 351:559–565
Kara FM, Chitu V, Sloane J, Axelrod M, Fredholm BB, Stanley ER, Cronstein BN (2010) Adenosine A1 receptors (A1Rs) play a critical role in osteoclast formation and function. FASEB J 24:2325–2333
Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA (2000) Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem 275:4429–4434
Morrison RR, Talukder MA, Ledent C, Mustafa SJ (2002) Cardiac effects of adenosine in A(2A) receptor knockout hearts: uncovering A(2B) receptors. Am J Physiol Heart Circ Physiol 282:H437–H444
Ramanathan M, Pinhal-Enfield G, Hao I, Leibovich SJ (2007) Synergistic up-regulation of vascular endothelial growth factor (VEGF) expression in macrophages by adenosine A2A receptor agonists and endotoxin involves transcriptional regulation via the hypoxia response element in the VEGF promoter. Mol Biol Cell 18:14–23
Ernens I, Leonard F, Vausort M, Rolland-Turner M, Devaux Y, Wagner DR (2010) Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem Biophys Res Commun 392:351–356
Burnstock G (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87:659–797
Acknowledgments
The authors are grateful for the funding from Arthritis Research UK (Career Developmental Fellowship, 19205, IRO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajjawi, M.O.R., Patel, J.J., Corcelli, M. et al. Lack of effect of adenosine on the function of rodent osteoblasts and osteoclasts in vitro. Purinergic Signalling 12, 247–258 (2016). https://doi.org/10.1007/s11302-016-9499-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-016-9499-2