Abstract
Gastrointestinal symptoms have a major impact on the quality of life and are becoming more prevalent in the western population. The enteric nervous system (ENS) is pivotal in regulating gastrointestinal functions. Purinergic neurotransmission conveys a range of short and long-term cellular effects. This study investigated the role of the ADP-sensitive P2Y13 receptor in lipid-induced enteric neuropathy. Littermate P2Y13 +/+ and P2Y13 −/− mice were fed with either a normal diet (ND) or high-fat diet (HFD) for 6 months. The intestines were analysed for morphological changes as well as neuronal numbers and relative numbers of vasoactive intestinal peptide (VIP)- and neuronal nitric oxide synthase (nNOS)-containing neurons. Primary cultures of myenteric neurons from the small intestine of P2Y13 +/+ or P2Y13 −/− mice were exposed to palmitic acid (PA), the P2Y13 receptor agonist 2meSADP and the antagonist MRS2211. Neuronal survival and relative number of VIP-containing neurons were analysed. In P2Y13 +/+, but not in P2Y13 −/− mice, HFD caused a significant loss of myenteric neurons in both ileum and colon. In colon, the relative numbers of VIP-containing submucous neurons were significantly lower in the P2Y13 −/− mice compared with P2Y13 +/+ mice. The relative numbers of nNOS-containing submucous colonic neurons increased in P2Y13 +/+ HFD mice. HFD also caused ileal mucosal thinning in P2Y13 +/+ and P2Y13 −/− mice, compared to ND fed mice. In vitro PA exposure caused loss of myenteric neurons from P2Y13 +/+ mice while neurons from P2Y13 −/− mice were unaffected. Presence of MRS2211 prevented PA-induced neuronal loss in cultures from P2Y13 +/+ mice. 2meSADP caused no change in survival of cultured neurons. P2Y13 receptor activation is of crucial importance in mediating the HFD- and PA-induced myenteric neuronal loss in mice. In addition, the results indicate a constitutive activation of enteric neuronal apoptosis by way of P2Y13 receptor stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gastrointestinal (GI) tract is an emerging key organ in whole body homeostasis. The basis of optimal GI regulation is an intact enteric nervous system (ENS). The ENS innervates the entire digestive tract and plays pivotal roles in coordinating motility, secretion and blood flow. The prevalence of GI symptoms is high in the Western world, substantially affecting quality of life [1, 2]. Patients with overweight and/or diabetes report high incidences of GI symptoms compared with the normal population [3].
Purinergic signalling was first described 40 years ago [4], since then, the field has gained substantial interest [5]. ATP was the first nucleotide shown to act as a sole or co-transmitter in central and peripheral, including autonomic, nervous systems [5, 6]. ATP is not only freed from damaged or dying cells, but a physiological release of ATP can be triggered from both neuronal and non-neuronal cells [7]. Extracellularly, ATP is degraded by ectonucleotidases to ADP, AMP and adenosine rendering the system a high flexibility in the ensuing response [8]. A large number of selective purinergic receptors have been identified. P1 receptors are adenosine sensitive while P2 receptors are preferentially activated by ATP/ADP. P2 receptors are further subdivided into X, ionotropic and Y, G-protein-coupled receptors (GPCRs) [5, 9]. P2Y13 is a GPCR activated by ADP and coupled to Gi/Go signalling [10]. It was initially identified in the spleen and brain but is also expressed in the dorsal root ganglia, microglia, pancreatic beta cells, hepatocytes and mast cells [10–15]. In central neurons, P2Y13 has been shown to be involved in neuronal differentiation, axonal elongation, oxidative stress and inflammatory pain behaviour [11, 16–18]. Peripherally, the receptor plays key roles in lipoprotein metabolism and reverse cholesterol transport [19]. Activation of the P2Y13 receptor reduces high-density lipoprotein (HDL) levels and increases hepatic HDL uptake in mice [13, 19–21]. Research obtained, either by silencing the P2Y13 receptors through targeted gene deletion or small interfering RNA, suggests a pro-apoptotic role of P2Y13 receptor activation. In contrast, P2Y13 receptor activation was found to elicit neuroprotection in cerebellar granule neurons [18].
It was recently shown that feeding mice with high-fat diet (HFD) for 6 months results in a substantial loss of myenteric neurons in both the ileum and colon [22]. The saturated free fatty acid (FFA), palmitic acid (PA), was put forward as a putative mediator of this effect, as PA exposure in vitro caused substantial neuronal loss. These findings and the role of P2Y13 receptor that have been attributed in apoptosis, neuroprotection and lipid transport initiated the current study. By using P2Y13 +/+ and P2Y13 −/− mice fed with either a normal diet (ND) or HFD, we investigated the possible role of the P2Y13 receptor in HFD-induced enteric neuropathy. In addition, we used primary myenteric neuronal cultures from P2Y13 +/+ and P2Y13 −/− mice to investigate survival and relative numbers of vasoactive intestinal peptide (VIP)-expressing neurons in response to PA, P2Y13 receptor agonist and antagonist.
Methods
Ethics statement
Experimental design was approved by the animal ethics committee, Lund and Malmö, Sweden. All animals were used in accordance with the European Community Council Directive (86/609/EEC and 2010/63/EU) and the Swedish Animal Welfare Act (SFS 1988:534).
In vivo experiments
P2Y13 −/− mice were backcrossed into the C57BL/6J background generating P2Y13 −/+ genotypes which were subsequently used to produce P2Y13 +/+ and P2Y13 −/− littermates [19].
At 1 month of age, mice were divided into four groups: two ND groups (P2Y13 +/+ n = 6, P2Y13 −/− n = 7) continuing on standard chow (Lactamin R36) and two HFD groups (P2Y13 +/+ n = 6, P2Y13 −/− n = 6) changing to a chow containing elevated levels of fat (Research diets D12451, USA); see Table 1 for a survey on nutritional content. After 6 months on either ND or HFD, mice were killed by cervical dislocation, weighed and body composition determined by dual-energy X-ray absorpmetry [23] using Lunar PIXImus scanner (GE lunar, USA).
The abdominal cavity was opened, and the GI tract from lower oesophageal sphincter to the anal canal was collected, opened along the mesenteric border, emptied and weighed before rinsed in cold saline. The intestines were fixed in Stefaninis fixative (0.2 % picric acid, 2 % formaldehyde in 0.1 M phosphate buffer, pH 7.2) for 24 h at 4 °C and rinsed three times in Tyrode’s solution containing 10 % sucrose at 4 °C. Segments from the ileum and transverse colon were orientated for longitudinal and cross-sectioning, embedded in Tissue-Tek (Histolab, SE), frozen at −80 °C and sectioned (10 μm). Sections were processed for histochemistry and immunocytochemistry.
In vitro experiments
P2Y13 +/+ (n = 10, 20–23 g) and P2Y13 −/− mice (n = 8, 20–23 g) on ND were used for in vitro experimentation. Primary cultures of myenteric neurons were prepared from the small intestine. Neurons were dissociated using a modification of a previously described method [24, 25]. In brief, anaesthetized mice (Ketalar®/Rompun®) had their small intestine exposed via midline incisions. The longitudinal muscle layer with attached myenteric ganglia was stripped from approximately 15 cm of the distal small intestine. The tissues were placed in Ca2+- and Mg2+-free Hank’s Balanced Salt Solution (HBSS) containing 1.9 CDU/mL collagenase 1-A (1.5 mg/mL, Sigma-Aldrich, SE) and 4.7 μU/mL protease IX (1.5 mg/mL, Sigma-Aldrich, SE) and enzymatically and mechanically separated. Trypsin (0.4 mg/mL, BioChrom AG) and EDTA (0.003 %, Sigma-Aldrich) were added. Inactivation of trypsin was by adding 50 % v/v fetal bovine serum (FBS, Life Technologies, SE). Cell suspension was centrifuged at 15.6g for 10 min. Supernatant was removed, and pellet was washed three times in HBSS, centrifuged at 15.6g for 10 min and diluted to 0.8 mL in Neurobasal A (NBA) cell culture medium (Life Technologies, SE) containing 10 % FBS, 0.5 mM l-glutamine (BioChrom AG), 50 U/mL penicillin and 50 μg/mL streptomycin (BioChrom AG). Cell cultures were prepared by adding 50 μL of a constantly mixed cell suspension into 8-well chambers (BD Falcon, VWR) pre-filled with 450 μL of the NBA cell culture medium. From each animal, two 8-well chambers (69 mm2/well) were prepared. Suspensions from multiple animals were never pooled. Cultures were incubated 4 days prior to experimental treatments.
Stock solutions of PA (Sigma-Aldrich, SE), 2-methylthioadenosine diphosphate trisodium salt (2meSADP, Tocris, UK) and MRS2211 (Tocris UK), were prepared, aliquoted and stored at −20 °C.
PA was conjugated to bovine serum albumin (BSA, Merck, SE) by adding PA stock (10−2 M) together with 20 % BSA w/v to the medium and mixed at 37 °C 1 h prior to use. Final PA/BSA molar ratio was 4–5:1. Final BSA concentration was ≤2 % v/v. Different concentrations (10−4–10−3 M) of albuminated PA were added to neuronal cultures from P2Y13 +/+ to P2Y13 −/− mice; controls received medium containing 2 % BSA. In a separate set of experiments, neuronal cultures were grown in control or in 2meSADP (10−7–10−5 M)-enriched medium. Further, cultures from P2Y13 +/+ mice were exposed to control or MRS2211 (10−5 M)-supplemented medium with or without PA (10−4–10−3 M) or 2meSADP (10−7–10−5 M).
All experimental treatments lasted for 4 days after which cells were fixed in Stefaninis fixative for 30 min and rinsed for 2 × 10 min in Tyrode’s solution containing 10 % sucrose. To enhance antibody penetration, cultures were frozen and thawed prior to immunocytochemical processing.
Immunocytochemistry
For details on primary and secondary antibodies, see Table 2. All antibodies were diluted in phosphate buffered saline containing 0.25 % Triton X-100 and 0.25 % BSA (PBS-T-B). For visualization of submucous and myenteric neurons in mice on ND or HFD, cryosections were immunostained with HuC/HuD-biotin and Vectastain ABC kit (Vector laboratories inc., USA) according to the manufacturer’s protocol. To estimate the relative numbers of neurons expressing neuronal nitric oxide synthase (nNOS), a modified immunolabelling protocol was used allowing for a fluorescent HuC/HuD-biotin detection. Cryosections were washed in wash buffer (WB) consisting of PBS with 0.25 % Triton X-100 and 0.5 % BSA. Antigen retrieval was performed by 2 × 8 min microwaving in citrate buffer (0.1 M citrate, pH 6.5) followed by 10-min cooling in water bath and 20-min rinsing in running tap water. To block unspecific background staining occurring in sectioned mouse tissues when using monoclonal HuC/HuD antibodies, sections were washed in WB and incubated for 40 min in 0.1 mg/mL Streptavidin (Sigma-Aldrich, SE), washed for 3 × 10 min in WB, incubated for 120 min in 0.5 mg/mL biotin (Sigma-Aldrich, SE) and washed again for 3 × 10 min in WB. Antigenicity of VIP was destroyed by antigen retrieval; therefore, the protocol described above for double labelling with HuC/HuD could not be applied for estimating the relative numbers of VIP-containing neurons in cryosections. Instead, single immunolabelling of VIP and HuC/HuD, respectively, was performed on consecutive sections.
Neuronal cell cultures are devoid of mouse IgG thus allowing for visualization of antigen-antibody complexes using indirect immunofluorescence also of monoclonal HuC/HuD antibodies. Double immunolabelling of cultures and sections was performed by overnight incubation in a moist chamber at 4 °C with a mixture of HuC/HuD and VIP (cultures only) or nNOS antibodies. Secondary antibodies and/or Alexa Fluor conjugated streptavidin (1:1,000, Life Technologies, SE) was mixed, and slides were incubated for 1 h at RT. All slides were mounted in PBS/glycerol 1:1 and analysed using fluorescence microscopy (Olympus BX43, LRI, SE) with appropriate filter setting.
Histochemistry
Morphometric analyses of the mouse intestine after ND or HFD were on toluidine-blue-stained cryosections. Cryosections were washed in PBS-T-B prior to immersion in 0.1 % toluidine blue in 60 % ethanol for 20 min, dehydrated, cleared and mounted (Pertex, Histolab, SE).
Intracellular lipid accumulations were visualized on intestinal cryosections from ND and HFD mice using Bodipy® 493/503 (Life Technologies, SE) diluted 1:1,000 in PBS-T-B, incubated for 1 h, washed in PBS-T and mounted on ProLong® Gold (Life Technologies, SE).
Morphometic analyses and cell calculations
The heights of mucosa and muscularis propria were estimated using the mean from ten representative measurements from each animal (Aperio ScanScope CS/GL SS5082 and Aperio ImageScope platform; www.aperio.com). Immunocytochemical visualization of the enteric nerve cell bodies in submucous and myenteric ganglia was used to estimate neuronal number per millimetre section length. The relative numbers of nNOS-immunoreactive neurons were estimated using double immunolabelling of HuC/HuD in combination with nNOS antibodies. All HuC/HuD-immunoreactive neurons were counted and checked for double labelling with nNOS; values are expressed in percentage. The relative numbers of VIP-immunoreactive neurons in cryosections were estimated by counting the numbers of VIP-immunoreactive and the number of HuC/HuD-immunoreactive neurons on consecutive sections. From each mouse, cryosections cut longitudinally at 3–4 different depths, comprising a total length of 25–30mm, were used.
Neuronal survival in vitro was calculated by counting the total number of surviving neurons in the entire culture well (69 mm2) and expressed as percentage of the number of neurons in the control well run in parallel. The relative number of neurons expressing VIP in vitro was estimated using HuC/HuD and VIP double labelling.
Statistical analyses
Data are presented as means ± SEM and analysed by GraphPad Prism (GraphPad Software Inc, USA). Statistical significance was determined using two-way analysis of variance followed by Bonferroni’s post hoc test (in vivo data) or one-way analysis of variance followed by Dunnet’s post hoc test towards controls (in vitro data). A confidence level of 95 % was considered significant.
Results
In vivo findings
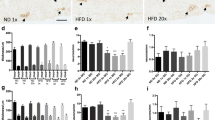
P2Y13 +/+ and P2Y13 −/− mice fed with HFD had increased body weight (p < 0.001, ND P2Y13 +/+ 32.1 ± 2.8 g, P2Y13 −/− 33.6 ± 2.5 g; HFD P2Y13 +/+ 46.2 ± 2.0 g, P2Y13 −/− 49.7 ± 1.6 g) and body fat percentage (p < 0.001, ND P2Y13 +/+ 24.0 ± 2.5 %, P2Y13 −/− 21.7 ± 1.8 %; HFD P2Y13 +/+ 47.5 ± 1.8 %, P2Y13 −/− 44.8 ± 1.7 %) compared with P2Y13 +/+ and P2Y13 −/− ND mice. Weights of the digestive tract did not differ between the four groups (ND P2Y13 +/+ 3.3 ± 0.3 g, P2Y13 −/− 3.7 ± 0.3 g; HFD P2Y13 +/+ 3.6 ± 0.3 g, P2Y13 −/− 3.0 ± 0.1 g). Morphometry on intestinal sections showed that HFD causes a modest ileal mucosal thinning, in both P2Y13 +/+ and P2Y13 −/− mice, that was absent in colon. No alterations in muscularis propria thickness were observed in the ileum or colon in any of the groups. HFD caused a significant loss of myenteric neurons in the ileum and colon of P2Y13 +/+, but not in P2Y13 −/− mice. In the colon from P2Y13 −/− mice, both on ND and HFD, a slight but significant increase in myenteric neurons per millimetre section was noted compared with P2Y13 +/+ mice. No change in the numbers of submucous neurons in the ileum and colon was observed in any of the groups. Results are summarized in Fig. 1 and Table 3.
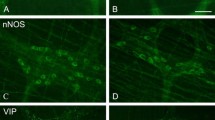
Upper panel shows representative micrographs of the ileum from P2Y13 +/+ (a, b, e and f) and P2Y13 −/− (c, d, g and h) mice fed with either normal diet (ND) or high-fat diet (HFD) for 6 months. Lower panel shows the effects of ND and HFD on the thicknesses of intestinal mucosa and muscularis propria in the ileum (i) and colon (j). a–d Cryosections immunostained for enteric neurons using the pan neuronal marker HuC/HuD. P2Y13 +/+ mice fed with HFD (b) have markedly less myenteric neurons per millimetre compared with ND-fed mice (a). HFD does not change neuronal density in P2Y13 −/− mice (d). e–h Cryosections stained with Bodipy® to illustrate lipid droplets within muscularis propria. P2Y13 +/+ mice fed with HFD (f) have increased intramuscular lipid droplet formation, compared with P2Y13 +/+ ND mice (e). P2Y13 −/− mice do not accumulate lipid droplets in muscularis propria (g, h). i, j Except a slight but significant reduction in the ileal mucosa height of HFD-fed animals, no structural remodelling was noted in any of the groups. Data presented as means ± SEM, asterisk denotes p < 0.05, all compared with P2Y13 +/+ ND; n = 5–12 in each group. Bar represents 20 μm
Both ND- and HFD-fed P2Y13 −/− mice displayed lower relative numbers of colonic VIP-containing submucous neurons compared with P2Y13 +/+ mice. HFD did not cause any change in the relative numbers of VIP-containing enteric neurons compared with ND mice. The relative numbers of myenteric neurons expressing nNOS were similar in the ileum and colon of P2Y13 +/+ and P2Y13 −/− mice irrespective of diet. P2Y13 +/+ mice fed with HFD had higher relative number of nNOS-containing submucous neurons in the colon as compared with the other three groups. Results are summarized in Table 3.
Bodipy staining of the ileum and colon from P2Y13 +/+ mice on HFD displayed a marked lipid droplet accumulation in muscularis propria while the other three groups (P2Y13 +/+ ND, P2Y13 −/− ND and P2Y13 −/− HFD) showed no or low levels of droplet accumulation (Fig. 1e–h).
In vitro findings
All in vitro investigations included a 4-day pre-treatment culture period followed by 4 days in treatment conditions. Untreated controls were always run in parallel, and neuronal survival was estimated as percent of control.
Survival of myenteric neurons from P2Y13 +/+ and P2Y13 −/− mice
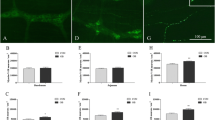
Survival of cultured myenteric neurons from ND-fed P2Y13 +/+ and P2Y13 −/− mice was determined after exposure to PA (10−4–10−3 M) or 2meSADP (10−7–10−5 M) with or without MRS2211 (10−5 M). Control wells displayed a large number (P2Y13 +/+ 6.0 ± 0.1 neurons/mm2, n = 31; P2Y13 −/− 5.9 ± 0.2 neurons/mm2, n = 20) of evenly distributed neurons. Exposing cultures from P2Y13 +/+ mice to the increasing concentrations of PA (10−4–10−3 M) caused a severe and concentration-dependent loss of neurons. In contrast, in neuronal cultures from P2Y13 −/− mice, no loss of myenteric neurons was observed after PA (10−4–10−3 M) exposure. The combined exposure of PA (10−4–10−3 M) and MRS2211 (10−5 M) protected P2Y13 +/+ cultures against PA-induced neuronal loss. Addition of 2meSADP (10−7–10−5 M) did not affect neuronal survival in either P2Y13 +/+ or P2Y13 −/− cultures. Neither did the combined exposure of 2meSADP (10−7–10−5 M) and MRS2211 (10−5 M) to P2Y13 +/+. MRS2211 (10−5 M) per se increased the number of surviving neurons in P2Y13 +/+ cultures. Results are summarized in Fig. 2 and Table 4.
Representative micrographs of cultured myenteric neurons from the small intestines of P2Y13 +/+ (a–d) and P2Y13 −/− mice (e, f). Neurons are visualized using HuC/HuD immunostaining. Exposure to palmitic acid (PA) (b) causes loss of P2Y13 +/+ neurons, compared with control (a). Exposure to the P2Y13 antagonist MRS2211 increases survival of P2Y13 +/+ neurons (c). MRS2211 also protects P2Y13 +/+ neurons against PA-induced loss (d). Neurons from P2Y13 −/− mice are protected against PA-induced neuronal loss. Bar represents 20 μm
VIP expression in cultured myenteric neurons from P2Y13 +/+ and P2Y13 −/− mice
The relative numbers of cultured myenteric VIP-containing neurons from P2Y13 +/+ and P2Y13 −/− mice were determined after exposure to PA (10−4–10−3 M) or 2meSADP (10−7–10−5 M) with or without MRS2211 (10−5 M). A reduction in the relative numbers of VIP-containing neurons was observed after exposure to PA (10−3 M) in both P2Y13 +/+ and P2Y13 −/− cultures compared with controls. In P2Y13 +/+ cultures, addition of MRS2211 (10−5 M) reversed PA-induced reduction of VIP-containing neurons at all PA concentrations except at 10−3 M. No change in the relative numbers of VIP-containing neurons was observed in P2Y13 +/+ or P2Y13 −/− cultures exposed to 2meSADP (10−4–10−3 M) compared with controls. P2Y13 +/+ cultures exposed to MRS2211 (10−5 M) with or without 2meSADP (10−4–10−3 M) did not change the relative number of VIP-containing neurons. Results are summarized in Fig. 3 and Table 4.
Representative micrographs of VIP immunoreactive cultured myenteric neurons from the small intestines of P2Y13 +/+ (a, b) and P2Y13 --/-- mice (c, d). No difference in the relative number of neurons immunoreactive for VIP was observed between P2Y13 +/+- (a) or P2Y13 --/-- (c) derived cultures. In contrast to the reduced relative numbers of VIP-immunoreactive neurons seen by exposure to 10-3M palmitic acid (PA), exposure to PA (10-4 – 4x10-4M) did not change the percentage of neurons immunoreactive for VIP in P2Y13 +/+- (b) or P2Y13 --/--- (d) derived cultures
Discussion
The current study investigated the effects of HFD on enteric neuronal survival in P2Y13 −/− and P2Y13 +/+ littermate mice. In addition, pharmacological in vitro studies on myenteric neurons from ND-fed P2Y13 −/− and P2Y13 +/+ were undertaken to reveal possible changes in neuronal survival after PA exposure and stimulation or inhibition of the P2Y13 receptor using the agonist 2meSADP or the antagonist MRS2211.
Lipid-induced neuronal loss
The study confirmed previous findings on HFD-fed C57BL/6 mice [22], in that HFD induced a significant loss of myenteric neurons in both the ileum and colon in the P2Y13 +/+ mice. Surprisingly, the P2Y13 −/− mice showed a remarkable protection against HFD-induced neuronal loss. It is also notable that the P2Y13 −/− mice fed with ND displayed an even higher number of neurons per millimetre section in colon compared with ND-fed P2Y13 +/+ littermates.
The finding that PA induces loss of myenteric neurons from P2Y13 +/+ mice in vitro is well in line with previous findings, describing PA-induced loss of cultured myenteric neurons from rat [22]. The current study also shows that cultured myenteric neurons from P2Y13 −/− mice are resistant to PA-induced loss. In rat, the mechanisms behind PA-induced neuronal loss was found to be complex, but excessive palmitoylcarnitine formation and exhausted l-carnitine stores leading to energy depletion, low acetylcholine synthesis and oxidative stress were identified as important events [22]. The present study shows that absence of the P2Y13 receptor or presence of MRS2211 abolished PA-induced neuronal loss in vitro. This indicates an unpredicted contribution of P2Y13 receptor activation in executing PA-induced loss of myenteric neurons. MRS2211-induced inhibition of P2Y13 receptors resulted in increased survival of cultured P2Y13 +/+-derived myenteric neurons, indicating constitutive activation of neuronal loss by way of P2Y13 receptor stimulation. Such constitutive activation could possibly explain the finding that P2Y13 −/− mice in vivo display increased myenteric neuronal density in the colon compared with P2Y13 +/+ mice.
The neuroprotection observed in P2Y13 −/− mice against the previously described, and here confirmed, PA- and HFD-induced loss of myenteric neurons is enigmatic. Compensatory mechanisms without any direct involvements of neurons may operate. The in vivo data have to be placed in light of a non-tissue-specific removal of the receptor, and the observed neuroprotection may be due to changes in lipid metabolism, altered insulin and glucose levels affecting whole body metabolism, or embryonic changes altering the prerequisites for PA-mediated neuronal loss. This is illustrated by the finding that though phenotypic analysis of P2Y13 −/− mice did not show changed insulin sensitivity or glucose tolerance, inhibition of P2Y13 receptor activity in mice using MRS2211 leads to increased insulin secretion and reduced plasma glucose [15, 20]. However, the here presented in vitro findings excitingly link PA exposure and neuronal P2Y13 receptor activation with a pro-apoptotic outcome.
Curiously, exposing P2Y13 +/+-cultured myenteric neurons to the stable and selective P2Y13 agonist 2meSADP did not hamper neuronal survival in vitro, while exposing cells to the antagonist MRS2211 enhanced the number of surviving neurons. This somewhat contradictory finding can partly be explained by MRS2211 being an inverse agonist rather than a pure antagonist (Olde B, et al. unpublished). Exposure thereby decreases constitutive apoptotic P2Y13 signalling. The absence of the effect of 2meSADP exposure may indicate that the protective effect of the P2Y13 −/− phenotype is indirect. Complex links between P2Y13 intracellular lipid and energy metabolism and/or neuronal transmembrane lipid transport may be operating. Altered lipid transport and metabolism in P2Y13 −/− mice have been highlighted in multiple studies. Activation of the P2Y13 receptor in mice leads to an augmented hepatic HDL uptake and bile acid secretion [21]. Silencing the P2Y13 receptor results in reduced TG-HDL endocytosis and internalization [13]. Gene profiling and phenotypic analysis of P2Y13 −/− mice confirm that they hold altered lipoprotein, cholesterol and HDL metabolism [20]. HFD has previously been shown to increase lipid droplet accumulation in intestinal muscularis propria in C57BL/6 mice [22]. In contrast, P2Y13 −/− mice fed with HFD did not accumulate lipid droplets in muscularis propria, which suggestively underlines the possibility of an altered lipid metabolism. An interesting finding was the ileal mucosal thinning observed in both P2Y13 +/+ and P2Y13 −/− mice fed with HFD. Previous studies have shown lipid infusions, unlike other nutrients, to cause a mucosa barrier impairment in the rat small intestine [26]. Whether the observed thinning of the mucosa is due to sustained lipid exposure when on HFD needs further investigations.
Lipid-induced alterations in VIP- and nNOS-containing neurons
Interestingly, the numbers of VIP-containing colonic neurons in submucous ganglia were significantly lower in the P2Y13 −/− mice than in the P2Y13 +/+ mice, irrespective of diet. This probably reflects a neuronal VIP content below the immunocytchemical detection limit and may be a compensatory mechanism due to genetic modification causing, e.g. lack of growth/survival factors or altered functional demands. A low-VIP content may be due to a low expression of VIP messenger RNA (mRNA) or excessive release of the peptide not matched by VIP mRNA expression. It may also reflect a low expression of PACAP or PAC1 receptors since PACAP by PAC1 receptor activation increases VIP mRNA expression [27]. In addition, present results suggest that P2Y13 receptor activation influences VIP expression. VIP expression is under calcium response element-binding protein’s (CREB) control, and the P2Y13 receptor, being Gi coupled, may through cAMP modulation regulate the expression of VIP. Present in vitro results show that the relative numbers of VIP-containing neurons are equal in both P2Y13 +/+- and P2Y13 −/−-derived cultures and that it decreases after high-PA exposure in both genotypes. In myenteric neurons from P2Y13 +/+ cultures, the selective P2Y13 receptor antagonist reversed the PA-induced decrease of VIP-containing neurons at all PA concentrations, except the highest. Inactivating the P2Y13 receptor may thus change the expression of VIP through CREB pathway activation. VIP asserts, as has previously been described in several in vivo and in vitro systems, a potent role in neuroprotection [28–30].
With the exception of an increased number of colonic nNOS-containing submucous neurons in P2Y13 +/+ mice on HFD, the relative numbers of nNOS-containing enteric neurons were similar in both the ileum and colon irrespective of diet. Why the relative numbers of submucous nNOS-containing neurons increase to 26 % after HFD cannot easily be explained. A dramatic increase in the relative frequency of nNOS-containing submucous neurons has previously been reported to occur during culture [31]. After 8 days in culture conditions, 50 % of all submucous neurons were found to express nNOS. This suggests a role of nitric oxide (NO) in enteric neuronal plasticity and survival.
In contrast to a previous report on mice in which nNOS-containing submucous neurons were reported to be approximately 3 % [32], we detected high (15–30 %) relative numbers of nNOS-containing neurons. This could be explained by strain differences or by technical differences due to the antibodies used in the immunocytochemical detection of nNOS-containing neurons.
Purinergic transmission was first discovered and described in the GI tract by Professor G. Burnstock et al. in 1970 [4]. Since ATP was recognized as a non-adrenergic non-cholinergic (NANC) inhibitory transmitter, our knowledge on the importance of purine nucleotides and nucleosides in the GI tract has significantly expanded. Purinergic pathways have been elucidated in depth both concerning normal intestinal physiology and with focus on a number of GI diseases; for a review, Antonioli et al. [33]. Purinergic signalling has also been emphasized as highly associated with diabetes and its adverse effects [34]. In the gut, ATP is mainly regarded a co-transmitter of NO and VIP eliciting non-nitrergic NANC inhibitory motor responses. In addition, purines and purinergic receptors are important in regulating intestinal secretion as well as vasoconstriction. Both P2X and P2Y receptors are found to be expressed in the GI tract, and for most of the purinergic receptors, a functional role has been described. With the exception of a vague suggestion that purines may act synergistically with glia-derived growth factors [35], none of the purines or the purinergic receptors has so far been ascribed any protective or hazardous role in the ENS. In the current study, we present strong evidence for the involvement of the P2Y13 receptor in lipid-induced loss of myenteric neurons both in vivo and in vitro.
Conclusion
Myenteric neurons from mice lacking the P2Y13 receptor or treated with a selective P2Y13 receptor antagonist are resistant against HFD- and PA-induced loss. The cellular and molecular mechanisms behind, rendering them this unique feature, are at present not understood. Strong evidence supports that intestinal neuropathy and neurodegeneration are instrumental in several intestinal diseases. Consequently, it may be speculated that individuals with inactivated P2Y13 receptors would better withstand injurious events involving lipotoxicity and metabolic stress. Also, P2Y13 antagonism might constitute a novel therapeutic strategy to patients affected by intestinal dysmotility involving neuropathy. Further exploration of the purinergic pathways and possible enteric nerve/glia/immune cell interplays would bring on important clues on how to prevent or treat gastrointestinal dysfunction.
References
Frank L, Kleinman L, Ganoczy D et al (2000) Upper gastrointestinal symptoms in North America—prevalence and relationship to healthcare utilization and quality of life. Dig Dis Sci 45:809–818
Tougas G, Chen Y, Hwang P, Liu MM, Eggleston A (1999) Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the DIGEST study. Am J Gastroenterol 94:2845–2854
Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M (2001) Prevalence of gastrointestinal symptoms associated with diabetes mellitus—a population-based survey of 15,000 adults. Arch Intern Med 161:1989–1996
Burnstock G, Campbell G, Satchell D, Smythe A (1970) Evidence that adenosine triphosphate or a related nucleotide is transmitter substance released by non-adrenergic inhibitory nerves in gut. Br J Pharmacol 40:668
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (2009) Purinergic signalling in the nervous system: an overview. Trends Neurosci 32:19–29
Burnstock G, Cocks T, Kasakov L, Wong HK (1978) Direct evidence for ATP release from non-adrenergic, non-cholinergic (purinergic) nerves in guinea-pig taenia-coli and bladder. Eur J Pharmacol 49:145–149
Abbracchio MP, Burnstock G (1998) Purinergic signalling: pathophysiological roles. Jpn J Pharmacol 78:113–145
Zimmermann H (1996) Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol 49:589–618
Gourine AV, Wood JD, Burnstock G (2009) Purinergic signalling in autonomic control. Trends Neurosci 32:241–248
Communi D, Gonzalez NS, Detheux M et al (2001) Identification of a novel human ADP receptor coupled to G(i). J Biol Chem 276:41479–41485
Malin SA, Molliver DC (2010) Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain 6:12
Kobayashi K, Yamanaka H, Yanamoto F, Okubo M, Noguchi K (2012) Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia 60:1529–1539
Jacquet S, Malaval C, Martinez LO et al (2005) The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell Mol Life Sci 62:2508–2515
Gao ZG, Ding Y, Jacobson KA (2010) P2Y(13) receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res 62:500–505
Amisten S, Meidute-Abaraviciene S, Tan C et al (2010) ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice. Diabetologia 53:1927–1934
Yano S, Tsukimoto M, Harada H, Kojima S (2012) Involvement of P2Y13 receptor in suppression of neuronal differentiation. Neurosci Lett 518:5–9
del Puerto A, Diaz-Hernandez JI, Tapia M et al (2012) Adenylate cyclase 5 coordinates the action of ADP, P2Y1, P2Y13 and ATP-gated P2X7 receptors on axonal elongation. J Cell Sci 125:176–188
Espada S, Ortega F, Molina-Jijon E et al (2010) The purinergic P2Y(13) receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med 49:416–426
Fabre AC, Malaval C, Ben Addi A et al (2010) P2Y13 receptor is critical for reverse cholesterol transport. Hepatology 52:1477–1483
Blom D, Yamin TT, Champy MF et al (2010) Altered lipoprotein metabolism in P2Y(13) knockout mice. Biochim Biophys Acta 1801:1349–1360
Serhan N, Cabou C, Verdier C et al (2013) Chronic pharmacological activation of P2Y(13) receptor in mice decreases HDL-cholesterol level by increasing hepatic HDL uptake and bile acid secretion. Biochim Biophys Acta 1831:719–725
Voss U, Sand E, Olde B, Ekblad E (2013) Enteric neuropathy can be induced by high fat diet in vivo and palmitic acid exposure in vitro. PloS One 8(12):e81413
Brommage R (2003) Validation and calibration of DEXA body composition in mice. Am J Physiol Endocrinol Metab 285:E454–E459
Sand E, Themner-Persson A, Ekblad E (2009) Mast cells reduce survival of myenteric neurons in culture. Neuropharmacology 56:522–530
Voss U, Sand E, Hellström PM, Ekblad E (2012) Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons. BMC Gastroenterol 12:30
Kvietys PR, Specian RD, Grisham MB, Tso P (1991) Jejunal mucosal injury and restitution—role of hydrolytic products of food digestion. Am J Physiol 261:G384–G391
Falktoft B, Georg B, Fahrenkrug J (2009) Signaling pathways in PACAP regulation of VIP gene expression in human neuroblastoma cells. Neuropeptides 43:387–396
Ekblad E, Mulder H, Sundler F (1996) Vasoactive intestinal peptide expression in enteric neurons is upregulated by both colchicine and axotomy. Regul Pept 63:113–121
Lin Z, Sandgren K, Ekblad E (2003) Increased expression of vasoactive intestinal polypeptide in cultured myenteric neurons from adult rat small intestine. Auton Neurosci 107:9–19
Sandgren K, Lin Z, Svenningsen AF, Ekblad E (2003) Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J Neurosci Res 72:595–602
Lin Z, Sandgren K, Ekblad E (2004) Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton Neurosci 114:29–38
Young HM, Ciampoli D (1998) Transient expression of neuronal nitric oxide synthase by neurons of the submucous plexus of the mouse small intestine. Cell Tissue Res 291:395–401
Antonioli L, Colucci R, Pellegrini C et al (2013) The role of purinergic pathways in the pathophysiology of gut diseases: pharmacological modulation and potential therapeutic applications. Pharmacol Ther 139:157–188
Burnstock G, Novak I (2013) Purinergic signalling and diabetes. Purinergic Signal 9:307–324
Höpker VH, Saffrey MJ, Burnstock G (1996) Neurite outgrowth of striatal neurons in vitro: involvement of purines in the growth-promoting effect of myenteric plexus explants. Int J Dev Neurosci 14:439–451
Sand E, Voss U, Hammar O et al (2012) Gonadotropin-releasing hormone analog buserelin causes neuronal loss in rat gastrointestinal tract. Cell Tissue Res 351(3):521–534
Ekblad E, Ekman R, Håkansson R, Sundler F (1988) Projections of peptide-containing neurons in rat colon. Neuroscience 27:655–674
Ekblad E, Alm P, Sundler F (1994) Distribution, origin and projections of nitric-oxide synthase-containing neurons in gut and pancreas. Neuroscience 63:233–248
Acknowledgments
We thank Anna Themner-Persson for excellent technical assistance.
Funding
This study was supported by Påhlsson foundation, Royal Physiographic Society and Faculty of Medicine, Lund University.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voss, U., Turesson, M.F., Robaye, B. et al. The enteric nervous system of P2Y13 receptor null mice is resistant against high-fat-diet- and palmitic-acid-induced neuronal loss. Purinergic Signalling 10, 455–464 (2014). https://doi.org/10.1007/s11302-014-9408-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-014-9408-5