Abstract
In plants, sucrose synthase (SUS, EC 2.4.1.13) is widely considered a multifunctional protein involved in modulating sink strength, cellulose biosynthesis, and carbon partitioning. However, supporting genetic evidence regarding the role of SUS from bamboo in fiber development is lacking. Here, we obtained transgenic poplar lines overexpressing the bamboo BeSUS5 gene and conducted functional analysis. We found that overexpression of BeSUS5 enhanced the activity of SUS and significantly promoted the growth of the plants, especially xylem growth. In BeSUS5 overexpressed poplar plants, the total soluble sugar (TSS) and starch contents were decreased in leaves, while the cellulose content was increased in stems, indicating that overexpression of BeSUS5 might enhance the partitioning of carbon to cellulose in poplar. Consistent with these results, the expression of cellulose biosynthesis and phloem loading–related genes, such as cellulose synthase (CesA7), KORRIGAN (KOR), and sucrose transporter (SUT1), was upregulated in transgenic plants. As a result, transgenic poplars displayed not only an increase in cell wall thickness and cell wall crystallinity but also an altered stem fiber phenotype. Taken together, our results imply the vast potential of BeSUS5 for the genetic improvement of wood cellulose production and fiber quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant cell wall is mainly composed of cellulose, hemicellulose, and lignin, which not only provide mechanical support for the plant and aid in avoiding biotic and abiotic stresses but also provide plentiful raw materials (Cassab 1998; Somerville 2006; Kumar et al. 2016). Any changes in the composition or structural properties of cell walls may have significant effects on plant morphology, growth potential, and biomass (Niu et al. 2018). Among these compounds, cellulose is the major carbon reservoir in plants, and its content and availability can impact the conversion and production of biofuel as well as affect the yield of pulp and the quality of paper (Jin et al. 2017).
Cellulose is a type of polysaccharide that is composed of β,1-4-glucan chains and gives rise to microfibrils. Each microfibril consists of 36 nonbranched β-1,4-glucan chains, and each of them contains numerous glucose residues (Li et al. 2014; Kumar and Turner 2015). The synthesis of cellulose requires a series of enzymes, such as CesA, COBRA-like (COBL), KORRIGAN (KOR), SUS, and some NAC and MYB transcription factors. Generally, its synthesis in higher plants is primarily dependent on glycosyltransferase (GT). CesA belongs to the GT2 subfamily (Kudlicka and Brown 1996; Scheible and Pauly 2004). It is generally accepted that the microfilament or the glucan chain is formed by cellulose synthase complex (CSC) at the plasma membrane (Somerville 2006; McFarlane et al. 2014; Xi et al. 2017). Moreover, COBL is a glycosylphosphatidylinositol (GPI)-anchored protein that can promote cell elongation and thickening (Niu et al. 2018) and affect cellulose microfibril orientation (Roudier et al. 2005; Li et al. 2013; Glass et al. 2015). KOR, an endo-1,4-β-glucanase (EGase), is associated with CSC and affects the content, crystallinity, and degree of polymerization of cellulose (Szyjanowicz et al. 2004; Robert et al. 2005; Maloney et al. 2012). Additionally, the domain of unknown function 231 (DUF231) family proteins, PdDUF231A (Yang et al. 2017) and some transcription factors, such as AtNAST1, AtNST2, and AtMYB46, could affect cellulose biosynthesis (Mitsuda et al. 2005; Zhong et al. 2007; Kim et al. 2013; Yang et al. 2017).
SUS is another member of the GT family (belonging to the GT4 subfamily) and plays an important role in the synthesis of cellulose in plants. It has been widely accepted that SUS can degrade sucrose and provide a UDP-glucose substrate for cellulose synthesis (Albrecht and Mustroph 2003; Persia et al. 2008; Coleman et al. 2009; Fujii et al. 2010; Ma et al. 2014). However, two separate studies have described the role of SUS in cellulose biosynthesis (Xu et al. 2012; Ruan et al. 2003; Coleman et al. 2009; Barratt et al. 009; Kumar et al. 2014). Previous studies in cotton have shown that increased SUS activity could enhance leaf expansion and fiber elongation (Xu et al. 2012). Additionally, deficient sucrose synthase activity represses cotton fiber cell initiation (Ruan et al. 2003). Similarly, evidence from Populus indicated that overexpression of the Gossypium hirsutum SUS gene in Populus could increase SUS enzyme activity in developing xylem, which affects carbon partitioning, increasing cellulose production and altering the cell wall ultrastructure (Coleman et al. 2009). However, some evidence from transgenic Arabidopsis and Populus plants suggests that SUS is not essential for cellulose biosynthesis. For example, in Arabidopsis sus1sus2sus3sus4 mutant plants lacking SUS activity, obvious changes in phenotypes and cellulose have not been found (Barratt et al. 2009). In Populus, reduced native SUS activity in developing wood has suggested that SUS plays a role in defining total carbon incorporation into wood cell walls but is not essential for cellulose biosynthesis (Kumar et al. 2014). Therefore, the role of SUS in the synthesis of cellulose remains partly unclear.
Bamboo, often considered the fastest growing woody plant, requires the partitioning of large amounts of carbon to cellulose production (Chiu et al. 2006). Moreover, bamboo fiber is widely used in pulping owing to its high cellulose content, permeability, tensile strength, and so on (Singh et al. 2004). Thus, bamboo is an ideal plant with which to investigate the mechanisms of carbon partitioning or cellulose synthesis. In our previous studies, seven BeSUSs genes were identified from bamboo, and the expression of BeSUS5 was highest during the development of bamboo shoots; after treatment with a cellulose synthesis inhibitor, BeSUS5 was clearly induced (Huang et al. 2018). These data suggested that BeSUS5 may have a unique role in fiber development. Therefore, we transformed BeSUS5 with the cauliflower mosaic virus (CaMV) 35S promoter into Chinese white poplar (P. tomentosa Carr.), which is an important pulp species and a good model system for studying wood development. We found that heterologous expression of BeSUS5 in poplar not only enhanced the activity of SUS but also improved cellulose production, cell wall thickness, and fiber quality in transgenic poplar. These data confirmed that bamboo BeSUS5 is unequivocally involved in determining carbon partitioning to wood cell walls and improving fiber quality.
Materials and methods
Plant material and growth conditions
Bamboo (Bambusa emeiensis) shoots (170 cm) used in this research were collected from the Botanical Resource Center of Southwest University of Science and Technology, Mianyang, Sichuan, China. After removing their sheaths, the top, middle, and base of the shoots were cut into smaller pieces and frozen in liquid nitrogen immediately for RNA extraction.
Aseptic seedlings of poplar (Populus tomentosa Carr.) were used for plant transformation. Poplar plantlets were grown on fresh woody plant medium (WPM) at 25 °C under a 16/8 h light/dark cycle with 5000 lux supplemental light at a relative humidity of approximately 60% for optimum growth (Li et al. 2015). Each of the following analyses was performed on at least three separate genotypes of each transgenic line and control plants.
Transgenic vector construction and plant transformation
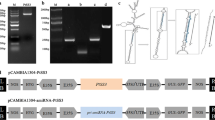
BeSUS5 was cloned from Bambusa emeiensis (GenBank KJ525750) as previously described (Huang et al. 2018) and was inserted into pCAMBIA1303N under the regulation of CaMV35S promoters. The resulting vector p35S:BeSUS5 with the Hpt (hygromycin phosphotransferase) gene (Fig.S1a), which confers resistance to hygromycin, was introduced into Agrobacterium tumefaciens strain EHA105 for poplar transformation.
P. tomentosa Carr. was transformed using Agrobacterium tumefaciens EHA105 as described previously (Huang et al. 2012). Transgenic plants were screened on WPM supplemented with 9 mg/L hygromycin for 3–5 weeks, and the survivors were transferred into soil for propagation.
DNA isolation and PCR analysis
Genomic DNA was isolated from leaves of putative transgenic plants using the CTAB method (Jia et al. 2010), and then the plantlets were confirmed as transgenic by polymerase chain reaction (PCR) screening of genomic DNA using gene-specific primers for the BeSUS5 gene (Table S1).
RNA extraction, cDNA synthesis, and qRT-PCR analysis
For expression analysis of BeSUS5 in transgenic plants, the leaves of 2-month-old transgenic plants were used for RNA extraction using TRIzol reagent. For reverse transcription PCR (RT-PCR), 1 μg of total RNA was reverse transcribed in a total volume of 20 μL using the PrimeScript® RT reagent kit (Perfect DNA) (Takara, Dalian, China) according to the instructions. The quality of the RNA was analyzed with 1% agarose gel electrophoresis and confirmed spectrophotometrically. cDNA was synthesized using a FastQuant RT kit (TianGen, Beijing, China).
A quantitative real-time PCR (qRT-PCR) assay was performed in a 20 μL reaction volume containing 9 μL of × 2.5 Real Master Mix/20 × SYBR solution (TianGen, Beijing, China), 8 μL of nuclease-free H2O, 1 μL of each primer, and 1 μL of cDNA, and run on a CFX connect™ Real-Time PCR detection system (Bio-Rad, Singapore). The poplar Actin gene was used as an internal standard. The gene-specific primers are listed in Supplemental Table S1. Student’s t test program (http://www.graphpad.com/quickcalcs/ttest1.cfm) was used for statistical analysis of the qRT-PCR data.
To analyze the expression pattern of SUS1-7 in bamboo shoots, the bamboo constitutive tubulin gene was used as an internal standard, and the gene-specific primers are displayed in Table S1.
Subcellular localization of BeSUS5
The open reading frame (ORF) of BeSUS5 was amplified with gene-specific primers (Table S1) and cloned into the pTEX vector to create a 35S:BeSUS5-GFP fusion vector (Fig. S1b). The recombinant plasmid 35S: BeSUS5-GFP and the control plasmid 35S: GFP were then bombarded into onion epidermal cells using a Gene Gun PDS-1000/He (Bio-Rad, USA). The onion skin was observed with a confocal laser microscope (Leica TCSSP8, Germany).
Enzyme assay
The activities of soluble SUS and membrane-bound SUS were measured. Briefly, the different tissues, including bamboo shoots, leaves, and stems of transgenic poplars, were ground into fine powder in liquid nitrogen and homogenized with 2-mL solution I (50 mM HEPES 5 mM MgCL2, 1 mM EGTA, 1 mM EDTA, 5 mM DTT, 10% glycerin, 40 mg/mL PVPP, 1 mM PMSF) and 20 μL 1% PMSF. After centrifugation at 12,000 rpm at 4 °C for 3 min, the supernatant was washed with 0.75 mL solution I and centrifuged further at 12,000 rpm at 4 °C for 3 min again, and a 0.5-mL supernatant was used as the soluble fraction. The precipitate was homogenized with 2.5 mL solution II (50 mM HEPES, 5 mM MgCL2, 1 mM EGTA, 1 mM EDTA, 10% glycerin). The sample was centrifuged for 4 min at 12,000 rpm in a precooled centrifuge at 4 °C; the supernatant was discarded, and the pellet was retained. This step was repeated twice. Then, the pellet was homogenized with 2 mL solution III (20 mM MES/KOH pH 0.6, 0.1 M MES, 2.5 M NaCl) and stored at − 4 °C for at least 16 h. After centrifugation, the supernatant fraction was used as the crude microsomal membrane fraction.
Reaction and reading
The enzyme preparation was incubated at 30 °C for 30 min with 50 μL reaction buffer (50 mM HEPES/KOH pH 7.0, 8% Suc and 10 mM UDP), and the reaction was stopped at 100 °C for 2 min. Then, 680 μL distilled water and 750 μL DNS were added to the reaction mixture and further incubated at 100 °C for 5 min. After cooling, the optical density (OD) of the sample was measured at 540 nm by a Multiskan EX spectrophotometer (ThermoScience, Rockford, IL, USA). In the control, the reaction buffer was replaced by 50 μL 50 mM HEPES, and the other steps were the same as those above.
Photosynthetic rates, soluble carbohydrate, and starch analysis
The photosynthetic rate was determined by Li-6400 portable photosynthesis system (USA, LiCOR) according to the manufacturer’s instructions.
The leaves and stems of transgenic poplars were ground into fine powder in liquid nitrogen, and 0.2 g of samples was incubated with 80% (v/v) ethanol at 80 °C for 10 min. After centrifugation at 6500 rpm for 15 min, the supernatant fraction was kept. The sample was further washed with 50% (v/v) ethanol, and the supernatant fractions were combined. Then, acetone was added to the fractions to further remove protein. Finally, the content of total soluble sugar (TSS) was determined by anthrone colorimetry at 620 nm. Furthermore, the pellet was further used to measure the starch content by the perchloric acid hydrolysis-anthranone colorimetric method. The pellet was incubated with 9.2 mol/L perchloric acid at 100 °C for 15 min. After cooling, the pellet was centrifuged further at 6500 rpm for 15 min, and the supernatant was used to measure the starch content at 620 nm.
Cellulose determination and FTIR analysis
The cellulose contents of the stems were measured following the protocol of the Updegraff method with appropriate modifications (Updegraff 1969; Ambavaram et al. 2011; Niu et al. 2018). The stems were ground to fine powder in liquid nitrogen, and 3.0 g of sample was incubated at 37 °C for 30 min with 5 mL 0.1 M phosphoric acid buffer (pH 7.2). Then, the sample was incubated with 80% (v/v) ethanol at 80 °C for 1 h, and the cell wall material (CWM) was suspended in acetone and dried at 60 °C for 12 h. The dried CWM was digested in acetic acid/water/nitric acid (8/2/1, v/v) for 30 min in a boiling water bath and dried at 60 °C. The samples were hydrolyzed by incubation with 72% H2SO4 (v/v) at room temperature for 1 h. Then, the sample was diluted, 10 mL precooled anthrone solution (0.2 g of anthrone was dissolved in 100 mL H2SO4) was added, and the sample was incubated for 16 min at 100 °C of water. Finally, the sample was measured at 625 nm, and α-cellulose was used as a standard.
FTIR analysis was performed according to Huang et al. (2018). FTIR spectra were acquired in the range of 4000~400 cm−1 (Perkin Elmer, Spectrum one). An area of 100 × 100 μm was selected for FTIR analysis, and the acquisition parameters were 4 cm+ resolution. The experiment was performed three times with control and transgenic plants.
Anatomical observations
For histological observations, stems from the same parts (the 14th internode) of 2-month-old wild-type and transgenic plants grown in a glasshouse were fixed with 2% formaldehyde and subsequently passed over a graded ethanol series. Then, the sections were embedded in paraffin. Eight-micrometer-thick sections were cut out with a rotary microtome. After the paraffin was removed, sections were examined with a light microscope. Images were captured under bright field using a Leica DM2500 (Leica, German). The radial widths of the bark, pith, and xylem were measured using ImageJ.
For histochemical staining, stem (the 5th and 12th internodes) sections from 2-month-old plants grown in the greenhouse were hand-cut with a razor blade. The microsections were stained for 15 s with 1.0% (w/v) phloroglucinol after dissociation for 60 s by 40% (v/v) HCl (hydrochloric acid) and then observed under a Leica DM2500 (Leica, German).
For scanning electron microscopy (SEM), the stem sections were attached to the scanning electron microscope using double-sided tape. Samples were viewed under Sigma 300 (Carl Zeiss, German) following the manual’s recommendations. Eight areas of lines were randomly selected for measurement of the number of layers and the cell wall thickness (μm) using ImageJ.
Lignin analysis
Lignin analysis was performed using thioglycolic acid (TGA) (Rueda-López et al. 2015). Briefly, the CWM was suspended with acetone and dried at 35 °C for 12 h. The pellet (crystalline cellulose) was added to 750 μL water, 250 μl HCl, and 100 μL thioglycolic acid and then further incubated at 80 °C for 3 h. After centrifugation at 12,000 rpm for 3 min, the supernatant fraction was washed with water and centrifuged further at 12,000 rpm for 3 min. The supernatant was added to 1 mL 1 M NaOH and placed in a shaker overnight at room temperature and centrifuged further at 12,000 rpm for 3 min. The supernatant was added to 200 μL 1 M HCl and incubated at 4 °C for 4 h. The sediment was collected, and 1 mL of 1 M NaOH was added. Finally, the sample was measured at 280 nm.
Determination of the fiber morphology and crystallinity of cellulose
To analyze the fiber of the transgenic plants, the CWM of stems was obtained as mentioned above, and a fiber quality analyzer (code LDA02) was used to determine the fiber morphology, including fiber length, width, fiber fine content, curl index and kink index, according to the operating instructions. The crystallinity of cellulose was estimated by the Segal method (Segal et al. 1959). This is an empirical method to quickly determine the crystallinity by X-ray diffraction (XRD). The crystallinity index was calculated according to (I002-Iam)/I002 × 100%
where I002 is the peak intensity of the maximum diffraction intensity on plane 002, and Iam is the diffraction intensity at 2θ = 18°.
Statistical analysis
Statistical analysis was performed using SPSS 13.0. In all these experiments, quantitative differences between the two groups of data for comparison were calculated using Student’s t test at p = 0.05 or p = 0.01. The differences were considered statistically significant when p < 0.05 and extremely significant when p < 0.01 (*P < 0.05; **P < 0.01.)
Results
Expression pattern of BeSUS5 in different tissues and the subcellular localization of BeSUS5
In this work, we analyzed the total activity of SUS and the transcript abundance of BeSUSs by qRT-PCR (Fig. S2). We found that SUS activity was not obviously different in any part of the bamboo shoot examined (Fig. S2a, b). BeSUS5 was ubiquitously expressed in all parts of the shoots and preferentially expressed in the middle of the shoots (Fig. S2c). Furthermore, a BeSUS5:GPF fusion protein driven by the CaMV 35S promoter was constructed and transformed into onion epidermal cells to investigate the subcellular localization of BeSUS5 in vivo. As shown in Fig. 1, the control protein (pTEX-35S: GFP) was distributed in both the cytoplasm and the nucleus of the onion cells, while the BeSUS5:GFP fusion protein was only detected in the membrane. These results indicate that BeSUS5 is targeted to the membrane and might have a unique role in bamboo shoot development.
Generation of transgenic poplar plants overexpressing BeSUS5
To study the essential role of BeSUS5 in cellulose synthesis, we constructed the plant expression vector pCAMBIA1303N under BeSUS5 and then introduced it into P. tomentosa Carr. using an Agrobacterium-mediated transformation method as described previously (Jia et al. 2010). In total, more than 14 independent putative transgenic plants were obtained and were further verified at the genomic and transcriptional levels by PCR and qRT-PCR with gene-specific primers (Fig. 2a, b). Three of the transformed lines with high expression of the BeSUS5 gene were selected for in-depth analysis (L2, L3, and L11) (Fig. 2c). Furthermore, SUS enzyme activity was measured as the soluble fraction and membrane fraction activities in leaves. Figure 3 shows that there were various degrees of increase in SUS activity, especially the membrane-bound SUS activities and total SUS activities, in comparison with that of the wild-type (WT). The membrane-bound SUS activities were increased by 49–131% in the transgenic plants compared to those in the WT (Fig. 3a). Successful heterologous expression of the bamboo BeSUS5 gene in poplar resulted in a significant upregulation of SUS activities in transgenic plants.
Molecular analyses of BeSUS5 transgenic trees and growth comparisons of wild-type (WT) and BeSUS5 transgenic plants. Genomic DNAs were isolated from hygromycin-resistant plants transformed with the 35S:BeSUS5 vector. a PCR analysis of wild-type and different independently regenerated transgenic lines. M, DL2000 DNA marker; +, corresponding plasmid DNA (positive control); -, ddH2O (negative control). b Quantitative RT-PCR analyses of 35S:BeSUS5 transgenic poplar plants. The results are presented as the mean values ± SE. Student’s t test: *P < 0.05; **P < 0.01. c Phenotypes of wild-type and three representative 35S:BeSUS5-overexpressing lines (lines 2, 3, and 11) at the 2nd month
Phenotype analysis of transgenic plants overexpressing BeSUS5
To investigate whether BeSUS5 would affect the normal growth and development of transgenic plants, we examined the phenotypes of 2-month-old transgenic poplars in a greenhouse. As shown in Table 1, transgenic plants (lines L2, L3, and L11) grew faster than WT plants and showed highly significant increases in plant height, leaf area, and stem diameter. The plant height was increased from 36.77 cm in WT plants to 39.94– 41.8 cm in transgenic plants, and the leaf areas of the transgenic plants (including the second, fourth, sixth, and eighth leaves) ranged from 13.78 to 154.05% larger than that of the WT. Most importantly, the stem diameter was increased from 1.97 mm in WT to 2.03–2.34 mm in BeSUS5 transgenic plants (Table 1). As shown in Fig. S3, all tested transgenic plants showed increased xylem growth, whereas there was no significant difference between the bark and pith compared to the WT (Fig. S3a, b). These findings suggested that overexpression of BeSUS5 in transgenic poplar plants might promote plant growth, perhaps due to increased SUS activity.
Overexpression of BeSUS5 increased photosynthetic capability, cellulose contents, and cell wall thickness in poplar
The photosynthetic rates and the levels of nonstructural carbohydrates (NSCs) were quantified in the transgenic lines to investigate the effect of increased SUS activity. We found that the net photosynthetic rates increased 19.53–62.14% in the transgenic lines (Fig. S4). However, there were marked decreases in soluble sugar and starch contents in the leaves of the transgenic lines compared to those of WT, whereas no significant changes were found in stems, contrary to the results of previous studies. In detail, the TSS in the leaves of the three transgenic lines was 3.12–3.68 mg/g FW and a decrease of 9.8–23.53% compared with WT (average 4.08 mg/g FW, Fig. 4a), The starch in leaves was also clearly decreased, ranging from 2.24 to 4.45 mg/g FW, compared with 5.62 mg/g FW in WT (Fig. 4b). However, neither TSS nor starch content was significantly changed in the stem.
Soluble sugar and starch contents in WT and transgenic plants. a The soluble sugar and starch contents in leaves, and the total soluble sugar content (TSS), determined as the sum of soluble sucrose, b the soluble sugar and starch contents in stems. The results are presented as the mean values ± SE. Student’s t test: *P < 0.05; **P < 0.01
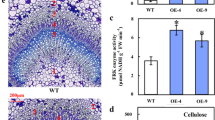
To examine whether the change in carbon partitioning observed in the transgenic line leaves was due to xylem growth, microscopic analyses were undertaken with the stems of WT and transgenic plants. As shown in Fig. 5, xylem growth was indeed improved in transgenic lines, especially in the mature stem. Histochemical staining revealed that the 12th percentile of stem sections of transgenic lines was wider than that of WT plants (Fig. 5a), and an increased number of cell layers was observed in transgenic plants (Fig. 6a). Scanning electron microscopy (SEM) analyses demonstrated that the thickness of secondary cell walls of 35S:BeSUS5 plants was dramatically increased compared with that of the control lines (Fig. 5b). The cell wall thickness of BeSUS5 overexpression plants was 46.7–55.4% thicker than that of the WT control (Fig. 6b).
Effects of BeSUS5 overexpression on secondary cell wall formation in poplar. a Phloroglucinol staining of stem sections of transgenic plants and wild-type plants (the 5th internode and the 12th internode). Ph, phloem; Xy, xylem. b SEM of the stem sections (the 12th internode) of wild-type and transgenic plants. Bars: a = 200 μm; b = 10 μm
The number of cell layers and cell wall thickness in stems of WT and transgenic poplar plants. a The number of cell layers in stems of wild-type and transgenic plants; b the cell wall thickness in stems of wild-type and transgenic plants. The results are presented as the mean values ± SE. Student’s t test: *P < 0.05; **P < 0.01
To examine the changes in the composition of the secondary cell wall, the lignin and cellulose contents were measured. As shown in Fig. 7a, we found that there were no statistically significant changes in the overall lignin contents. However, the cellulose content in the stem was obviously increased (increased by 19.66–58.85%) compared with that of the control (Fig. 7b). Furthermore, FTIR analysis showed that the FTIR spectrum of transgenic plant stems changed in absorbance intensity and locations of specific peaks (Fig. 7c). Therefore, these data suggest that the higher activities of SUS might cause a decrease in the biosynthesis of TSSs and starch in leaves and promote an increase in the cellulose content in stems, resulting in secondary wall thickening.
Cellulose and lignin contents in the stems of WT and transgenic poplar plants. a The content of lignin in stems; b the content of cellulose; c the Fourier transform infrared (FTIR) spectra of the stems of wild-type and transgenic poplar plants. Error bars represent ± SE of three biological replicates. Student’s t test: *P < 0.05; **P < 0.01
Overexpression of BeSUS5 altered the fiber phenotype and increased cell wall crystallinity in poplar
To further investigate the potential function of BeSUS5 during fiber growth, fiber phenotypes were observed. Table 2 shows that although no significant changes in the fiber length as well as in the curl index and kink index were observed between WT and transgenic plants, the content of fine fiber and the fiber width decreased in transgenic plants. Moreover, the aspect ratio (fiber L/W) increased obviously in transgenic plants compared to that of the WT (Table 2). Taken together, these results indicate that the fibers of transgenic plants became thicker than those of WT plants. Furthermore, the cell wall crystallinity was significantly elevated in all transgenic lines; the average crystallinity degree was increased by up to 1.1-fold compared with that of the WT control trees (Table 2). All these results indicated that SUS activity indeed plays a crucial role in carbon partitioning and cellulose biosynthesis and even modulates the fiber phenotype.
Overexpression of BeSUS5 could promote the expression of cellulose synthesis genes in poplar
To seek further evidence to verify the role of BeSUS5 in cellulose synthesis and carbon transfer, the xylem and leaf expression levels of several genes involved in carbohydrate metabolism and cellulose biosynthesis were determined by qRT-PCR, including poplar sucrose phosphate synthase (SPS), CesA, and KOR in the stem, and SUT in the leaf. As shown in Fig. 8, the SPS was significantly downregulated in transgenic plants, while the cellulose biosynthesis gene CesA7, an ortholog of AtCesA7, was three- to four-fold higher in the transgenic lines than in the WT. Furthermore, CesA3 (an ortholog of AtCesA3) was downregulated in all transgenic plants, and the expression level of KOR was most dramatically enhanced (11- to 17-fold) in all of the transgenic plants. In source leaves, SUT1 which associated with phloem upload was significantly upregulated. These results indicated that BeSUS5 plays an important role in cellulose biosynthesis and carbon transfer.
Relative gene expression of cellulose biosynthesis-related genes in stems of WT and transgenic poplar plants. Approximately 2.0-cm segments from the base stem were sampled for qRT-PCR analysis of four genes: CesA3 (KF809715), CesA7 (KF613995), KOR (HQ380298), and SPS (MF463440) in wild-type plants and different transgenic lines. Error bars represent ± SE of three biological replicates. Student’s t test: *P < 0.05; **P < 0.01
Discussion
The shoot of a bamboo plant often arises from the rhizome of its mother plant. As such, the mother plant is required to produce a large proportion of the sucrose needed to support the rapid growth rate of the shoot (Chiu et al. 2006), and the dynamic allocation and transfer of nonstructural carbohydrates (NSC) may be a possible mechanism for the explosive growth of bamboo. When new shoots develop into mature bamboo culms, the culms are used in pulping because of their high-quality fibers (Rai et al. 2011), so the development of bamboo shoots is inevitably associated with the rapid partitioning of carbon and the accumulation of enzymes related to cellulose synthesis (Song et al. 2016a). Among the many genes involved in sucrose metabolism, SUS has long been considered a biochemical marker for sink strength, but to some extent, the role of SUS in the synthesis of cellulose is still controversial (Baroja-Fernandez et al. 2012; Smith et al. 2012; Kumar et al. 2014). In bamboo, Bambusa emeiensis, 7 BeSUS genes were identified in a previous study, and in the current work, we found that BeSUS5 was ubiquitously expressed in the rapid growth stage of bamboo shoots (170 cm) and was especially accumulated in the middle part of the bamboo shoot, which is an important area for bamboo fiber development and maturity (Fig. S2c). Furthermore, BeSUS5 is targeted to the membrane (Fig. 1), and this is consistent with the results of subsequent enzyme analysis of transgenic plants that showed that heterologous expression of the BeSUS5 gene resulted in a significant upregulation of membrane-bound SUS activities of transgenic poplar. The SUS protein has two distinct forms, a soluble form and a membrane-bound form. The membrane-bound SUS is generally thought to provide a substrate (UDPG) for cellulose synthesis and callose formation. Therefore, we speculated that BeSUS5 may directly supply UDP-glucose to CSC, thus promoting bamboo cellulose synthesis and shoot development.
The function of BeSUS5 was investigated by overexpressing this gene in poplar, which is a good model system for studying wood development. In this study, the BeSUS5 overexpressed poplar plants showed significant growth acceleration, including increases in plant height, leaf area and stem diameter (Table 1). These findings concur with some previous studies in Populus alba, tobacco and cotton. Konishi et al. (2004) observed an increase in plant height when overexpressing the modified mung bean SUS gene in Populus alba. Similarly, Nguyen et al. (2016) also found an improvement in height growth in transgenic tobacco overexpressing the A. thaliana SUS gene. In cotton, overexpression of a potato SUS gene not only enhanced plant height but also accelerated leaf expansion (Xu et al. 2012). However, the growth phenotypes of some SUS transgenic plants showed no dramatic changes. For instance, Coleman et al. (2009) transformed the Gossypium hirsutum SUS gene into hybrid poplar with the 35S promoter, and transgenic poplars exhibited a normal phenotype. Similarly, the downregulation of SUS in Populus has also shown no influence on plant height (Kumar et al. 2014). Some studies suggest that this discrepancy among the phenotypes of transgenic plants largely depends on the exogenous gene source or the type of transgenic plants (Coleman et al. 2009; Li et al. 2019). SUS may be necessary in crops or fast-growing tree species for the development of bulky sink organs (Bieniawska et al. 2007; Ruan et al. 2010) or for a fast growth rate, but not in other species. Bamboo is a typical plant with a fast growth rate, while poplar grows relatively slowly. We observed that the BeSUS5 overexpressed poplar plants grew rapidly and that the xylem widened (Fig. 5a), indicating that BeSUS5 might perform similar functions in bamboo. In addition, some studies showed that a decrease in SUS activity did not affect the growth of transgenic poplar, which may be due to the role of invertases in sucrose cleavage after the strong reduction in SUS activity (Kumar et al. 2014).
Plant NSC contents are essential indicators for evaluating SUS transgenic plants (Xu et al. 2012; Nguyen et al. 2016). Some studies showed that the overexpression of SUS in plants resulted in a significant change in NSC levels. For example, Coleman et al. (2009) found that poplar overexpressing GhSUS under the control of the 4CL promoter had a significant increase in total soluble carbohydrates in the stem and no statistically significant changes the leaves (only 1 line showed statistical significance), while the starch content did not change in either the leaves or stems. Additionally, the overexpression of AtSUS in tobacco resulted in increases in the total soluble carbohydrate content in leaves and starch content in stems (Nguyen et al. 2016). In the current work, the larger leaf area of the BeSUS5 overexpressed poplar plants with a higher net photosynthetic rate (Fig. S4) might mean the amount of TSS produced from the source leaves would also increase. However, we observed decreases in soluble sugar and starch in the leaves, whereas no significant changes were found in the stems (Fig. 4). Further analysis revealed that the cellulose content of the stems was obviously increased (Fig. 7b). Thus, one possibility is that the decreased amount of NSCs in leaves might cause an increase in cellulose in the stem. That is, the decrease in NSC accumulation in transgenic plant sink leaves might mean a higher export rate. Subsequent gene expression analysis in leaves further confirmed our hypothesis (Fig. 8). SUTs are H+-coupled sucrose symporters and are essential for sucrose translocation (Lalonde et al. 2004). The SUT1 belongs to group II transporters and is thought to be involved in the loading of sucrose phloem (Gottwald et al. 2000). In the current work, the expression level of SUT1 significantly increased (Fig. 8), indicating that overexpression of BeSUS5 might improve stem xylem growth and cellulose synthesis through enhanced sucrose supply from source leaves. In plants, carbohydrates have two main forms, including both SCs and NSCs (Dietze et al. 2014). NSCs, composed of monosaccharides, disaccharides, and starch, are the main energy substances in plant energy metabolism (Raessler et al. 2010). SCs are the principal substances of plant morphogenesis and are mainly composed of cellulose (Li et al. 2008; Song et al. 2016). Cellulose synthesis generally relies on NSCs as substrates, so the increase in cellulose content in stems may be one of the reasons for the decrease in NSCs in leaves. Furthermore, the rapid growth of plants may also lead to a relative decrease in NSCs as energy sources. For example, moso bamboo shows rapidly increased height and diameter in a short time, which is attributed to the mature bamboo transferring almost all NSCs from the source and sink tissues, such as leaves and branches, to the “explosively growing” branches through underground rhizomes (Song et al. 2016). Taken together, these results suggest that overexpression of the BeSUS5 gene could increase the activities of SUS and regulate carbon transfer.
The CesA and KOR (Robert et al. 2005; Yu et al. 2014) genes were found to be involved in cellulose synthesis. Hence, we further analyzed the expression of CesA3, CesA7, and KOR, and the results showed that overexpression of BeSUS5 resulted in an increase in the expression of KOR and CesA7 and a slight decrease in CesA3 expression (Fig. 8). Considering that SUS is not a transcription factor, its influence on KOR and CesA expression might be an indirect effect. In Arabidopsis, the AtCesA3 and AtCesA7 (Brown et al. 2005; Taylor-Teeples et al. 2015) genes are known to be associated with the regulation of primary and secondary cell wall biosynthesis, respectively. Additionally, the function of PtrCesA7 has been characterized in poplar, and it is considered to be involved in the formation of the poplar secondary wall (Abbas et al. 2019). Moreover, the crystallinity of cellulose might be related to CesA (Arioli et al. 1998). During the development of the plant cell wall from the primary wall to secondary wall, the crystallinity and polymerization of cellulose increase (Blaschek et al. 1982; Zhang et al. 2013), which means that the expression levels of different members of the CESA family may change. KOR, a membrane-bound endo-1,4-β-D-glucanase, has been proposed to remove noncrystalline glucan chains and/or relieve tensional stress, which may be generated during the assembly of microfibrils (Mølhøj et al. 2001; Robert et al. 2005). Glass et al. (2015) pointed out that downregulation of AtGH9C2 (an ortholog of KOR) led to a decrease in cell wall crystallinity. In the current work, thickening of the secondary cell wall in the mature poplar stem and an increase in the crystallinity of cellulose were found (Figs. 5 and 6). Therefore, we inferred that overexpression of BeSUS5 affected the expression levels of CesA7, KOR, and CesA3 to different degrees, which promoted cellulose deposition in the secondary xylem and further resulted in increased thickness of xylem cell walls (Fig. 6b) and cell wall crystallinity (Table 2). These observations are consistent with those of the previous study by Coleman et al. (2009), who found that in transgenic hybrid poplar, the elevated concentration of cellulose was associated with an increase in cell wall crystallinity. Furthermore, overexpression of BeSUS5 in poplar reduced the expression of SPS in the stem (sink) (Fig. 8), whereas a previous study showed that SPS expression was upregulated in SUS overexpressed tobacco leaves (source) (Nguyen et al. 2016). Haigler et al. (2001) proposed that the main role of SPS is to control fructose recycling, which could lead to the continuous synthesis of large amounts of sucrose. However, the results of this study differ from the hypothesis to some extent. We suggest that the differences in SPS expression in different transgenic plants may be ascribed to the differences growth states of plants or carbon levels between source and sink tissues. In plant sink tissue, fructose is involved not only in sucrose synthesis but also in different metabolic pathways, such as respiration (Ahmed et al. 2018). In the current work, we inferred that the accelerated growth of BeSUS5 overexpressed poplar plants might require a large amount of energy, possibly from respiration. Moreover, the reduced amount of NSC in sink leaves might be further transported to the stem, where the rich carbon might affect the expression of SPS. In summary, the present results imply that the reduced amount of NSCs in leaves might be further transported to the stem through the symplastic or apoplastic pathways and further used in cellulose synthesis or rapid growth, thus indirectly affecting the expression of SPS, CesA, KOR, or other genes (Fig. 9).
A hypothetical model of how overexpression of BeSUS5 genes affects plant growth. The model highlights that overexpressing the bamboo BeSUS5 gene in poplar can regulate carbon transfer and affect the expression of CesA, KOR, and SPS or other genes, resulting in increased cellulose synthesis, improved cell wall crystallinity and changed fiber phenotype, and finally promote plant growth. PM, plasma membrane
Furthermore, another important observation of this study is that the fiber phenotype of transgenic poplar was altered. Increased activities of SUS not only resulted in a decreased fiber width and fine fiber content but also in an increased fiber aspect ratio (Table 2). In the pulp and paper industry, the fiber aspect ratio is closely linked to paper quality. The increase in the fiber aspect ratio promotes the interlacing between fibers and enhances paper strength. Notably, these results are contrary to a recent study of tobacco transgenic lines overexpressing PsnSuSy1 and 2, which found that the fiber length was elongated whereas the fiber width did not significantly change (Li et al. 2019). Therefore, the different patterns of change of fiber phenotypes in various SUS-overexpressing transgenic plants need to be further studied.
Conclusions
In summary, combining the experimental results in this study, we speculated that overexpression of BeSUS5 would promote NSC partitioning to the stem and affect the expression of cellulose biosynthesis genes, which consequently results in increased cellulose synthesis and cell wall crystallinity and an altered fiber phenotype in transgenic poplars (Fig. 9). The information shown in this work implies the great potential of BeSUS5 in the genetic improvement of woody biomass and fiber quality.
References
Abbas M, Peszlen I, Shi R, Kim H, Katahira R, Kafle K, Xiang Z, Huang X, Min D, Mohamadamin M, Yang C, Dai X, Yan X, Park S, Li Y, Kim SH, Davis M, Ralph J, Sederoff RR, Chiang VL, Li Q (2019) Involvement of CesA4, CesA7-A/B and CesA8-A/B in secondary wall formation in Populus trichocarpa wood. Tree Physiology 40:73–89. https://doi.org/10.1093/treephys/tpz020
Ahmed M, Shahid AA, Akhtar S, Latif A, Din S, Fanglu M, Rao AQ, Sarwar MB, Husnain T, Xuede W (2018) Sucrose synthase genes: a way forward for cotton fiber improvement. Biologia 73:703–713. https://doi.org/10.2478/s11756-018-0078-6
Albrecht G, Mustroph A (2003) Sucrose utilization via invertase and sucrose synthase with respect to accumulation of cellulose and callose synthesis in wheat roots under oxygen deficiency. Russian Journal of Plant Physiology 50:813–820. https://doi.org/10.1023/B:RUPP.0000003280.10924.03
Ambavaram MMR, Krishnan A, Trijatmiko KR, Pereira A (2011) Coordinated activation of cellulose and repression of lignin biosynthesis pathways in rice. Plant Physiol 155:916–931. https://doi.org/10.1104/pp.110.168641
Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W, Camilleri C, Höfte H, Plazinski J, Birch R, Cork A, Glover J, Redmond J, Williamson RE (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279:717–720. https://doi.org/10.1126/science.279.5351.717
Baroja-Fernandez E, Munoz FJ, Li J, Bahaji A, Almagro G, Montero M, Etxeberria E, Hidalgo M, Sesma MT, Pozueta-Romero J (2012) Sucrose synthase activity in the sus1/sus2/sus3/sus4 Arabidopsis mutant is sufficient to support normal cellulose and starch production. Proceedings of the National Academy of Sciences 109:321–326. https://doi.org/10.1073/pnas.1117099109
Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis: the sucrose synthase gene family in Arabidopsis. The Plant Journal 49:810–828. https://doi.org/10.1111/j.1365-313X.2006.03011.x
Blaschek W, Koehler H, Semler U, Franz G (1982) Molecular weight distribution of cellulose in primary cell walls: Investigations with regenerating protoplasts, suspension cultured cells and mesophyll of tobacco. Planta 154:550–555. https://doi.org/10.1007/BF00402999
Brown DM, Zeef LAH, Ellis J, Goodacre R, Turner SR (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17:2281–2295. https://doi.org/10.1105/tpc.105.031542
Cassab GI (1998) Plant cell wall proteins. Annu Rev Plant Physiol Plant Mol Biol 49:281–309. https://doi.org/10.1146/annurev.arplant.49.1.281
Chiu W-B, Lin C-H, Chang C-J, Hsieh MH, Wang AY (2006) Molecular characterization and expression of four cDNAs encoding sucrose synthase from green bamboo Bambusa oldhamii. New Phytol 170:53–63. https://doi.org/10.1111/j.1469-8137.2005.01638.x
Coleman HD, Yan J, Mansfield SD (2009) Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proceedings of the National Academy of Sciences 106:13118–13123. https://doi.org/10.1073/pnas.0900188106
Dietze MC, Sala A, Carbone MS, Czimczik CI, Mantooth JA, Richardson AD, Vargas R (2014) Nonstructural carbon in woody plants. Annu Rev Plant Biol 65:667–687. https://doi.org/10.1146/annurev-arplant-050213-040054
Fujii S, Hayashi T, Mizuno K (2010) Sucrose synthase is an integral component of the cellulose synthesis machinery. Plant and Cell Physiology 51:294–301. https://doi.org/10.1093/pcp/pcp190
Glass M, Barkwill S, Unda F, Mansfield SD (2015) Endo-β-1,4-glucanases impact plant cell wall development by influencing cellulose crystallization: endoglucanses alter cell wall formation. J Integr Plant Biol 57:396–410. https://doi.org/10.1111/jipb.12353
Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proceedings of the National Academy of Sciences 97:13979–13984. https://doi.org/10.1073/pnas.250473797
Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin K, Delmer DP (2001) Carbon partitioning to cellulose synthesis. In: Carpita NC, Campbell M, Tierney M (eds) Plant cell walls. Springer Netherlands, Dordrecht, pp 29–51
Huang Y, Liao Q, Hu S, Cao Y, Xu G, Long Z, Lu X (2018) Molecular cloning and expression analysis of seven sucrose synthase genes in bamboo ( Bambusa emeiensis ): investigation of possible roles in the regulation of cellulose biosynthesis and response to hormones. Biotechnology & Biotechnological Equipment 32:316–323. https://doi.org/10.1080/13102818.2017.1412271
Jia Z, Sun Y, Yuan L, Tian Q, Luo K (2010) The chitinase gene (Bbchit1) from Beauveria bassiana enhances resistance to Cytospora chrysosperma in Populus tomentosa Carr. Biotechnol Lett 32:1325–1332. https://doi.org/10.1007/s10529-010-0297-6
Jin Y-L, Tang R-J, Wang H-H, Jiang CM, Bao Y, Yang Y, Liang MX, Sun ZC, Kong FJ, Li B, Zhang HX (2017) Overexpression of Populus trichocarpa CYP85A3 promotes growth and biomass production in transgenic trees. Plant Biotechnology Journal 15:1309–1321. https://doi.org/10.1111/pbi.12717
Kim W-C, Kim J-Y, Ko J-H, Kim J, Han KH (2013) Transcription factor MYB46 is an obligate component of the transcriptional regulatory complex for functional expression of secondary wall-associated cellulose synthases in Arabidopsis thaliana. Journal of Plant Physiology 170:1374–1378. https://doi.org/10.1016/j.jplph.2013.04.012
Konishi T, Ohmiya Y, Hayashi T (2004) Evidence that sucrose loaded into the phloem of a poplar leaf is used directly by sucrose synthase associated with various β-Glucan synthases in the stem. Plant Physiol 134:1146–1152. https://doi.org/10.1104/pp.103.033167
Kudlicka K, Brown RM (1996) Cellulose biosynthesis in higher plants. 65:149–156
Kumar M, Turner S (2015) Plant cellulose synthesis: CESA proteins crossing kingdoms. Phytochemistry 112:91–99. https://doi.org/10.1016/j.phytochem.2014.07.009
Kumar, Manoj, Gorzsas, et al (2014) Deficient sucrose synthase activity in developing wood does not specifically affect cellulose biosynthesis, but causes an overall decrease in cell wall polymers. 203:1220–1230
Kumar M, Campbell L, Turner S (2016) Secondary cell walls: biosynthesis and manipulation. EXBOTJ 67:515–531. https://doi.org/10.1093/jxb/erv533
Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55:341–372. https://doi.org/10.1146/annurev.arplant.55.031903.141758
Li M-H, Xiao W-F, Wang S-G, Cheng GW, Cherubini P, Cai XH, Liu XL, Wang XD, Zhu WZ (2008) Mobile carbohydrates in Himalayan treeline trees I. Evidence for carbon gain limitation but not for growth limitation. Tree Physiology 28:1287–1296. https://doi.org/10.1093/treephys/28.8.1287
Li S, Ge F-R, Xu M, Zhao XY, Huang GQ, Zhou LZ, Wang JG, Kombrink A, McCormick S, Zhang XS, Zhang Y (2013) Arabidopsis COBRA-LIKE 10, a GPI-anchored protein, mediates directional growth of pollen tubes. Plant J 74:486–497. https://doi.org/10.1111/tpj.12139
Li S, Bashline L, Lei L, Gu Y (2014) Cellulose synthesis and its regulation. The Arabidopsis Book 12:e0169. https://doi.org/10.1199/tab.0169
Li C, Wang X, Ran L, Tian Q, Fan D, Luo K (2015) PtoMYB92 is a Transcriptional activator of the lignin biosynthetic pathway during secondary cell wall formation in Populus tomentosa. Plant Cell Physiol 56:2436–2446. https://doi.org/10.1093/pcp/pcv157
Li M, Wang S, Liu Y, Zhang Y, Ren M, Liu L, Lu T, Wei H, Wei Z (2019) Overexpression of PsnSuSy1, 2 genes enhances secondary cell wall thickening, vegetative growth, and mechanical strength in transgenic tobacco. Plant Mol Biol 100:215–230. https://doi.org/10.1007/s11103-019-00850-w
Ma Y, Wang Y, Liu J, Lv F, Chen J, Zhou Z (2014) the effects of fruiting positions on cellulose synthesis and sucrose metabolism during cotton (Gossypium hirsutum L.) Fiber Development. PLoS ONE 9:e89476. https://doi.org/10.1371/journal.pone.0089476
Maloney VJ, Samuels AL, Mansfield SD (2012) The endo-1,4-β-glucanase Korrigan exhibits functional conservation between gymnosperms and angiosperms and is required for proper cell wall formation in gymnosperms. New Phytologist 193:1076–1087. https://doi.org/10.1111/j.1469-8137.2011.03998.x
McFarlane HE, Döring A, Persson S (2014) The cell biology of cellulose synthesis. Annu Rev Plant Biol 65:69–94. https://doi.org/10.1146/annurev-arplant-050213-040240
Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2005) The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell 17:2993–3006. https://doi.org/10.1105/tpc.105.036004
Mølhøj M, Ulvskov P, Dal Degan F (2001) Characterization of a functional soluble form of a Brassica napus membrane-anchored endo-1,4-β-glucanase heterologously expressed in Pichia pastoris. Plant Physiol 127:674–684. https://doi.org/10.1104/pp.010269
Nguyen QA, Luan S, Wi SG, Bae H, Lee DS, Bae HJ (2016) Pronounced Phenotypic changes in transgenic tobacco plants overexpressing sucrose synthase may reveal a novel sugar signaling pathway. Front Plant Sci 6: https://doi.org/10.3389/fpls.2015.01216
Niu, Erli, Fang, et al (2018) Ectopic expression of GhCOBL9A, a cotton glycosyl-phosphatidyl inositol-anchored protein encoding gene, promotes cell elongation, thickening and increased plant biomass in transgenic Arabidopsis. 1–14
Persia D, Cai G, Del Casino C et al (2008) Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiol 147:1603–1618. https://doi.org/10.1104/pp.108.115956
Raessler M, Wissuwa B, Breul A, Unger W, Grimm T (2010) Chromatographic analysis of major non-structural carbohydrates in several wood species – an analytical approach for higher accuracy of data. Anal Methods 2:532. https://doi.org/10.1039/b9ay00193j
Rai V, Ghosh JS, Pal A, Dey N (2011) Identification of genes involved in bamboo fiber development. Gene 478:19–27. https://doi.org/10.1016/j.gene.2011.01.004
Robert S, Bichet A, Grandjean O, Kierzkowski D, Satiat-Jeunemaître B, Pelletier S, Hauser MT, Höfte H, Vernhettes S (2005) An Arabidopsis endo-1,4-β-d-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell 17:3378–3389. https://doi.org/10.1105/tpc.105.036228
Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GHH, Schindelman G, Song S, Baskin TI, Dupree P, Wasteneys GO, Benfey PN (2005) COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell 17:1749–1763. https://doi.org/10.1105/tpc.105.031732
Ruan Y-L, Llewellyn DJ, Furbank RT (2003) Suppression of sucrose synthase gene expression represses cotton fiber cell initiation, elongation, and seed development. Plant Cell 15:952–964. https://doi.org/10.1105/tpc.010108
Ruan Y-L, Jin Y, Yang Y-J, Li GJ, Boyer JS (2010) sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Molecular Plant 3:942–955. https://doi.org/10.1093/mp/ssq044
Rueda-López M, Cañas RA, Canales J, Cánovas FM, Ávila C (2015) The overexpression of the pine transcription factor PpDof5 in Arabidopsis leads to increased lignin content and affects carbon and nitrogen metabolism. Physiol Plantarum 155:369–383. https://doi.org/10.1111/ppl.12381
Scheible W-R, Pauly M (2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Current Opinion in Plant Biology 7:285–295. https://doi.org/10.1016/j.pbi.2004.03.006
Segal L, Creely JJ, Martin AE, Conrad CM (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Textile Research Journal 29:786–794. https://doi.org/10.1177/004051755902901003
Singh S, Bhatt, Singha (2004) Commercial edible bamboo species of the North-Eastern Himalayan Region. India. Part I: young shoot sales. Journal of Bamboo and Rattan 3:337–364. https://doi.org/10.1163/1569159042464680
Smith AM, Kruger NJ, Lunn JE (2012) Source of sugar nucleotides for starch and cellulose synthesis. Proceedings of the National Academy of Sciences 109:E776–E776. https://doi.org/10.1073/pnas.1200878109
Somerville C (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22:53–78. https://doi.org/10.1146/annurev.cellbio.22.022206.160206
Song X, Peng C, Zhou G, Gu H, Li Q, Zhang C (2016) Dynamic allocation and transfer of non-structural carbohydrates, a possible mechanism for the explosive growth of Moso bamboo (Phyllostachys heterocycla). Sci Rep 6:25908. https://doi.org/10.1038/srep25908
Szyjanowicz PMJ, McKinnon I, Taylor NG, Gardiner J, Jarvis MC, Turner SR (2004) The irregular xylem 2 mutant is an allele of korrigan that affects the secondary cell wall of Arabidopsis thaliana. Plant J 37:730–740. https://doi.org/10.1111/j.1365-313X.2003.02000.x
Taylor-Teeples M, Lin L, de Lucas M, Turco G, Toal TW, Gaudinier A, Young NF, Trabucco GM, Veling MT, Lamothe R, Handakumbura PP, Xiong G, Wang C, Corwin J, Tsoukalas A, Zhang L, Ware D, Pauly M, Kliebenstein DJ, Dehesh K, Tagkopoulos I, Breton G, Pruneda-Paz JL, Ahnert SE, Kay SA, Hazen SP, Brady SM (2015) An Arabidopsis gene regulatory network for secondary cell wall synthesis. Nature 517:571–575. https://doi.org/10.1038/nature14099
Updegraff DM (1969) Semimicro determination of cellulose inbiological materials. Analytical Biochemistry 32:420–424. https://doi.org/10.1016/S0003-2697(69)80009-6
Xi W, Song D, Sun J, Shen J, Li L (2017) Formation of wood secondary cell wall may involve two type cellulose synthase complexes in Populus. Plant Mol Biol 93:419–429. https://doi.org/10.1007/s11103-016-0570-8
Xu S-M, Brill E, Llewellyn DJ, Furbank RT, Ruan YL (2012) Overexpression of a potato sucrose synthase gene in cotton accelerates leaf expansion, reduces seed abortion, and enhances fiber production. Molecular Plant 5:430–441. https://doi.org/10.1093/mp/ssr090
Yang Y, Yoo CG, Winkeler KA, Collins CM, Hinchee MAW, Jawdy SS, Gunter LE, Engle NL, Pu Y, Yang X, Tschaplinski TJ, Ragauskas AJ, Tuskan GA, Chen JG (2017) Overexpression of a domain of unknown function 231-containing protein increases O-xylan acetylation and cellulose biosynthesis in Populus. Biotechnol Biofuels 10:311. https://doi.org/10.1186/s13068-017-0998-3
Yu L, Chen H, Sun J, Li L (2014) PtrKOR1 is required for secondary cell wall cellulose biosynthesis in Populus. Tree Physiology 34:1289–1300. https://doi.org/10.1093/treephys/tpu020
Zhang W, Yi Z, Huang J, Li F, Hao B, Li M, Hong S, Lv Y, Sun W, Ragauskas A, Hu F, Peng J, Peng L (2013) Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresource Technology 130:30–37. https://doi.org/10.1016/j.biortech.2012.12.029
Zhong R, Richardson EA, Ye Z-H (2007) The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell 19:2776–2792. https://doi.org/10.1105/tpc.107.053678
Funding
This work was supported by the National Natural Science Found of China (31400257), the Science and Technology Project of Sichuan Province, China (18YYJC0920), and the Demonstration Project of the Industrial Chain of Sichuan Province, China (19Z21842).
Author information
Authors and Affiliations
Contributions
HY and WLL designed the experiments and performed the study; HY and CY drafted the manuscript; WLL performed the transgene study; HSL and LXG proposed and supervised the overall project. All authors read and approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Data archiving statement
The full-length CDS of BeSUS5 (accession number KJ525750) has been submitted to GenBank.
Additional information
Communicated by A. De La Torre
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, Y., Wang, L., Hu, S. et al. Overexpression of the bamboo sucrose synthase gene (BeSUS5) improves cellulose production, cell wall thickness and fiber quality in transgenic poplar. Tree Genetics & Genomes 16, 75 (2020). https://doi.org/10.1007/s11295-020-01464-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11295-020-01464-w