Abstract
Beech is one of the most important trees in the temperate and subtropical forests of the Northern Hemisphere. Despite Chinese beeches have the particularity that only grow in subtropical areas, they have received few phylogeographic research. In this study, we sampled 25 populations of the northernmost-distributed Chinese beech, Fagus engleriana, and detected six haplotypes across 350 individuals by using sequences of two chloroplast intergenic spacers. The chloroplast genetic diversity was relatively low (h T = 0.659), with most genetic variance residing among populations (G ST = 0.831, N ST = 0.855, G ST≈N ST). SAMOVA analysis indicated that populations clustered into six groups with little admixture among them (most groups were characterized by a unique hapotype). Pairwise difference among haplotypes and Fu’s Fs statistic indicated that populations of F. engleriana have not experienced recent sudden expansions. Both the phylogeographic and demographic patterns found in this study suggest that F. engleriana remained fragmented in multiple refugia throughout the Pleistocene climatic changes, and experienced limited both glacial and interglacial/postglacial expansion. The results of this study imply that long-term isolation among multiple refugia, coupled with little admixture among populations of different refugia provided numerous opportunities for population divergence and allopatric speciation, which might be a driving factor for the exceptionally broad temperate species diversity in southern China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Sino-Japanese Floristic Region (SJFR) of East Asia harbors the most diverse flora among all the regions in the northern temperate zone (López-Pujol et al. 2011; Qian and Ricklefs 2000; Qiu et al. 2011). Subtropical China, a hilly mid-elevation area ranging between the Qingling Mountains-Huai River line (at ca. 34° N) and the tropical south (≤22° N), is a core part of SJFR (Wu and Wu 1996). Being characterized by subtropical climate and a complex topography, this area interestingly sustains numerous temperate forests at high elevations which are separated by extensive tropical and subtropical forests occupying lower altitudes (Qian and Ricklefs 2000). The particular combination of climate and geomorphology, coupled with much less impact of glaciations during the Pleistocene could explain why the temperate flora of subtropical China have responded to the Pleistocene climatic changes in a different and more complex way (e.g. Gong et al. 2008; Harrison et al. 2001; Qiu et al. 2009a, b; Tian et al. 2010; Wang and Ge 2006; Wang et al. 2009; Zhou et al. 2010) compared with the European, North American and even Japanese counterparts. In those regions, survival in the south during the glacials (in ‘glacial refugia’; Bennett and Provan 2008) followed by postglacial recolonization of northern regions is assumed to be the general pattern among a variety of temperate plant species (Hewitt 2004; Hu et al. 2009; Okaura and Harada 2002; Soltis et al. 2006).
Based on present-day temperate taxa distributions, Qian and Ricklefs (2000) suggested that temperate forests (particularly the temperate-deciduous forests) in subtropical China would have spread to lower elevations during glacial periods of the Pleistocene, allowing isolated patches to join together (and forming thus a continous band of vegetation); in contrast, warmer climates in the interglacials would have forced them back to refugia (‘interglacia refugia’ sensu Bennett and Provan 2008) at higher elevations, as occurs today. However, simulated palaeovegetation maps based on fossil pollen remains suggest that temperate-deciduous forests in subtropical latitudes suffered a considerable range constriction during glacial periods, being either replaced by non-forest biomes (Ni et al. 2010) or by mixed forests (boreal and mixed temperate-boreal forests; Harrison et al. 2001). Further north (>31° N) temperate-deciduous forests were entirely wiped out and replaced by steppe/desert vegetation. Although palaeodata provide some evidence for both range and altitudinal shift of temperate deciduous forests in subtropical China (Harrison et al. 2001; Ni et al. 2010; Zheng 2000), reconstructions of entire palaeo-forest biomes cannot be accurate because of the scarce pollen fossil records from China (Liu et al. 2003; Ni et al. 2010; Qian and Ricklefs 2001). Furthermore, distribution limits of a particular species are difficult to reconstruct using palaeodata owing to problems of pollen representation and dispersal, as well as to poor taxonomic resolution (Comes and Kadereit 1998).
Molecular phylogeographic studies have provided independent evidence for identifying possible refugia and migration routes for many plant species. To date, the range-shift histories of European, Northern American and Japanese temperate flora have been outlined by numerous phylogeographic studies, which began to be compiled more than one decade ago (e.g. Okaura and Harada 2002; Petit et al. 2005; Soltis et al. 2006). In contrast, in subtropical China, which hosts a much larger diversity of temperate species, just a small sample has been studied during recent times (Gong et al. 2008; Qiu et al. 2009a, b; Tian et al. 2010; Wang and Ge 2006; Wang et al. 2009; Zhou et al. 2010; see Qiu et al. 2011 for a review). These pioneering studies have suggested a general pattern of multiple refugia and little admixture among refugial populations. However, we must be aware that this pattern is based on a relatively few case studies, mostly focused on endangered species with narrow distribution ranges (Gong et al. 2008; Wang and Ge 2006). Therefore, it would be of great interest to know if this pattern is also applicable to the typical and dominant species in temperate deciduous forests, which may be of greater diagnostic value for identifying the putative refugial areas for this biome in subtropical China during the Pleistocene glaciations. An accurate delimitation of glacial refugia is a high priority for conservation because these are key areas for the persistence and evolution of biodiversity (López-Pujol et al. 2011).
Fagus (beech) is one of the most important components of the temperate deciduous forests in the Northern Hemisphere (Denk 2003; Shen 1992). In North America, Europe and Japan, Fagus species are widespread in the temperate zone with a cool and moist climate. In China, however, they are absent in the deciduous forest of the temperate zone, but occur in mountainous areas in the moist subtropical zone, south of 34° N (Cao et al. 1995; Fang and Lechowicz 2006; Guo and Werger 2010; Liu et al. 2003). Ecological studies suggest that the prevailing Pacific monsoon and the complex topography of southern China could be the responsible factors for their occurrence within subtropical mountains (Cao et al. 1995; Fang and Lechowicz 2006; Guo and Werger 2010). Chinese beeches represent a typical component in temperate deciduous forests of subtropical China, rendering them an ideal choice for depicting the range shift history of such vegetation type during the Pleistocene. However, despite their biogeographic significance, Chinese beeches have received very little attention from the phylogeographers (but see Liu 2008).
Fagus engleriana Seem. is the only representative of subgen. engleriana among Chinese beeches (Denk 2003; Denk et al. 2005; Shen 1992) and occurs in the northernmost part of the Chinese beech range (i.e. in the coolest climate) (Liu et al. 2003, see Fig. 1). Similar to other Chinese beeches, the altitude of the lowest occurrence of F. engleriana increases from about 800 m in the southeast to about 2,550 m in the northwest, forming isolated patches among extensive evergreen forests at the lowlands (Guo and Werger 2010). We chose F. engleriana among Chinese beeches because of two reasons. First, given that interspecific sharing of chloroplast variants in woody genera is common (particularly among closely related species) due to incomplete lineage sorting and/or introgression (Funk and Omland 2003), it is less likely for F. engleriana to hybridize with other beech species and more likely for lineage sorting to complete since subgen. engleriana is a divergent lineage within Fagus (Denk 2003; Denk et al. 2005). The uniqueness of subgen. engleriana is represented by its multistemmed growth form, rougher, darker bark and thinner leaves than the members of subgen. Fagus, which includes single-stemmed beeches (Hiraoka and Tomaru 2009; Ohkawa et al. 2006; Shen 1992). Second, many populations of F. engleriana are located to the north of 31° N, where extensive expansion of boreal, mixed forest and/or treeless vegetation have been proposed to occur during the LGM (Harrison et al. 2001; Ni et al. 2010). Therefore, the distribution range of F. engleriana might have been severely fragmented or displaced during glacial periods, leaving profound genetic imprints on the current populations.
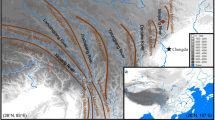
Distribution of six haplotypes within and among populations of F. engleriana. Pie sizes are proportional to the corresponding sample sizes. Right top is the network of six haplotypes of F. engleriana (H1–H6) and one haplotype of F. longipetiolata (Long). The pie size is proportional to the haplotype frequency. Acronyms in red italics: TP Tibetan Plateau, MCS Micangshan Mountains, QL Qinling Mountains, DBS Dabashan Mountaisn, WLS Wulingshan Mountains, DBIES Dabieshan Mountains, TMS Tianmushan Mountains. Dashed lines encircle SAMOVA-identified groups: pink, ‘Northwest group’; black, ‘Northwest-central group’; violet, ‘Southeast-central group’; orange, ‘East group’; green, ‘Easternmost group’; and red, ‘Central group’. Black lines indicate the distribution range of this beech
We used chloroplast sequences of atpI-atpH and ndhJ-trnF intergenic spacers (IGSs) to examine the phylogeographic pattern of 25 populations of F. engleriana across its distribution range. Our specific objectives were to address the following questions: (1) What is the genetic structure of a typical temperate deciduous species of subtropical China? (2) Did the fragments of F. engleariana coalesce during the glacial periods (as suggested by Qian and Ricklefs 2000) or remained isolated (as pointed out by vegetation reconstructions, e.g. Harrison et al. 2001)?
Materials and methods
Population sampling
We made extensive field surveys throughout the entire range of F. engleriana in China from 2006 to 2008. Fresh leaves of F. engleriana were collected from 25 populations across north subtropical China (Table 1, Fig. 1). In spite of great efforts, inaccessibility prevented the sampling of the two southern populations of Fanjingshan (Guizhou Province) and Maoershan (Guangxi Province). Because F. engleriana has a multi-stemmed growth form (Cao and Peters 1998), sampled trees were spaced by ca. 50–100 m to avoid collecting clones. To test the extent of haplotype sharing among beech species of China, three congeneric populations sympatric to F. engleriana were sampled: Taoyuan (Sichuan, 14 individuals) for Fagus lucida, Shimen (Hunan, 10 individuals) for Fagus longipetiolata, and Wangchang (Sichuan, 15 individuals) for Fagus hayatae. All samples were desiccated in silica gel and stored at −20°C until being processed.
DNA extraction, amplification and sequencing
Genomic DNA was extracted using a modified CTAB procedure (Doyle and Doyle 1987). Two IGSs of chloroplast genome, ndhJ-trnF and atpI-atpH, were selected for phylogeographic analysis after a screening of eight IGSs. The selected IGSs were amplified and sequenced, using the primers described in Taberlet et al. (1991) and Shaw et al. (2007), respectively. Amplification reactions were carried out in a volume of 25 μl containing 12.5 μl 2 × Taq PCR MasterMix (Tiangen, Shanghai, China), 1 μl forward and reverse primer each (0.2 uM), template DNA 2 μl (ca. 50–100 ng) and ddH2O 8.5 μl. Amplification was carried out in a Bioer XP cycler (Bioer, Hangzhou, China) programmed for an initial 240 s at 94°C, followed by 30 cycles of 60 s at 94°C, 45 s at 54°C (atpI-atpH) or 55°C (ndhJ-trnF), 120 s at 72°C and a final 600 s at 72°C. Sequencing reactions were conducted with the corresponding forward and reverse primers commercially by Sangon Biotech Co., Ltd. (Shanghai, China).
Data analysis
Because chloroplast genome is regarded as a single locus, the two fragments were pooled to define cpDNA haplotypes. A haplotype frequency map was constructed using ArcGIS 8.3. Total genetic diversity (h T) and within-population diversity (h S) were calculated with HAPLONST (Pons and Petit 1996). To determine phylogenetic relationships among haplotypes, median-joining networks were constructed using Network version 4.6 (Bandelt et al. 1999).
The geographical structure of chloroplast DNA variation in F. engleriana was investigated using two approaches. First, we evaluated whether there is a phylogeographic signal in the haplotype distribution by comparing G ST with N ST using the software HAPLONST. A higher N ST than G ST usually indicates the presence of phylogeographic structure (Pons and Petit 1996), with closely related haplotypes being found more often than less closely related haplotypes in the same area. Second, to explore the partitioning of the genetic variance into different geographically and genetically distinguishable groups, we analysed the populations using SAMOVA (Dupanloup et al. 2002). Various SAMOVA were run, increasing the number of K groups until the percentage of explained variance among groups reached a limit (K = 2–9).
To test whether F. engleriana has undergone recent demographic population expansion events, we conducted mismatch analysis using DNASP (version 5.0, Librado and Rozas 2009). Multimodal mismatch distributions of pairwise differences between individuals are expected for populations at demographic equilibrium with a relatively stable size over time, whereas unimodal distributions are expected for populations that have experienced recent demographic expansions (Harpending 1994). The goodness-of-fit of observed mistmatch distributions to the theoretical distributions under a model of sudden expansion was tested with the raggedness index (r) (Rogers and Harpending 1992). The significance of the raggedness index (r) was obtained by examining the null distribution of 5,000 coalescent simulations of these statistics. to complement this method, we calculated Fs statistic of Fu (1997) using Arlequin ver. 3.1 (Excoffier et al. 2005), which is based on the probability of having a number of haplotypes greater or equal to the observed number of samples drawn from a constant-sized population. The Fs is suggested to be more sensitive to population expansion than other statistics (Pilkington et al. 2008). The significance of Fs was assessed with coalescent simulations.
Results
Chloroplast haplotype variation, network and distribution
The aligned sequences of ndhJ-trnF and atpI-atpH intergenic spacers from 25 populations of F. engleriana were 1,045 and 1,104 bp in length, respectively. Two substitutions at sites 24 and 609 of ndhJ-trnF defined three variants, while two variants of atpI-atpH resulted from two substitutions at sites 216 and 413. Pooled sequences of two fragments allowed the identification of six chlorotypes among all individuals examined. Across 39 congeneric individuals, two haplotypes were detected, and H4 of F. engleriana was shared by F. lucida in Taoyuan and F. hayatae in Wangchang, indicating that H4 might be an ancestral haplotype among Chinese beeches. The haplotype found in Shimen of F. longipetiolata is absent in F. engleriana. The resulting network of six F. engleriana haplotypes and one F. longipetiolata haplotype (Fig. 1) was quite simple. H4 and H1 connected to H5 (the central haplotype) by one step. H6 connected to H4, and three tip nodes (H2, H3 and Long) to H1, each by one mutational step. All the chloroplast sequences have been deposited in GenBank under accession numbers HM347323-HM347328.
Among 25 populations of F. engleriana, 16 were fixed by a single haplotype, while the remaining nine were polymorphic (Table 1; Fig. 1). The most common haplotypes were H1, H2 and H4. H1 occurred at high frequency in FJ, SM, YC and XS, all located within southeastern Dabashan and Wulingshan Mountains, but also appeared at low frequency in WF, WX and WC. H2 was found in populations of northwestern Dabashan Mountains (CK, WF, YL, SNJ and WS). H4 was the most widespread haplotype, fixed or prevailing in the mountains of the eastern fringe of the Tibetan Plateau, Micangshan Mountains and Qinling Mountains (EMS, DJY, MY, QC, WX, KX, WC, ZB, LB, WJB, NZ, TY and LS). This haplotype also occurred in several populations in different parts of the species range at variable frequencies (CK, WF, FJ, YC and AJ). H3, H5 and H6 were low-frequency haplotypes; H3 was fixed in two populations of Dabieshan Mountains, YX and SC. H5 was disjunctly distributed: common in AJ of Tianmushan Mountains and rare in ZB of Micangshan Mountains. H6 occurred at low frequency in WF and SNJ, within Dabashan Mountains.
Genetic diversity and genetic structure
Total genetic diversity h T (0.659) across all populations was much higher than average within-population diversity h S (0.112). Consequently, both G ST (0.831) and N ST (0.855) were high. A permutation test indicated that N ST was not significantly higher than G ST (P = 0.29). In the SAMOVA analysis, F CT reached almost plateau value at K = 5 (F CT = 0.870); however, at K = 6, F SC was much minimized, approaching to zero (F SC = 0.07) and corresponded well with the geographical distribution of haplotypes (Table 2; Fig. 1). Thus we used K = 6 as the best grouping scheme. The first group (‘Northwest group’) was characterized by H4, and its populations (MS, DJY, MY, QC, WX, KX, WC, ZB, LB, WJB, NZ, TY and LS) were mostly distributed in the mountains of eastern Tibetan Plateau, the Micangshan and the Qinling Mountains. The second group (‘Northwest-central group’) contained four populations from northwestern Dabashan Mountains (CK, SNJ, WS and YL), where H2 was ubiquitous. The third group (‘Southeast-central group’) was found in southeastern Dabashan Mountains (XS and YC) and Wulingshan Mountains (SM and FJ), and was dominated by H1. The two populations of Dabieshan Mountains, SC and YX, constituted the ‘East group’, where H3 was exclusively found. WF and AJ constituted two separate groups, namely the ‘Central group’ and the ‘Easternmost group’, respectively. WF was closely located to the first three groups, exhibiting their dominant haplotypes (H1, H2 and H4) and the rare H6, suggesting that this population could have resulted from the admixture of divergent lineages from separate refugia (Chen et al. 2008). Although AJ contained high proportion of H4 (46.7 %), it deserved a separate group due to the large occurrence of the H5 haplotype, which, apart from there, is only present in a sole individual in the ZB population (Table 2). This is the reason why in the K = 5 grouping scheme AJ is merged with the northwest group (Table 2).
Historical demography
The mismatch distribution for chlorotypes of F. engleriana was bimodal, indicating this species differs from that predicted under a model of sudden population expansion (Fig. 2). Fu's Fs value (1.881; P = 0.805) for F. engleriana also supports that F. engleriana did not experience sudden population expansion. In contrast, a nonsignificant raggedness value (r = 0.128, P = 0.146) suggests that F. engleriana might conform to the model of sudden population expansion. However, the raggedness value may not be a good indicator for population expansion (e.g. Qiu et al. 2009b). In our analyses, the raggedness value was nonsignificant for all population groups (data not shown). The Easternmost group, which is the smallest group and most unlikely to experience any population expansion, still got a nonsignificant value (r = 0.289, P = 0.150). Thus, the nonsignificant raggedness value is not taken here as strong evidence of expansion.
Discussion
Genetic diversity and genetic structure
In this study, six haplotypes were found across 350 individuals in 25 populations of F. engleriana by aligning 2,149 bp chloroplast sequences. The number of haplotypes is lower than that found in three other beech species also studied by means of chloroplast sequences across their distribution ranges: the Japanese Fagus crenata (7 haplotypes/21 populations/351 individuals/1,515 bp; Okaura and Harada 2002), the Chinese F. longipetiolata (13 haplotypes/26 populations/201 individuals/1,664 bp; Liu 2008), and the American Fagus grandifolia (20 haplotypes/137 populations/232 individuals/1920 bp; Morris et al. 2010). In a survey of Fagus sylvatica by chloroplast PCR-RFLPs, 20 different haplotypes were detected in 1,800 beech trees from 352 populations (Magri et al. 2006), a figure much higher than that for F. engleriana. Although F. japonica and F. multinervis (the other two members of subgen. Engleriana endemic to Japan and Ullung Island of South Korea, respectively) have not been investigated by means of chloroplast sequencing, available data showed that both of them possess high genetic diversity (Hiraoka and Tomaru 2009; Ohkawa et al. 2006). Accordingly, genetic diversity of F. engleriana (h T = 0.659) is low compared with other beech species (cf. F. crenata: h T = 0.771, Okaura and Harada 2002; F. longipetiolata: h T = 0.906, Liu 2008; both values were calculated from the raw data provided by the authors and measured by chloroplast sequences).
The relatively low genetic diversity is contrary to our expectations, because the samples of F. engleriana are quite large both in terms of studied populations and sampled individuals per population. In addition, the sequences used in this study are also long compared with those of F. crenata, F. longipetiolata and F. grandifolia. This finding is also somewhat inconsistent with the results of many widespread plants in subtropical China (Gao et al. 2007; Qiu et al. 2009a; Wang et al. 2009; Yuan et al. 2008; Zhou et al. 2010), an unglaciated region which probably enjoyed relatively stable environmental conditions throughout the Pleistocene (López-Pujol et al. 2011). A possible explanation is that the distribution range of F. engleriana may have been severely fragmented or displaced during the LGM (and likely the previous glacial maxima) as the pollen fossil records suggest (Harrison et al. 2001; Ni et al. 2010; Yu et al. 2000), instead of expanding as proposed by Qian and Ricklefs (2000). F. engleriana occurs in the northernmost part of the Chinese beech range, under the coolest climate conditions (Cao et al. 1995), and this would have maximized its displacement, replacement and/or fragmentation in an scenario of much colder but especially drier glacial climates (López-Pujol et al. 2011; Ni et al. 2010). On the contrary, the much higher genetic diversity of F. longipetiolata (Liu 2008), a Chinese beech which today occupies southern and warmer habitats (Guo and Werger 2010), may be the result of its less extensive southwards displacement during glacial stages. An alternative or complementary explanation for the relatively low genetic diversity found in F. engleriana is the strong competition ability of evergreen trees and understory shrubs (such as bamboos) during the warm periods –such as the Holocene–, thus constraining the development of F. engleriana stands (Cao et al. 1995; Guo and Werger 2010).
Consistent with other beech species, the chloroplast genetic differentiation is very high (G ST = 0.831 and N ST = 0.855) in F. engleriana. Chloroplast genome is maternally inherited in beeches and, therefore, moved by seeds only (Morris et al. 2010). Most of the beech seeds simply drop to the ground under the parent trees and a few may roll down on steep terrain. Rodents may carry some of them short distances, and occasionally birds (e.g. blue jays for F. grandifolia) may transport beech seeds several kilometers (Johnson and Adkisson 1985). While the inefficient seed dispersal mechanisms are primarily responsible for the high chloroplast divergence observed in other beeches (Liu 2008; Morris et al. 2010; Magri et al. 2006; Okaura and Harada 2002), this may also be the case for F. engleriana, although its seed dispersal mode is still to be studied in detail.
Limited admixture among multiple refugia
Similar to many temperate plant species of subtropical China (Qiu et al. 2009a; Tian et al. 2010; Wang and Ge 2006; Wang et al. 2009; Zhou et al. 2010) and a few examples from northern China (e.g. Chen et al. 2008), SAMOVA analysis identified that most of the genetic variance (F CT = 0.874) of F. engleriana is among population groups. More significantly, and with the exception of population WF and the occasional sharing of three haplotypes among groups (H1, H4 and H5, which may be due to incomplete lineage sorting and/or interspecific hybridization; Jakob and Blattner 2006; Palme et al. 2004), most of the population groups of F. engleriana (which are generally characterized by a dominant single haplotype) are confined to different mountain ranges, showing a quite clear-cut phylogeographic structure (Fig. 1). In addition, bimodal distribution of pairwise difference among haplotypes (Fig. 2) and Fu's Fs statistic suggest that F. engleriana underwent limited range expansions (at the most, it spread locally within mountain ranges, as is apparent for Northwest group, see Fig. 1). Considering the low mutation rate of Fagaceae chloroplast genomes (2.36 × 10−10 substitutions−1 site year−1, one substitution represents 1.97 million years, Frascaria et al. 1993), the results pinpoint that F. engleriana may have experienced long-term isolation among multiple refugia (at least five) throughout the Pleistocene and undergone limited glacial expansions, with little admixture among populations of different origins. Therefore, the phylogeographic structure of F. engleariana supports the hypothesis that temperate deciduous forests in subtropical China remained isolated during glacial periods as pointed out by vegetation reconstructions (e.g. Harrison et al. 2001) rather than coalesced as suggested by Qian and Ricklefs (2000). This study highlights that molecular phylogeographic studies, particularly on dominant species of a given biome, can provide independent evidence for resolving biogeographical debates (e.g. Harrison et al. 2001; Qian and Ricklefs 2000).
The particular phylogeographic structure found in F. engleriana is consistent with the dynamics of Fagus forests during the past few million years. Fossil pollen records indicate that Fagus occurred even in northeast China (at latitudes over 45°) during late Tertiary but migrated southwards to mountainous areas of subtropical China (i.e. ‘rear edge’ sensu Hampe and Petit 2005, see one example of Gugger et al. 2011) during the early Pleistocene when the climate became colder (Liu et al. 1998, 2003). The heterogeneous topography in subtropical China may have provided stable climatic conditions for long-term persistence of Chinese beeches during both glacial and interglacial/postglacial periods of the Quaternary. The emerging scenario of multiple plant refugia hold by mountains of subtropical China, both depicted by DNA-based data (reviewed by Qiu et al. 2011) and by patterns of endemism (López-Pujol et al. 2011), fits well for F. engleriana.
Beeches, however, never came back to northern latitudes in the climatic warm periods, e.g. the Holocene (but which occurred in Europe and N America), and this is generally attributed to the development of monsoon climate during the Pleistocene (Fang and Lechowicz 2006; Guo and Werger 2010). The length of water stress period during the early growth season is the main limiting factor for Chinese beeches: it cannot be longer than 60 days, but in the modern temperate China (i.e. at latitudes over 34°) this lasts for >70 days (Guo and Werger 2010). Also, its development at low elevations in the subtropical latitudes has been avoided by the expansion of evergreen tree species because of their higher competition ability (Fang and Lechowicz 2006; Liu et al. 2003). Within subtropical mountains, the occasional occurrence of Fagus pollen at most sites during the Holocene implies that Fagus has never experienced extensive postglacial expansion (Liu et al. 2003; Zhao et al. 2009). In addition, the pollen records also indicate that the expansion of Fagus during the glacial periods of the Pleistocene was generally limited (with a few exceptions in some places, see below) and often circumscribed to areas close to mountain ranges, rarely entering lowlands because of lacking the adequate moisture for its development (Liu et al. 2003; Zheng 2000). This scenario specific for Fagus spp. matches well with the vegetation reconstructions of Harrison et al. (2001) and Ni et al. (2010), suggestive of a long-term fragmentation of temperate deciduous forests at the current subtropical latitudes. Reduced gene flow among fragments, in association with selection for local adaptation, may result in the development of remarkably distinct ecotypes and even species (Hampe and Petit 2005), which might be a driving factor for the exceptionally broad temperate species diversity in southern China (Qiu et al. 2009a).
The local conditions of LGM in the eastern fringe of the Tibetan Plateau could have contributed to some extent to the limited demographic expansion detected for the Northwest group of populations (Fig. 1). According to most of the LGM climate reconstructions for China using both proxy data and climate modelling, the LGM precipitation shows a dual model: whereas it was considerably reduced in most of China, in the Tibetan Plateau it maintained stable or even increased (Ju et al. 2007; Yu et al. 2003; Zheng et al. 2004). Since an enough level of precipitation is critical for the occurrence of beeches (Fang and Lechowicz 2006; Guo and Werger 2010), the eastern rim of the Tibetan Plateau offered the most suitable environmental conditions during the glacial periods, which allowed some demographic expansion. However, due to the limitation of low sequence variation in cpDNA, more variable markers (such as nuclear sequences) are needed to investigate the demographic history of Northwest group with more accuracy.
References
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Bennett KD, Provan J (2008) What do we mean by ‘refugia’? Quat Sci Rev 27:2449–2455
Cao KF, Peters R, Oldeman RAA (1995) Climatic range and distribution of Chinese Fagus species. J Veget Sci 6:317–324
Cao KF, Peters R (1998) Structure and stem growth of multi-stemmed trees of Fagus engleriana in China. Plant Ecol 139:211–220
Chen K, Abbott RJ, Milne RI, Tian X-M, Liu J (2008) Phylogeography of Pinus tabulaeformis Carr. (Pinaceae), a dominant species of coniferous forest in northern China. Mol Ecol 17:4276–4288
Comes HP, Kadereit JW (1998) The effect of Pleistocene climatic changes on plant distribution and evolution. Trends Plant Sci 3:432–438
Denk T (2003) Phylogeny of Fagus L. (Fagaceae) based on morphological data. Plant Syst Evol 240:55–81
Denk T, Grimm GW, Hemleben V (2005) Patterns of molecular and morphological differentiation in Fagus (Fagaceae): phylogenetic implications. Am J Bot 92:1006–1016
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Dupanloup I, Schneider S, Excoffier L (2002) A simulated annealing approach to define the genetic structure of populations. Mol Ecol 11:2571–2581
Fang J, Lechowicz MJ (2006) Climatic limits for the present distribution of beech (Fagus L.) species in the world. J Biogeogr 33:1804–1819
Frascaria N, Maggia L, Michaud M, Bousquet J (1993) The rbcL gene sequence from chestnut indicates a slow rate of evolution in the Fagaceae. Genome 36:668–671
Fu YX (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147:915–925
Funk DJ, Omland KE (2003) Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol System 34:397–423
Gao LM, Möller M, Zhang XM, Hollingsworth ML, Liu J, Mill RR, Gibby M, Li DZ (2007) High variation and strong phylogeographic pattern among cpDNA haplotypes in Taxus wallichiana (Taxaceae) in China and North Vietnam. Mol Ecol 16:4684–4698
Gong W, Chen C, Dobeš C, Fu C-X, Koch MA (2008) Phylogeography of a living fossil: Pleistocene glaciations forced Ginkgo biloba L. (Ginkgoaceae) into two refuge areas in China with limited subsequent postglacial expansion. Mol Phylogenet Evol 48:1094–1105
Gugger P, González-Rodríguez A, Rodríguez-Correa H, Sugita S, Cavender-Bares J (2011) Southward Pleistocene migration of douglas-fir into Mexico: phylogeography, ecological niche modeling, and conservation of ‘rear edge’ populations. New Phytol 189:1185–1199
Guo K, Werger MJA (2010) Effect of prevailing monsoons on the distribution of beeches in continental East Asia. Forest Ecol Manag 259:2197–2203
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467
Harpending HC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Harrison SP, Yu G, Takahara H, Prentice IC (2001) Diversity of temperate plants in east Asia. Nature 413:129–130
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Pleistocene. Philos Trans R Soc London (Biol) 359:183–195
Hiraoka K, Tomaru N (2009) Population genetic structure of Fagus japonica revealed by nuclear microsatellite markers. Int J Plant Sci 170:748–758
Hu FS, Hampe A, Petit RJ (2009) Paleoecology meets genetics: deciphering past vegetational dynamics. Front Ecol Environ 7:371–379
Jakob SS, Blattner FR (2006) A chloroplast genealogy of Hordeum (Poaceae): long-term persisting haplotypes, incomplete lineage sorting, regional extinction, and the consequences for phylogenetic inference. Mol Biol Evol 23:1602–1612
Johnson WC, Adkisson CS (1985) Dispersal of beech nuts by blue jays in fragmented landscapes. Am Midl Nat 113:319–324
Ju L, Wang H, Jiang D (2007) Simulation of the Last Glacial Maximum climate over East Asia with a regional climate model nested in a general circulation model. Palaeogeogr Palaeoclim Palaeoecol 248:376–390
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism. Bioinformatics 25:1451–1452
Liu Y-S, Wang W-M, Arata M (1998) China’s beech forests in the Pre-Pleistocene. Foss Rec 1:151–165
Liu H, Xing Q, Ji Z, Xu L, Tian Y (2003) An outline of Pleistocene development of Fagus forest in China: palynological and ecological perspectives. Flora 198:249–259
Liu M-H (2008) Phylogeography of Fagus longipetiolata: insights from nuclear DNA microsatellites and chloroplast DNA variation. Dissertation, East China Normal University, Shanghai
López-Pujol J, Zhang F-M, Sun H-Q, Ying T-S, Ge S (2011) Centres of plant endemism in China: places for survival or for speciation? J Biogeogr 38:1267–1280
Magri D, Vendramin GG, Comps B et al (2006) A new scenario for the quaternary history of European beech populations: palaeobotanical evidence and genetic consequences. New Phytol 171:199–221
Morris AB, Graham CH, Soltis DE, Soltis PS (2010) Reassessment of phylogeographical structure in an eastern North American tree using Monmonier's algorithm and ecological niche modelling. J Biogeogr 37:1657–1667
Ni J, Yu G, Harrison SP, Prentice IC (2010) Palaeovegetation in China during the late Pleistocene: biome reconstructions based on a global scheme of plant functional types. Palaeogeogr Palaeoclim Palaeoecol 289:44–61
Okaura T, Harada K (2002) Phylogeographical structure revealed by chloroplast DNA variation in Japanese Beech (Fagus crenata Blume). Heredity 88:322–329
Ohkawa T, Kitamura K, Takasu H, Kawano S (2006) Genetic variation in Fagus multinervis Nakai (Fagaceae), a beech species endemic to Ullung Island, South Korea. Plant Species Biol 21:135–145
Palme AE, Su Q, Palsson S, Lascoux M (2004) Extensive sharing of chloroplast haplotypes among European birches indicates hybridization among Betula pendula, B. pubescens and B. nana. Mol Ecol 13:167–178
Petit RJ, Hampe A, Cheddadi R (2005) Climate changes and tree phylogeography in the Mediterranean. Taxon 54:877–885
Pilkington MM, Wilder JA, Mendez FL et al (2008) Contrasting signatures of population growth for mitochondrial DNA and Y chromosomes among human populations in Africa. Mol Biol Evol 25:517–525
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Qian H, Ricklefs RE (2000) Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 407:180–182
Qian H, Ricklefs RE (2001) Diversity of temperate plants in East Asia (Qian and Ricklefs reply). Nature 413:130
Qiu Y-X, Guan B-C, Fu C-X, Comes HP (2009a) Did glacials and/or interglacials promote allopatric incipient speciation in East Asian temperate plants? Phylogeographic and coalescent analyses on refugial isolation and divergence in Dysosma versipellis. Mol Phylogenet Evol 51:281–293
Qiu Y-X, Sun Y, Zhang X-P, Lee J, Fu C-X, Comes HP (2009b) Molecular phylogeography of East Asian Kirengeshoma (Hydrangeaceae) in relation to Pleistocene climate change and landbridge configurations. New Phytol 183:480–495
Qiu Y-X, Fu C-X, Comes HP (2011) Plant molecular phylogeography in China and adjacent regions: tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol Phylogenet Evol 59:225–244
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Shaw J, Lickey EB, Schilling EE, Small RL (2007) Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am J Bot 94:275–288
Shen CF (1992) A monograph of the genus Fagus Tourn. ex L. (Fagaceae). The City University of New York, New York
Soltis DE, Morris AB, McLachlan JS, Manos PS, Soltis PS (2006) Comparative phylogeography of unglaciated eastern North America. Mol Ecol 15:4261–4293
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Tian S, López-Pujol J, Wang H-W, Ge S, Zhang Z-Y (2010) Molecular evidence for glacial expansion and interglacial retreat during Pleistocene climatic changes in a montane temperate pine (Pinus kwangtungensis Chun ex Tsiang) in southern China. Plant Syst Evol 284:219–229
Wang H-W, Ge S (2006) Phylogeography of the endangered Cathaya argyrophylla (Pinaceae) inferred from sequence variation of mitochondrial and nuclear DNA. Mol Ecol 15:4109–4122
Wang J, Gao P, Kang M, Lowe AJ, Huang H (2009) Refugia within refugia: the case study of a canopy tree (Eurycorymbus cavaleriei) in subtropical China. J Biogeogr 36:2156–2164
Wu ZY, Wu SG (1996) A proposal for a new floristic kingdom (realm)—the E. Asiatic kingdom, its delimitation and characteristics. In: Zhang AL, Wu SG (eds) Proceedings of the first international symposium on floristic characteristics and diversity of east asian plants. China Higher Education Press, Beijing, pp 3–42
Yu G, Chen X, Ni J, Cheddadi R et al (2000) Palaeovegetation of China: a pollen data-based synthesis for the mid-Holocene and Last Glacial Maximum. J Biogeogr 27:635–664
Yu G, Xue B, Liu J, Chen X (2003) LGM lake records from China and an analysis of climate dynamics using a modelling approach. Glob Planet Chang 38:223–256
Zhao Y, Yu Z, Chen F, Zhang J, Yang B (2009) Vegetation response to Holocene climate change in monsoon-influenced region of China. Earth-Sci Rev 97:242–256
Yuan QJ, Zhang ZY, Peng H, Ge S (2008) Chloroplast phylogeography of Dipentodon (Dipentodontaceae) in southwest China and northern Vietnam. Mol Ecol 17:1054–1065
Zheng Z (2000) Vegetation and climate since the late Pleistocene in southern China. J Geosci China 2:7–20
Zheng YQ, Yu G, Wang SM, Xue B, Zhuo DQ, Zeng XM, Liu HQ (2004) Simulation of paleoclimate over East Asia at 6 Ka BP and 21 Ka BP by a regional climate model. Clim Dynam 23:513–529
Zhou T-H, Li S, Qian Z-Q, Su H-L, Huang Z-H, Guo Z-G, Dai P-F, Liu Z-L, Zhao G-F (2010) Strong phylogeographic pattern of cpDNA variation reveals multiple glacial refugia for Saruma henryi Oliv. (Aristolochiaceae), an endangered herb endemic to China. Mol Phylogenet Evol 57:176–188
Acknowledgements
The authors thank Dr. Deng-Mei Fan for her help in drawing the haplotype distribution map. This study was funded by a project of National Science Foundation of China (30760016) and the Cultivation Program for Young Scientists of Jiangxi Province (grants to Zhi-Yong Zhang, 2008DQ01500).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by A. Kremer
Ming Lei and Qun Wang are co-first authors.
Rights and permissions
About this article
Cite this article
Lei, M., Wang, Q., Wu, ZJ. et al. Molecular phylogeography of Fagus engleriana (Fagaceae) in subtropical China: limited admixture among multiple refugia. Tree Genetics & Genomes 8, 1203–1212 (2012). https://doi.org/10.1007/s11295-012-0507-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-012-0507-6