Abstract
Crown gall, caused by Agrobacterium tumefaciens, causes severe damage to apple saplings resulting in weak growth and loss of commercial value. Developing molecular markers linked to crown gall resistance genes, and establishing a marker-assisted selection (MAS) for such a trait would be an effective way to improve rootstock breeding for crown gall resistance. The wild apple Malus sieboldii Sanashi 63 carries the crown gall resistance gene Cg effective against the A. tumefaciens strain Peach CG8331 (biovar 2). Applying the genome scanning approach on the mapping population JM7 (cgcg) × Malus sieboldii Sanashi 63 (Cgcg), Cg was mapped on the linkage group (LG) 2. The constructed linkage map of LG 2 of Sanashi 63 spans 59.8 cM and has an average marker density of 3.5 cM per marker. The 191 bp allele of the simple sequence repeat (SSR) NZmsEB119405 co-segregated perfectly with Cg in a segregating population of 119 individuals. Quantitative trait loci, accounting for 75.3% to 84.3% of phenotypic variation were detected in the same position. Testing eight additional rootstocks with the NZmsEB119405 SSR marker revealed that the 191 bp allele is also present in crown gall-susceptible rootstock accessions. Only the markers CH03b01 and NZmsPal92 mapping at 0.9 and 4.3 cM from Cg, respectively, showed “private” alleles associated to Cg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crown gall, caused by Agrobacterium tumefaciens, occurs worldwide in apple and pear trees and in numerous species of other dicotyledonous plants (Stover and Walsh 1998). The damages on apple trees results in weak growth and loss of commercial value. This disease is of primary importance for nurseries and results in discarding of 1–10% of trees with peaks up to of 80% or more (Moore 1990). Compared with M.9 and M.26, the percentage of affected JM1 and JM7 saplings is higher, ranging from 48.1% to 70.9% (Nekoduka et al. 2001). The breeding of resistant rootstock is an effective strategy to control this disease. This approach has been studied in woody plants such as grapevine, peach, and aspen (Beneddra et al. 1996; Bliss et al. 1999; Mahmoodzadeh et al. 2004; Szegedi et al. 1984). In a field experiment with grapevine, the use of crown gall-resistant rootstocks reduced the disease; 79% of susceptible rootstocks had crown gall, whereas only 9% of resistant rootstocks showed symptoms (Sule and Burr 1998).
In apple, such a strategy just starts to be adopted (Viss et al. 2003). Recently, Moriya et al. (2008) identified a crown gall-resistant wild apple, Malus sieboldii Sanashi 63. Sanashi 63 is considered to carry a major gene at the heterozygous state for resistance to crown gall. The result of other testcrosses allowed us to hypothesized that resistance gene is dominant. However, this resistance is strain specific (Moriya et al. 2008). In fact, one of the tested strains (ARAT-001) is able to form galls on Sanashi 63, indicating that this strain is able to overcome this resistance. Nevertheless, the strategy focusing on Cg in the resistant breeding program is valuable, because Peach CG8331 is in fact virulent for major rootstock accessions. The supposed resistance source to ARAT-001 is M. sieboldii Mo-15, because Mo-15 did not exhibit gall by both Peach CG8331 and ARAT-001 (Moriya et al. 2008).
Crown gall-resistant rootstock breeding has certain drawbacks, such as the long time it takes and the difficulty in assessing the resistance level of numerous progeny plants. Therefore, developing molecular markers linked to the crown gall-resistant gene and applying a marker-assisted selection (MAS) system in the selection of crown gall-resistant plants would be an effective way to promote rootstock breeding for crown gall resistance.
Molecular markers are a powerful tool for breeding disease-resistant apples. In apple, disease resistance breeding has mainly focused on apple scab caused by Venturia inaquealis. Molecular markers linked to Vb, Vbj, Va, Vm, Vf, and so on, have been developed (Erdin et al. 2006; Gygax et al. 2004; Hemmat et al. 2003; Patocchi et al. 2005; Tartarini et al. 1999). For other diseases and insect damages, molecular markers and/or quantitative trait loci (QTL) linked to powdery mildew, fire bright, rosy leaf-curling aphid, and wooly apple aphid, have been studied (Calenge and Durel 2006; Calenge et al. 2005a; Khan et al. 2006; Cevik and King 2002; Bus et al. 2008). To readily map major resistance genes and identify molecular markers linked to them, the genome scanning approach (GSA) was proposed by Patocchi and Gessler (2003). GSA is based on the detection of distortion of the expected 1:1 segregation ratio of the alleles of the resistant parent for a simple sequence repeat (SSR) marker within a small subset of progeny plants. The GSA was successfully applied to identify the map position of Vb (Erdin et al. 2006), Vbj (Gygax et al. 2004), and Vm (Patocchi et al. 2005). In Japanese pear, the map position of genes for susceptibility to black spot disease was also determined by the GSA (Terakami et al. 2007).
In this study, we identified a SSR marker closely linked to crown gall resistance by GSA and mapped the resistance gene Cg of Sanashi 63 against Agrobacterium tumefaciens. To our knowledge, this is the first time that a crown gall resistance gene is mapped in apple.
Materials and methods
Plant material
One hundred and twenty progenies of JM7 (M.9 × M. prunifolia Morioka Seishi) × M. sieboldii Sanashi 63 were used for this study. Three replications of each plant were prepared by grafting dormant shoots on JM7 rootstock potted in fumigated soil. Each plant had three shoots.
DNA extraction
One hundred milligrams of young leaves were first crushed using an SH-48 machine (Kurabo, Osaka, Japan) using liquid nitrogen and the obtained fine leaf powder was incubated for 30 min at 37°C with 1 mL of isolation buffer (10% PEG#6000, 100 mM Tris-HCl (pH 8.0), 350 mM sorbitol, and 50 mM EDTA (pH 8.0)) as described by Moriya et al. (2009). Then, genomic DNA was extracted using the automated DNA extracting machine PI-50α (Kurabo) according to the manufacturer’s instructions. Extracted DNA was eluted in Tris-EDTA (TE) buffer and quantified by agarose gel electrophoresis as described by Yamamoto et al. (2006). Finally, quantified DNA was diluted to 10 ng/μL with 1/10 TE buffer.
Inoculation and evaluation of crown gall resistance
The A. tumefaciens strain Peach CG8331 (biovar 2), isolated from peach at Yamagata, Japan, was used for inoculation. The bacterial inoculum was cultured in a YP broth at 28°C for 2 days and adjusted to a 109 colony-forming unit (cfu)/mL. The inoculation was conducted when almost all the shoots were 30 cm long. The inoculation was performed as described by Moriya et al. (2008); the bacterial suspensions were taken up into sterilized disposable syringes (5 mL, Terumo, Tokyo, Japan) with a sterilized needle (0.55 × 25 mm, Terumo), the needle was inserted into the internode of each growing shoot, and then one drop of bacterial suspension was injected. Needles were replaced after each injection. Since nine sites per potted plant were inoculated, in total, 27 sites per genotype were inoculated. The inoculation test was repeated twice over 2 years.
Six months after inoculation, each inoculation site was visually assessed to determine whether a crown gall had formed and the number of galls at the inoculated sites was counted for each progeny (Fig. 1). The frequency of gall occurrence was then determined for each progeny. Since the distribution of individual phenotypic values indicated the putative presence of a major gene, these values were transformed into binary data for genetic mapping. Transformation was performed according to the scale of Moriya et al. (2008) slightly modified: resistant (0) and moderately resistant (>0 to ≤0.3) were classified as resistant in this study. The progenies showing a frequency of >0.3 were classified as susceptible.
Genome scanning approach
To identify the linkage group (LG) carrying the crown gall resistance Cg and then precisely map the gene, GSA was applied after Patocchi et al. (2005). For the first GSA round, 22 resistant plants and the two parents were used. SSRs used for GSA round 1 were chosen on the basis of a reference map published by Silfverberg-Dilworth et al. (2006). For each linkage group, SSRs were selected and tested until two to three informative (i.e., Sanashi 63 segregating alleles) SSRs per linkage group were identified (Table 1).
Polymerase chain reaction (PCR) was performed using two methods: the direct fluorescent primer method (Ziegle et al. 1992) and the M13-tailed primer method (Schuelke 2000) with the following modification: the M13-tailed sequence of the forward primer was substituted with the T7 promoter sequence. PCR products amplified by both methods were separated and detected using the auto-sequencer CEQ 8000 (Beckman-Coulter Inc., CA, USA). The sizes of the amplicons were determined on the basis of an internal standard DNA (DNA Size Standard Kit-400, Beckman-Coulter) with a CEQ Genetic Analyzer (Beckman-Coulter). The allele size obtained by the T7-tailed primer method contained 17–18 bp of tailed sequence length. Once segregation distortion (distortion above 16:6; p < 0.05) was detected, the distorted SSR were tested on the whole progeny (GSA round 2). Upon identification of the linkage group carrying Cg, SSRs previously mapped on this LG of apple and pear (Celton et al. 2009; Fernandez-Fernandez et al. 2008; Liebhard et al. 2003; Silfverberg-Dilworth et al. 2006; Yamamoto et al. 2007) were used to generate a genetic map of the LG of Sanashi 63 (Table 2).
Cleaved amplified polymorphic sequence analysis
The expressed sequence tag CN493139 was transformed in a cleaved amplified polymorphic sequence (CAPS) marker. Primers (F: 5′- AAA CTG GTA CAT ACC GCT GGA -3′ and R: 5′- GCA GGA TTT CTA TAA TAT CGG AAA AG -3′) were designed using the Primer3 software (Rozen and Skaletsky 2000). PCR was performed in a 20 μL solution of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.01% gelatin, 0.2 mM each dNTP, 10 pmol of each forward and reverse primer, 20 ng of genomic DNA, and 0.5 units of Taq DNA polymerase (Invitrogen Corp., CA, USA). The amplification was performed in 35 cycles at 94°C for 30 s (denaturing), 60°C for 30 s (annealing), and 72°C for 1 min (primer extension). PCR products were sequenced and a polymorphism was found in an intron. Amplicons were digested with Tsp 509I (New England BioLabs Inc., MA, USA) and separated on 1.5% agarose gels. The gels were stained with ethidium bromide and visualized with ultraviolet light.
Mapping and QTL analysis
JoinMap ver. 3.0 (Van Ooijen and Voorrips 2001) was used to calculate the genetic linkage maps. The Kosambi mapping function was used to convert recombination units into genetic distances. The map of Sanashi 63 was generated using MapChart 2.2 (Voorrips 2002). QTL were detected using the interval mapping procedure of MapQTL ver. 4.0 (Van Ooijen et al. 2002). QTL with a maximum logarithm of odds (LOD) score greater than or equal to 3.0 were declared significant. For each significant QTL, confidence intervals corresponding to a LOD score drop-off of 2 on both sides of the likelihood peak were calculated. The confidence interval is supposed to contain the QTL with a probability over 95% (Van Ooijen 1992).
Results
Segregation of crown gall resistance
As a positive correlation (R 2 = 0.68; data not shown) between the phenotyping data of the 2 years, has been observed, the average value of crown gall frequency over the 2 years was calculated and used to discriminate between resistant and susceptible plants for the GSA and the genetic mapping. A single progeny was clearly classified as an outlier and excluded from subsequent analysis. In total, 61 plants were scored as resistant to crown gall and 58 plants were scored as susceptible (Fig. 2). This segregation ratio does not significantly differ from the expected 1:1 ratio (χ2 = 0.076) and is indication of monogenic inheritance.
Distribution of the frequency of gall occurrence within the F1 progeny derived from JM7 and Malus sieboldii Sanashi 63 inoculated with Agrobacterium tumefaciens strain Peach CG8331. The frequency of each individual was calculated averaging the frequencies observed in 2 years (2005 and 2006). Close and open arrows indicate the frequency of Sanashi 63 and JM7, respectively. Reproduced by permission of Japanese Society for Horticultural Science
Genome scanning approach
In the first round of GSA, out of the 71 SSRs tested, 41 markers were informative for Sanashi 63 (Table 1). Two or three informative SSRs were obtained on every LG. Significant distortion from 1:1 of the alleles of Sanashi 63 was found for eight SSRs of five LGs: LG 1, 2, 7, 10, and 15. These SSRs were considered as putatively linked to Cg; therefore, the GSA round 2 was carried out with all progeny plants and the eight distorted SSRs. As a result, significant linkage to Cg was detected only on CH03d10 and CH05e03 of LG 2 with recombination values 0.202 (LOD 9.83) and 0.252 (LOD 6.63), respectively, while no significant linkage was found with the other SSRs. Recombination values between Cg and Hi02c07p (LG 1), Hi03a10 (LG7), CH04e05 (LG7), CH02d11 (LG 15), and CH03b10 (LG 15) were all higher than 0.445 (LOD ≤0.30).
Fine mapping of Cg on LG 2
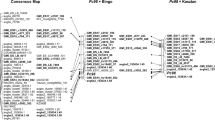
Publicly available SSRs located on LG2 were tested to better define the position of Cg on Sanashi 63 LG. Ten out of 18 tested CH or Hi SSRs (Liebhard et al. 2002; Silfverberg-Dilworth et al. 2006), four out of seven NZ SSRs (Celton et al. 2009), and one NH SSR (Yamamoto et al. 2002) were successfully mapped (Table 3, Fig. 3). Because the SSR CN493139 was mapped between Hi02a07 and AT000400-SSR by Silfverberg-Dilworth et al. (2006), this SSR should be located close to Cg. However, the SSR CN493139 showed an indistinguishable fragment pattern on tested plants. It was converted into a CAPS marker and mapped distinct position than in the previous publication. A SNP, made visible by a restriction digestion with the enzyme Tsp 509I, was identified. JM7 is A/A while Sanashi 63 is A/G, with the A allele of Sanashi 63 being in coupling with Cg. The constructed linkage map of LG 2 of Sanashi 63 spanned 59.8 cM and had an average marker density of 3.5 cM per marker (Fig. 3). The SSR NZmsEB119405 perfectly co-segregated with Cg (LOD 35.8).
a Genetic map of the genomic region around the crown gall resistance locus Cg of Malus sieboldii Sanashi 63. b Representation of the distribution of apple scab resistance genes on LG2. Genetic distances of apple scab resistance genes are represented as in Bus et al. (2005b). Map distances are indicated in centimorgans
QTL analysis
Since the gall occurrence frequency was a quantitative trait, a QTL analysis was also performed for LG 2. A QTL with a high LOD score was detected on the same position as in the previous mapping using the phenotype data of both years (Fig. 4). Their effect on the phenotypic variation was noteworthy strong; R2 = 84.3 and 75.3% in 2005 and 2006, respectively. The marker closest to the peak with the highest LOD is NZmsEB119405. The confidence intervals of two years overlapped and distribute around NZmsEB119405. Significantly skewed markers on LG 1, 7, 10, and 15 in the first round of GSA were also analyzed using interval mapping procedure. The LOD score of these markers ranged from 0.00 to 0.55, indicating no presence of QTL.
Verification of the allele coupling with Cg
Perfectly co-segregating SSR NZmsEB119405 and closely linked SSRs Hi02a07, AT000400-SSR, CH03b01, and NZmsPal92 were tested on additional 8 rootstock accessions (Table 4). All these accessions has been using as parents in rootstock breeding program, and considered to be crown gall susceptible by the inoculation test and/or field observations. For NZmsEB119405, three alleles were observed among 10 accessions. The 191 bp allele of NZmsEB119405, which is associated with Cg in Sanashi 63, was also observed in crown gall susceptible accessions of M. prunifolia Mo-84a, M. prunifolia Morioka Seishi, JM1, and JM5 (the latter 2 accessions are descendants of Morioka Seishi).The first SSRs most closely associated to Cg, showing “private” alleles among the tested rootstocks were CH03b01 (allele 193 bp), and NZmsPal92 (null allele), mapped at 0.9 cM and 4.3 cM from Cg, respectively,.
Discussion
In this study, we identified the map position of the crown gall resistance gene, Cg, derived from Malus sieboldii Sanashi 63. Testing as little as 22 resistant plants with 71 SSRs was sufficient to identify 5 LGs candidate to carry the Cg gene. After the second GSA round, only SSR on LG 2 of Sanashi 63 showed a significant linkage to Cg, indicating that the crown gall resistance gene was located on LG 2 of Sanashi 63. An SSR marker, NZmsEB119405, co-segregates in a population of 119 progeny plants has also been identified (Table 3). The same locus was identified also performing a QTL mapping. A strong QTL accounting for about 80% of the phenotypic variation was identified in the same region. The high phenotypic variation explained by the QTL is consistent with our hypothesis that a major gene is responsible for the resistance. To our knowledge, this is the first report of the mapping of resistance gene to crown gall disease in apple.
Genomic regions with distorted segregation were found on LG 1, 7, 10, and 15 of Sanashi 63. During other mapping experiments, regions with variable degree of marker segregation distortion were found on various linkage groups (Patocchi et al. 2005). Gao and van de Weg (2006) reported sub-lethal genes linked to Vf mapped on LG 1. The distortion detected on LG 7 and 15 was consistent with the reports of Igarashi et al. (2008). The segregation distortion on LG 10 was frequently detected (Kenis and Keulemans 2005; Liebhard et al. 2003; Maliepaard et al. 1998) and these distortions were explained by the presence of alleles of genes conferring low viability (Liebhard et al. 2003).
SSR markers tightly linked to crown gall resistance can be directly used in MAS. Therefore, the frequency of the alleles in coupling of the most closely linked SSRs was verified in 10 rootstock accessions (Table 4). The alleles 191 bp, 257 bp and 244 bp in coupling with Cg of NZmsEB119405, Hi02a07 and AT000400-SSR were also found in crown gall susceptible accessions of M. prunifolia Mo-84a, M. prunifolia Morioka Seishi, and Morioka Seishi descendants JM1 and JM5. Only CH03b01 that co-segregated with AT000400-SSR (at 0.9 cM from Cg) and NZmsPal92 (at 4.3 cM from, Cg) presented unique alleles among the investigated accessions. These results indicate that for MAS CH03b01 and NZmsPal92 may be better suited than NZmsEB119405, Hi02a07, and AT000400-SSR. However, CH03b01 and NZmsPal92 have both an inconvenience. At our conditions, CH03b01 amplified some weak bands of about the same size of the allele in coupling with Cg. These bands may complicate the scoring of CH03b01. The problem of NZmsPal92 is that the allele associated with the Cg is a null allele making impossible to distinguish amplification failure form resistant progenies. In MAS, therefore, using several SSRs to generate haplotypes is considered to be reasonable selection method; e.g., NZmsEB119405, CH03b01, and NZmsPal92.
LG 2 appears to be deeply involved in disease resistance in apple. In fact, on apple LG 2, six scab resistance major genes have been mapped, including Vh2, Vh4, Vh8, VT57, Vbj, and Vr2 (Bus et al. 2005a, b; Gygax et al. 2004; Patocchi et al. 2004; Fig. 3). Major QTL for apple scab resistance (Calenge et al. 2004) and powdery mildew (Calenge and Durel 2006) were also identified on LG 2 using Discovery × TN10-8 progeny (Fig. 3). Moreover, several resistance gene analogs of the NBS-type have been mapped on the LG 2 and particularly on the top of the LG (Baldi et al. 2004; Calenge et al. 2005b; Celton et al. 2009; Naik et al. 2006). Comparing the map position of the crown gall resistance Cg and apple scab resistance genes and QTL mapped on this LG, it can be observed that Cg maps about in the same position as Vr2, Vh4, and powdery mildew QTL. Therefore, on the top of LG 2, not only resistance genes against fungal disease are present but also resistance genes against a bacterial disease.
Sanashi 63 exhibits no gall at all with strain Peach CG8331. On the other hand, numerous moderately resistant progeny plants exhibited some crown gall (freqency >0 and ≤0.3). The genetic background of Sanashi 63 probably consisted of Cg major gene and putative polygenic factors having moderate/minor but complementary effects. The lack of these polygenic factors (modifiers) could decrease the level of resistance of some progenies carrying Cg. Modifiers have been proposed for the apple scab resistance gene Vf (Gessler 1989). Genome wide QTL study will reveal such minor resistance factors.
One of our main goals is the development of durable crown gall-resistant and dwarfing rootstock suitable for the Japanese climate. Rootstocks adapted to the Japanese climate can be obtained by crossing Sanashi 63 with rootstocks that are well adapted to the Japanese climate such as JM1, JM5, and JM7. Recently, the map position of Dw1, a gene conferring dwarf growth to the scion derived from M.9 rootstock, has been determined and molecular markers closely linked to the gene have been identified (Pilcher et al. 2008). Therefore, combining these markers and our Cg-linked markers will enable apple breeders to save time and labor in the selection of dwarfing rootstocks resistant to some strains of crown gall.
References
Baldi P, Patocchi A, Zini E, Toller C, Velasco R, Komjanc M (2004) Cloning and linkage mapping of resistance gene homologues in apple. Theor Appl Genet 109:231–239
Beneddra T, Picard C, Petit A, Nesme X (1996) Correlation between susceptibility to crown gall and sensitivity to cytokinin in aspen cultivars. Phytopathol 86:225–231
Bliss FA, Almehdi AA, Dandekar AM, Schuerman PL, Bellaloui N (1999) Crown gall resistance in accessions of 20 Prunus species. Hortscience 34:326–330
Bus VGM, Laurens FND, Van de Weg E, Rusholme RL, Rikkerink E, Gardiner S, Bassett H, Kodde L, Plummer K (2005a) The Vh8 locus of a new gene-for-gene interaction between Venturia inaequalis and the wild apple Malus sieversii is closely linked to the Vh2 locus in Malus pumila R12740-7A. New Phytol 166:1035–1049
Bus VGM, Rikkerink E, Van de Weg E, Rusholme RL, Gardiner S, Bassett H, Kodde L, Parisi L, Laurens F, Meulenbroek B, Plummer K (2005b) The Vh2 and Vh4 scab resistance genes in two differential hosts derived from Russian apple R12740-7A map to the same linkage group of apple. Mol Breed 15:103–116
Bus VGM, Chagne D, Bassett HCM, Bowatte D, Calenge F, Celton JM, Durel CE, Malone MT, Patocchi A, Ranatunga AC, Rikkerink EHA, Tustin DS, Zhou J, Gardiner SE (2008) Genome mapping of three major resistance genes to woolly apple aphid (Eriosoma lanigerum Hausm.). Tree Genet Genomes 4:223–236
Calenge F, Durel CE (2006) Both stable and unstable QTLs for resistance to powdery mildew are detected in apple after four years of field assessments. Mol Breed 17:329–339
Calenge F, Faure A, Goerre M, Gebhardt C, Van de Weg WE, Parisi L, Durel CE (2004) Quantitative trait loci (QTL) analysis reveals both broad-spectrum and isolate-specific QTL for scab resistance in an apple progeny challenged with eight isolates of Venturia inaequalis. Phytopathol 94:370–379
Calenge F, Drouet D, Denance C, Van de Weg WE, Brisset MN, Paulin JP, Durel CE (2005a) Identification of a major QTL together with several minor additive or epistatic QTLs for resistance to fire blight in apple in two related progenies. Theor Appl Genet 111:128–135
Calenge F, Van der Linden CG, Van de Weg E, Schouten HJ, Van Arkel G, Denance C, Durel CE (2005b) Resistance gene analogues identified through the NBS-profiling method map close to major genes and QTL for disease resistance in apple. Theor Appl Genet 110:660–668
Celton JM, Tustin DS, Chagne D, Gardiner SE (2009) Construction of a dense genetic linkage map for apple rootstocks using SSRs developed from Malus ESTs and Pyrus genomic sequences. Tree Genet Genomes 5:93–107
Cevik V, King GJ (2002) High-resolution genetic analysis of the Sd-1 aphid resistance locus in Malus spp. Theor Appl Genet 105:346–354
Erdin N, Tartarini S, Broggini GAL, Gennari F, Sansavini S, Gessler C, Patocchi A (2006) Mapping of the apple scab-resistance gene Vb. Genome 49:1238–1245
Fernandez-Fernandez F, Evans KM, Clarke JB, Govan CL, James CM, Maric S, Tobutt KR (2008) Development of an STS map of an interspecific progeny of Malus. Tree Genet Genomes 4:469–479
Gao ZS, Van de Weg E (2006) The Vf gene for scab resistance in apple is linked to sub-lethal genes. Euphytica 151:123–132
Gessler C (1989) Genetics of the interaction Venturia inaequalis-Malus: the conflict between theory and reality. In: Gessler, Butt and Koller (eds) Integrated control of pome fruit diseases II. IOBC-WPRS Bulletin, pp 168–190
Gygax M, Gianfranceschi L, Liebhard R, Kellerhals M, Gessler C, Patocchi A (2004) Molecular markers linked to the apple scab resistance gene Vbj derived from Malus baccata jackii. Theor Appl Genet 109:1702–1709
Hemmat M, Brown SK, Aldwinckle HS (2003) Identification and mapping of markers for resistance to apple scab from ‘Antonovka’ and ‘Hansen’s baccata #2’. Acta Hort 622:153–161
Igarashi M, Abe Y, Hatsuyama Y, Ueda T, Fukasawa-Akada T, Kon T, Kudo T, Sato T, Suzuki M (2008) Linkage maps of the apple (Malus × domestica Borkh.) cvs ‘Ralls Janet’ and ‘Delicious’ include newly developed EST markers. Mol Breed 22:95–118
Kenis K, Keulemans J (2005) Genetic linkage maps of two apple cultivars (Malus × domestica Borkh.) based on AFLP and microsatellite markers. Mol Breed 15:205–219
Khan MA, Duffy B, Gessler C, Patocchi A (2006) QTL mapping of fire blight resistance in apple. Mol Breed 17:299–306
Liebhard R, Gianfranceschi L, Koller B, Ryder CD, Tarchini R, Van de Weg E, Gessler C (2002) Development and characterisation of 140 new microsatellites in apple (Malus × domestica Borkh.). Mol Breed 10:217–241
Liebhard R, Koller B, Gianfranceschi L, Gessler C (2003) Creating a saturated reference map for the apple (Malus × domestica Borkh.) genome. Theor Appl Genet 106:1497–1508
Mahmoodzadeh H, Nazemieh A, Majidi I, Paygami I, Khalighi A (2004) Evaluation of crown gall resistance in Vitis vinifera and hybrids of Vitis spp. Vitis 43:75–79
Maliepaard C, Alston FH, Van Arkel G, Brown LM, Chevreau E, Dunemann F, Evans KM, Gardiner S, Guilford P, Van Heusden AW, Janse J, Laurens F, Lynn JR, Manganaris AG, APMd N, Periam N, Rikkerink E, Roche P, Ryder C, Sansavini S, Schmidt H, Tartarini S, Verhaegh JJ, Van Ginkel MV, King GJ (1998) Aligning male and female linkage maps of apple (Malus pumila Mill.) using multi-allelic markers. Theor Appl Genet 97:60–73
Moore LW (1990) Crown gall. In: Jones AL, Aldwinckle HS (eds) Compendium of apple and pear disease. APS press, St. Paul, USA, pp 64–65
Moriya S, Iwanami H, Takahashi S, Kotoda N, Suzaki K, Abe K (2008) Evaluation and inheritance of crown gall resistance in apple rootstocks. J Jpn Soc Hort Sci 77:236–241
Moriya S, Iwanami H, Kotoda N, Takahashi S, Yamamoto T, Abe K (2009) Development of a marker-assisted selection system for columnar growth habit in apple breeding. J Jpn Soc Hort Sci 78:279–287
Naik S, Hampson C, Gasic K, Bakkeren G, Korban SS (2006) Development and linkage mapping of E-STS and RGA markers for functional gene homologues in apple. Genome 49:959–968
Nekoduka S, Kawamura T, Nakatani F, Sasaki H, Onoda K (2001) Occurrence of crown gall on apple rootstock ‘JM’ strain. Ann Rep Soc Plant Protection North Jpn 52:105–108 (In Japanese)
Patocchi A, Gessler C (2003) Genome scanning approach (GSA), a fast method for finding molecular markers for any trait. Proceedings of the Plant and Animal Genomes XI Conference. Available via http://www.intl-pag.org/11/abstracts/P3b_P178_XI.html
Patocchi A, Bigler B, Koller B, Kellerhals M, Gessler C (2004) Vr2: a new apple scab resistance gene. Theor Appl Genet 109:1087–1092
Patocchi A, Walser M, Tartarini S, Broggini GAL, Gennari F, Sansavini S, Gessler C (2005) Identification by genome scanning approach (GSA) of a microsatellite tightly associated with the apple scab resistance gene Vm. Genome 48:630–636
Pilcher RLR, Celton JM, Gardiner SE, Tustin DS (2008) Genetic markers linked to the dwarfing trait of apple rootstock ‘Malling 9’. J Am Soc Hort Sci 133:100–106
Rozen S, Skaletsky H (2000) Primer 3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, New Jersey, pp 365–386
Stover E, Walsh C (1998) Crown gall in apple rootstocks: inoculation above and below soil and relationship to root mass proliferation. Hortscience 33:92–95
Silfverberg-Dilworth E, Matasci CL, Van de Weg WE, Van Kaauwen MPW, Walser M, Kodde LP, Soglio V, Gianfranceschi L, Durel CE, Costa F, Yamamoto T, Koller B, Gessler C, Patocchi A (2006) Microsatellite markers spanning the apple (Malus × domestica Borkh.) genome. Tree Genet Genomes 2:202–224
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Sule S, Burr TJ (1998) The effect of resistance of rootstocks to crown gall (Agrobacterium spp.) on the susceptibility of scions in grape vine cultivars. Plant Pathol 47:84–88
Szegedi E, Korbuly J, Koleda I (1984) Crown gall resistance in east-Asian Vitis species and in their V. vinifera hybrids. Vitis 23:21–26
Tartarini S, Sanasavini S, Vinatzer B, Gennari F, Domizi C (1999) Development of reliable PCR markers for the selection of the Vf gene conferring scab resistance in apple. Plant Breed 118:183–186
Terakami S, Adachi Y, Iketani H, Sato Y, Sawamura Y, Takada N, Nishitani C, Yamamoto T (2007) Genetic mapping of genes for susceptibility to black spot disease in Japanese pears. Genome 50:735–741
Yamamoto T, Kimura T, Shoda M, Ban Y, Hayashi T, Matsuta N (2002) Development of microsatellite markers in the Japanese pear (Pyrus pyrifolia Nakai). Mol Ecol Notes 2:14–16
Yamamoto T, Kimura T, Hayashi T, Ban Y (2006) DNA profiling of fresh and processed fruits in pear. Breed Sci 56:165–171
Yamamoto T, Kimura T, Terakami S, Nishitani C, Sawamura Y, Saito T, Kotobuki K, Hayashi T (2007) Integrated reference genetic linkage maps of pear based on SSR and AFLP markers. Breed Sci 57:321–329
Van Ooijen JW (1992) Accuracy of mapping quantitative trait loci in autogamous species. Theor Appl Genet 84:803–811
Van Ooijen JW, Voorrips RE (2001) JoinMap 3.0, Software for the calculation of genetic linkage maps. Plant Research International, Wageningen, The Netherlands
Van Ooijen JW, Boer MP, Jansen RC, Maliepaard C (2002) MapQTL 4.0, software for the calculation of QTL positions on genetic maps. Plant Research International, Wageningen, The Netherlands
Viss WJ, Pitrak J, Humann J, Cook M, Driver J, Ream W (2003) Crown-gall-resistant transgenic apple trees that silence Agrobacterium tumefaciens oncogenes. Mol Breed 12:283–295
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78
Ziegle JS, Su Y, Corcoran KP, Nie L, Mayrand PE, Hoff LB, McBride LJ, Kronick MN, Diehl SR (1992) Application of automated DNA sizing technology for genotyping microsatellite loci. Genomics 14:1026–1031
Acknowledgement
We would like to thank Dr. Y. Takikawa of Shizuoka University for providing the bacterial strain and Dr. S. Terakami of National Institute of Fruit Tree Science for valuable discussions and computer analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Dirlewanger
Rights and permissions
About this article
Cite this article
Moriya, S., Iwanami, H., Takahashi, S. et al. Genetic mapping of the crown gall resistance gene of the wild apple Malus sieboldii . Tree Genetics & Genomes 6, 195–203 (2010). https://doi.org/10.1007/s11295-009-0240-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-009-0240-y