Abstract
Conservation of Anatidae in North Africa is hindered by lack of information concerning population size, population trends, and species-habitat relationships. In this work, we used a 2-year survey data in 25 wetlands distributed throughout Morocco to model duck spatial distribution and to assess the relevance of a set of environmental and anthropogenic factors in predicting site occupancy, by means of Generalized Linear Mixed Models (GLMM). Mallards and Ferruginous ducks were the most commonly detected species, whereas White-Headed Ducks and Common Pochards were the least detected ones. An inter-annual variation in site occupancy was recorded for Ruddy Shelducks, Red-Crested Pochards and White-Headed Ducks. Geographical location (measured mainly as distance to the coastline and altitude) was the major predictor of the occurrence probability of Ruddy Shelducks, Marbled Teals and Red-Crested Pochards, while human presence and habitat features were the most relevant factors in shaping Mallard’s distribution. However, none of the considered environmental and anthropogenic factors explained the distribution patterns of the Ferruginous Duck, Common Pochard and Gadwall. The results of this study clearly show that there are still gaps in our knowledge on factors driving wetland occupancy by breeding Anatidae in Morocco. The pursuit of the investigations, while considering other explanatory factors such as water quality (limnological data), diet, predation, and conservation status, is of great importance to more profoundly understand the dynamics of Moroccan duck populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the environmental and anthropogenic factors affecting the distribution of waterbirds is of crucial importance in theoretical and applied ecology. Recently, patterns of spatial distribution and habitat use by waterbirds have generated numerous questions in community ecology (Cody 1985; Block and Brennan 1993), and the assessment of avian habitat relationships has become an important part of wildlife management and conservation planning (Verner et al. 1986; Hoffman et al. 1996; Marone et al. 1997), especially within the context of global change (Newbold et al. 2015). When choosing a site at which to settle, waterbirds need to simultaneously consider the quality of available sites in terms of their environmental conditions, resource availability and the presence/frequency of predators and/or parasites (Martin 1993; Penteriani et al. 2002; Cardador et al. 2011; Cherkaoui et al. 2013, 2015).
The Kingdom of Morocco, located in the extreme north-west of Africa, is bordered to the north by the Mediterranean Sea, to the west by the Atlantic Ocean, and to the south by Mauritania. It supports habitats ranging from high-altitude moorland through cork-oak forests to wetlands, deltas, arid steppes and deserts (Magin 2001). Morocco is located at the crossroads of several avian migratory routes between Europe and Africa and plays a major role in the migration of waterbirds as it supports numerous wetlands and 70% of its 3500 km of coastline lie within the East-Atlantic Flyway (Dakki et al. 2001). Wetlands are habitats of the highest conservation interest due to their high biological diversity (Weller 1999; Keddy 2010; García et al. 2015). They also play a vital role in the life cycle of many species during wintering, breeding and migration periods (Tucker and Evans 1997). Among them, Anatidae species are among the most important waterbirds groups in the Palearctic. Although this group plays a decisive role in the energy flow of wetland ecological systems and in maintaining their stabilities (Monfils and Prince 2009; McKinney et al. 2011; Liang et al. 2015), it remains nonetheless so sensitive to environmental variables (Lant et al. 2005).
Several studies on breeding ducks have recently emerged in numerous wetland sites in Morocco (Cherkaoui et al. 2013, 2015, 2016). Nonetheless, the ecological factors and processes shaping the patterns of spatial distribution of these bird species at the scale of the entire Moroccan wetland network remain poorly known. Such data are of great importance to more profoundly understand the ecology of duck species, not only at the scale of Morocco but also at the scale of the entire western Mediterranean. They are also essential to draw a more complete picture about the conservation status of these species and to propose large-scale conservation plans.
The aim of this study was not only to describe the patterns of geographical distribution of duck species in Morocco, but rather to identify the ecological factors that account in shaping these patterns. More specifically, we used a 2-year survey data collected in 25 wetlands distributed throughout Morocco to investigate the relevance of wetland geographic position, habitat features and human presence in predicting the occurrence probability of each duck species. Understanding the relationship between the presence of breeding Anatidae species and these variables would provide potential insights into protecting their diversity and the restoration of their habitats.
Waterfowl are known to be highly sensitive to environmental conditions during breeding period (Lant et al. 2005; Holopainen et al. 2015). For example, human disturbances are known to negatively affect breeding waterfowl by reducing hatching success and duckling survival (Korschgen and Dahlgren 1992; Fox et al. 2015), while wetland features are key determinants of habitat use in numerous breeding ducks [e.g., the Marbled Teal (Green 1998; Green and El Hamzaoui 2000; Sebastián-González et al. 2013), White-Headed Duck (Castro et al. 1994; Sebastián-González et al. 2013), Mallard (Amat 1982; Merendino and Ankney 1994), and Red-Crested Pochard (Amat 1982; Broyer and Curtet 2010)]. For these reasons, we hypothesized that wetland occupancy might be especially influenced by habitat features and human activities, but the significance and direction of these relationships may vary among species according to their ecological requirements.
Materials and methods
Study sites
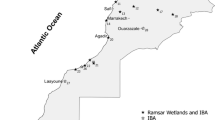
Data used in this work were collected in 25 wetlands of international conservation importance, based on their designation as both Ramsar sites (Ramsar Convention Bureau 2002; Ramsar 2015) and Important Bird Areas IBA (Fishpool and Evans 2001; www.birdlife.org). The studied wetlands represent 41% of the total Moroccan wetlands. 92% of these wetlands are Ramsar sites, 64% are protected, and 92% are IBA. Four wetlands are located in the Atlas Mountains at an altitude of 1462–2300 m (3 in the Middle Atlas mountains and one within the High Atlas mountains), five in inland areas from 100 to 356 m, five in Saharan zones and the others are coastal wetlands (Fig. 1).
Data collection
Anatidae data
Given that the phenology of breeding duck species is overall similar (Thévenot et al. 2003), surveys of all designated wetlands were conducted in 2012 and 2013 during the peak of breeding activity in May and June. Our sampling effort comprised 25 wetlands ×4 repetitions ×2 years, totaling 200 observations made over the whole study period. Each wetland was visited 4 times, and during each visit the observer (I. Cherkaoui) recorded the presence/absence of breeding duck species in 3–6 points (depending on wetland size), using a spotting scope (30X by 60 mm) and a telescope (60X). The range of duck observation varied from 20 to 400 m. Proofs of breeding for each species was confirmed by one of the following: (i) nest construction activity; (ii) presence of active nest; (iii) nest attendance by adults and (iv) presence of chicks nearby the adults or the behavioural breeding criteria (Cherkaoui et al. 2014) and (v) copulation. The Ruddy Shelduck Tadorna ferruginea is a cavity-nesting duck (Thévenot et al. 2003) so criteria (i), (ii) and (iii) were not appropriate for this species. All observations were made between sunrise and midday, and nearly always in good weather, ensuring that detection conditions were relatively homogeneous.
Environmental data
We collected data related to geographical characteristics, habitat features and human disturbance for each studied wetland. The location of each wetland and its elevation (ELEV) were determined using a Global Positioning System (GPS) device (Garmin eTrex HC). We also determined: (i) wetland size (WSIZE) in hectares from Dakki et al. (2011) for Ramsar sites, from Birdlife datasets (www.birdlife.com) for Important Bird Areas (IBA), and from the Moroccan Protected Areas Study (AEFCS 1996) for SBEI; (ii) the proportion of the water surface area (WAC); (iii) the proportion of the water surface area covered by emergent vegetation (VC) [(the ratio of vegetation coverage area to the wetland area) ×100] (Cherkaoui et al. 2015, 2016). To estimate the proportion areas of WAC and VC, we used Google Earth Pro (2013 cover), since high resolution images are available for all studied wetlands. We have determined (iv) the number of species contributing to emergent vegetation (e.g. Reedmace Typha sp., Reed Phragmites sp., Bulrush Scirpus sp., Rushes Juncus spp. and Tamarisk Tamarix sp.). This variable was checked during fieldwork by walking randomly within vegetated areas. We also measured (v) the distance (m) to the nearest road (DRD); (vi) the distance (m) to the nearest resident (DRES), and (vii) the distance (km) to the nearest coastline (DCOAST) by using the centroid of each studied wetland. All distances were measured using the application of Geographic Information Systems (QGIS, v1.7.3).
Statistical analyses
Site-based statistical models are commonly used to quantify relationships between site characteristics status and the probability that a particular species will be present (Petit et al. 2003; Lecis and Norris 2004; Reich et al. 2004; Wassens et al. 2010).
Prior to statistical analysis, we checked for normality and homogeneity of variance of all the variables. Variables that did not conform to the requirements for parametric tests were log-transformed (in the cases of altitude and area) or square root-transformed (% vegetation cover) to meet the assumptions of the analysis. We also checked for possible correlations among variables by using Pearson’s rank correlation (r) index. We collapsed all environmental variables into independent vectors using Principal Component Analysis (PCA), since this allowed us to: (i) reduce the dimensionality of the set of variables to a smaller number of ‘representative’ and ‘uncorrelated’ variables; (ii) prevent multi-collinearity; and (iii) describe dominant ecological gradients (Legendre and Legendre 1998).
For each PCA, a varimax normalised rotation was applied to the set of principal components with eigenvalues >1.0, to obtain simpler and more interpretable gradients (Legendre and Legendre 1998). We interpreted the biological meaning of the principal components, which explain the greatest amount of combined variation within the habitat structure data, by examining the component loadings of each variable (McGarigal et al. 2000). After this initial analysis, we modelled occupancy of wetlands separately for the eight study species as a function of geographical, anthropological and macrohabitat variables. For this we used the Generalised Linear Mixed Model (GLMM) with binomial error (a binary response variable: unoccupied = 0 or occupied = 1), using the glmer function to test the relationships between the occupation of wetlands by each species (response variable) and explanatory variables [PCA components, Julian date (day 1 = 1 May), and year (categorical variable)]. Each of the explanatory PCA components was tested alone, in addition with another component and in interaction with the categorical variable (year). We included site as a random effect to statistically control for between-site variation. In order to select the best GLMM models, we developed an all-inclusive design (all possible combination models) by using multimodel inference (Burnham and Anderson, 2002). Models were then ordered by increasing Akaike Information Criterion corrected for small sample sizes using AICc (Burnham and Anderson 2002). We considered all models with ΔAICc lower than 2 as equally good (Burnham and Anderson 2002), with the exclusion of the ‘uninformative parameters’ (cf. Arnold 2010), i.e. variables included only in models comprising more parsimonious nested models (Ficetola et al. 2011). Model averaging was used to generate estimates of function slopes for parameters of interest (Burnham and Anderson 2002). Model weights were used to define the relative importance of each explanatory variable across the full set of models evaluated by summing weight values of all models that included the explanatory variable of interest. Variance explained was calculated using the methods of Nakagawa and Schielzeth (2013). We calculated marginal R 2 (which describes the variance explained by fixed effects) and conditional R 2 (which describes variance explained by the full model).
One of the assumptions of parametric statistics is that observations are independent of each other. This assumption is often violated with spatial data, so it is important to test for and, where present, subsequently address spatial autocorrelation in data prior to data analysis. The spatial structure of the presence of breeding Anatidae was thereby quantified by the indicator semivariogram (Goovaerts 1998) by using the residual of the best model in terms of AICc value. The variogram model parameters are (1) the sill: the semivariance on the y-axis where the variogram reaches its asymptote, which is approximately the global variance of the data; (2) the range: the distance on the x-axis where the semivariogram reaches its asymptote and beyond which all points are spatially uncorrelated; and (3) the nugget: the semivariance on the y-axis at the ordinate of the variogram due to measurement error and fine spatial-scale fluctuations in the property of interest (Burgess and Webster 1980; Ooi et al. 2014). Nugget to total sill ratio (NSR) was expressed as the percentage of total semivariance and was used to define for spatial dependency: NSR < 0.25 indicated strong spatial dependence, 0.25 < NSR < 0.75 indicated moderate spatial dependence, and NSR > 0.75 indicated weak spatial dependence (Cambardella et al. 1994). When spatial autocorrelation was encountered, we used spatial generalized linear mixed models, fitted via penalized quasi-likelihood (glmmPQL), with a binomial error. glmmPQL enables the building of spatial models with dependent data not normally distributed, and is among the best techniques for this kind of data (Dormann 2007). We adopted a Gaussian spatial correlation structure, but tests with exponential and spherical structures led to the same results.
All statistical analyses were performed in R-3.0.2 software (R Development Core Team 2013). We used the package “ade4” for Principal Component Analysis (Dray and Dufour 2007), the package “lme4” for fitting generalized linear mixed models (Bates et al. 2014), and the package “MuMIn” to calculate AICc (Bartoń 2015). The top ranked models (within two points of AICc) were averaged as implemented in the MuMIn R-package (Bartoń 2015). The same package was used to calculate conditional R2 via the function “rsquared.glmm”.The packages “sp”, “lattice” and “gstat” were carried out to draw semivariograms (Pebesma 2006), and the package “MASS” was used to fit glmmPQL models (Venables and Ripley 2002). Means are quoted ± standard errors.
Results
In total, 8 species of breeding Anatidae were detected in the 25 monitored wetlands during the 1 years of study (Table 1). The most commonly recorded species were the Mallard and Ferruginous Duck, while the less common species were the White-Headed Duck and Common Pochard (Table 1).
The PCA summarized the studied field variables into three independent axes which accounted for 78% of the variance of the original dataset [30% (eigenvalue = 2.373), 25% (eigenvalue = 2.005), and 23% (eingenvalue = 1.842), respectively)]. The first gradient (Human presence) represented an axis of increasing distance to man-made structures (Table 2). The second gradient (geographic location) was characterized by high loadings of variables related to geographical location. The third gradient represented an axis of habitat features (Table 2). The Kaiser–Meyer–Olkin measure of sampling adequacy (KMO) indicated that our data were suitable for the PCA (PCA: n = 8, KMO = 0.415; Bartlett-test for sphericity, χ 2 = 1447.59, P < 0.0001).
For 3 duck species, namely the Ruddy Shelduck, Marbled Teal and Red-Crested Pochard, wetland geographic location (PC2) was the strongest predictor of occurrence probability. However, the direction of the relationship varied among species. Indeed, the occurrence probability of Ruddy Shelducks was positively related to PC2, while an opposite trend was found for Marbled Teals and Red-Crested Pochards (Table 3). In the latter species, the effect of geographical location was less pronounced in 2012 than in 2013 (Table 3). The year effect has also affected the occurrence probability of the White-Headed Duck and Ruddy Shelduck. These two species were more commonly recorded in 2013 than in 2012 (Table 3). Among the eight studied ducks, the Mallard was the sole species that nested in close proximity to man-made structures. Indeed, the human presence (PC1) did not seem to affect Mallard occurrence, which seemed positively related to habitat features (PC3) (Table 3).
For 3 species, namely the Ferruginous Duck, Common Pochard and Gadwall, the model that best described site occupancy was the null model (Table 3). None of the considered variables (i.e. PC1, PC2, PC3, Julian date and year) provided a significant predictor of the occurrence probability.
For 4 species, namely the Marbled Teal, Mallard, White-Headed Duck, and Red-Crested Pochard, we found no evidence of spatial autocorrelation in model’s residuals. Semi-variograms of residuals from the tops AICc ranked GLMMs showed no significant spatial autocorrelation (Fig. 2). Indeed, for these four species, nuggets to total sill ratios (NSR) are close to 1 (Table 3), which suggests that nearby wetlands were not similarly occupied. However, this was not the case for the Ruddy Shelduck. The semi variogram, established from the residuals of the best model, indicates a spatial pattern in the data (NSR: 0.641–0.663) (Fig. 2). When we explicitly considered spatial autocorrelation in the modeling through the glmmPQL models (Table 3), the effect of year has been highlighted (Table 3).
Discussion
Using survey data on duck species inhabiting the Moroccan wetland network, we aimed to investigate the role of wetland features in shaping the distribution patterns of these species at a relatively large geographic scale. Our results showed that different duck species responded differently to spatial variation in environmental factors. As throughout the world (Isenmann and Moali 2000; Thévenot et al. 2003; Isenmann et al. 2005; Bengtsson et al. 2014), the Mallard is the most common duck species in Morocco (Thévenot et al. 2003). The second most represented species is the Ferruginous Duck. Although it is classified as “Near Threatened” (BirdLife International 2012), its presence is currently confirmed in many wetlands throughout Morocco (Cherkaoui et al. 2016). The same distribution pattern is recorded for the Marbled teal and Common Pochard, which are classified as “Vulnerable” species by IUCN (BirdLife International 2012). The only endangered duck species, breeding in Morocco, is the White-headed Duck. Its presence is overall limited to Atlantic coastal wetlands. The Ruddy shelduck, Gadwall and Red-crested Pochard are common species in Morocco. Their presence is , respectively related to mountainous wetlands (Middle-Atlas) and Atlantic coastal wetlands.
Results of our modelling approch revealed that, among the considered parameters, the geographic location of the wetlands influences the presence of three duck species: the Ruddy Shelduck, Marbled Teal and Red-Crested Pochard. The two latter species have used exclusively coastal wetlands, which are known to be of higher quality (Cherkaoui et al. 2016). These wetlands are the most important in terms of vegetation cover and the diversity of submerged vegetation beds (Dakki et al. 2011; Cherkaoui et al. 2016). In contrast to these two species, Ruddy Shelducks used mountain wetlands. Indeed, this cavity-nesting duck species breeds within tall mature Cedar trees Cedrus atlantica [which only occur at 1500–2600 m (Benabid 1982)] (Thévenot et al. 2003; Cherkaoui et al. 2016). The presence of moderate spatial autocorrelation corroborated this finding, especially of the fact that Cedar forests in Morocco are chiefly distributed in Middle-Atlas mountainous areas (M’Hirit 1999). An opposite pattern in wetland occupancy was observed between Ruddy Shelducks, on the one hand, and Marbled Teals and Red-Crested Pochards on the other hand. This pattern was also recorded by Amat (1982) and BirdLife International (2015). This begs the question, why such a pattern? This could be attributed to a marked difference in breeding habitats requirements between these species. Indeed, the Ruddy Shelduck generally breeds, far away from wetlands, in the holes of trees and rocks, whereas the Marbled Teal and Red-Crested Pochard use exclusively habitats of coastal wetlands for nesting (e.g., Phragmites reedbeds and Scirpus vegetation) (Defos du Rau et al. 2005; Sebastián-González et al. 2013; Cherkaoui et al. 2016). The effect of geographical location has additionally differed among years, in particular for Red-Crested Pochards. The between-year variation in wetland occupancy is fairly frequent in this duck species (Defos du Rau et al. 2005). The availability of food resources would probably be behind this annual variation (Noordhuis et al. 2002). It seems nonetheless that, in Morocco, this spatiotemporal dynamic would much rather be explained by the occupancy, for the first time, of new wetlands for breeding. Although our study was carried out over a relatively short period of time, it would announce a spreading of this diving duck towards south. This tendency to extend breeding areas has been already recorded in some Moroccan wetlands, such as Douiyet, Merja Bergha (Qninba et al. 2008), Smir (El Agbani et al. 2009), Fouarate marshes (Lahrouz et al. 2012), and Sidi Boughaba (Cherkaoui et al. 2013).
Interannual variability in wetland occupancy has also been noted for Ruddy Shelducks and White-Headed Ducks. In the latter species, the literature clearly mentions the presence of variation in site selection according to whether it was a rainy or dry year (Newton 1998; Almaraz and Amat 2004; Atiénzar et al. 2012), or whether water quality and hydrological regime have undergone significant changes (Amat 1982). Moreover, for Ruddy Shelducks, the interannual variability would be tributary to survival of newly hatched ducklings and adults to roadways leading between nest-trees to nearest wetlands. According to Khaffou and Chahlaoui (2012), several potential predators exist within this mountainous forest such as Stray Dogs (Canis familiaris), Golden Jackal (Canis aureus) and raptors (e.g., Booted Eagles Hieraaetus pennatus, Black Kites Milvus migrans, Long-legged Buzzards Buteo rufinus; IC Pers. Obs.). The ability of Ruddy Shelducks to escape predators would be undoubtedly correlated with their probabilities of presence in closest wetlands.
Contrary to all studied duck species, Mallards showed a relative tolerance to human presence in Moroccan wetlands. This pattern was already observed by Amat (1982) and Caithness (1982). In addition, in their review, Holopainen et al. (2015) have also asserted that human-induced eutrophication may be favourable to increase Mallard density. The presence of Mallards was also positively correlated with habitat features (water surface area, surface area covered by emergent vegetation and number of emergent vegetation species). This is globally consistent with the existing literature on the selection of breeding habitats in Mallards (Rotella and Ratti 1992; Pöysä 2001; Ma et al. 2004; Sánchez-Zapata et al. 2005). Overall, Mallards are habitat generalists that are variable in terms of the number and type of wetlands they use (Newton and Campbell 1975; Amat 1982; Thorpe 1997).
Interestingly, in Ferruginous Ducks, Common Pochards and Gadwalls, the probability of presence was not related to human presence (PC1), geographical location (PC2), habitat features (PC3), year or Julian date. However, for Ferruginous Ducks, human presence and habitat features were present in the top models without being sufficiently important. The presence, in the top models, reinforces the findings of previous studies on this diving duck in Morocco (Green 1998; Cherkaoui et al. 2016). The presence of the null model in top models would suggest that other covariates, not taken into account, may play determinant roles in explaining the occupancy of studied wetlands by these duck species.
Therefore, consideration of further environmental variables will be valuable in improving our understanding of the effects of ecological factors on the processes of wetlands/habitats selection (see Pöysä et al. 1996; Green 1998). For instance, it is important to initiate quantitative investigations of the effects of conservation status (protected versus unprotected; large versus reduced protected area), water level fluctuation, water quality (e.g., clarity, temperature, dissolved oxygen, and pH), sediments (e.g., organic matter content and particle size), food availability (e.g., abundance, diversity and accessibility), weather (especially rainfall) and predation (e.g., dogs, cats, and wild boards) to better understand spatio-temporal dynamics of breeding ducks. It would be highly informative to conduct such research both at a regional (Maghreb region) level and at an international (Mediterranean area) level in order: (i) to allow a global-scale analysis and benchmarking, (ii) to improve the provision of information to support good management interventions, (iii) to contribute to conservation management planning for these waterbird species, and (iv) to assess how conservation benefits can be optimized. Setting up a scientific monitoring program devoted to the breeding ducks similar to those already established for wintering waterbirds will provide suitable data to adopt a much more effective management strategy. The role of scientific research is thus of paramount importance.
References
AEFCS (1996) Plan Directeur des Aires Protégées du Maroc. Administration des Eaux et Forêts et de la Conservation des Sols/ BCEOM/SECA/ISR/EPHE. (Unpublished report)
Almaraz P, Amat JA (2004) Multi-annual spatial and numeric dynamics of the White-headed duck (Oxyura leucocephala) in southern Europe: seasonality, density dependence and climatic variability. J Anim Ecol 73:1013–1023
Amat JA (1982) The nesting biology of ducks in the Marismas of the Guadalquivir, south-western Spain. Wildfowl 33:94–104
Arnold TW (2010) Uninformative parameters and model selection using Akaike’s information criterion. J Wildl Manag 74:1175–1178
Atiénzar F, Antón-Pardo M, Armengol X, Barba E (2012) Distribution of the white-headed duck Oxyura leucocephalais affected by environmental factors in a Mediterranean wetland. Zool Study 51:783–792
Bartoń K (2015) MuMIn: multi-model inference. R package version 1.9.13. http://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-ef-fects models using Eigen and S4. R package version 1.1–7. Retrieved 21 Oct 2016. http://cran.r-project.org/package=lme4
Benabid A (1982) Bref aperçu sur la zonation altitudinale de la végétation climacique au Maroc. Ecolg Med 8:301–315
Bengtsson D, Avril A, Gunnarsson G, Elmberg J, Söderquist P, Norevik G, Tolf C, Safi K, Fiedler W, Wikelski M, Olsen B, Waldenström J (2014) Movements, Home-Range Size and Habitat Selection of Mallards during Autumn Migration. PLoS One 9(6):e100764. doi:10.1371/journal.pone.0100764
BirdLife International (2012) IUCN red list for birds. http://www.birdlife.org/. Accessed 1 June 2015
BirdLife International (2015) IUCN red list for birds. http://www.birdlife.org/. Accessed 17 Nov 2016
Block WM, Brennan LA (1993) The habitat concept in ornithology: theory and applications. Curr Ornithol 11:35–91
Broyer J, Curtet L (2010) The influence of macrophyte beds on duck breeding in fishponds of the Dombes region, France. Wildfowl 60:136–149
Burgess TM, Webster R (1980) Optimal interpolation and isarithmic mapping of soil properties. I. The semi-variogram and punctual kriging. J Soil Sci 31:315–331
Burnham KP, Anderson DR (2002) Model Selection and Multimodel Inference. Springer, New York
Caithness TA (1982) Gamebird hunting. The Wetland Press, Wellington, p 96
Cambardella CA, Moorman TV, Novak JM, Parkin TB, Karlen DL, Turco RF, Konopka AE (1994) Field-scale variability of soil properties in Central Iowa soils. Soil Sci Soc Am J 58:1501–1511
Cardador L, Carrete M, Mañosa S (2011) Can intensive agricultural landscapes favour some raptor species? The marsh harrier Circus aeruginosus in Northeastern Spain. Anim Conserv 14:382–390
Castro H, Nevado JC, Paracuellos M, López JM (1994) La Malvasía (Oxyura leucocephala) en la provincia de Almería. Evolución poblacional, nidificación y selección de hábitat. Oxyura 7:119–133
Cherkaoui SI, Dakki M, Lahrouz S, Hanane S (2013) Ten-year survey of breeding Anatidae of Lake Sidi Boughaba (North-western Marocco): status, tendencies of change and avenues for future research. Rev d’Écol (Terre Vie) 68:167–180
Cherkaoui SI, Bouajaja A, El Banak A, Lahrouz S, Hanane S (2014) The Black-necked Grebe (Podiceps nigricollis): an expanding species in the Middle Atlas wetlands, Morocco. Wetl Ecol Manag J 22:93–98
Cherkaoui SI, Hanane S, Magri N, EL Agbani MA, Dakki M (2015) Factors influencing species richness of breeding waterbirds in Moroccan IBA and Ramsar wetlands. A macroecological approach. Wetlands 35:913–922
Cherkaoui SI, Magri N, Hanane S (2016) Factors predicting Ramsar site occupancy by threatened waterfowl: the case of the Marbled Teal Marmaronetta angustirostris and Ferruginous Duck Aythya nyroca in Morocco. Ardeola 63:295–309
Cody ML (1985) Habitat selection in birds. Academic Press, Orlando, p 558
Dakki M, Qninba A, El Agbani MA, Benhoussa A, Beaubrun PC (2001) Waders wintering in Morocco: national population estimates, trends and site-assessments. Wader Study Group Bull 96:47–59
Dakki M, El Agbani MA, Qninba A (eds) (2011) Zones humides du Maroc inscrites jusqu’en 2005 sur la liste de la Convention de Ramsar. Travaux Institut Scientifique, Rabat, Série Générale 7: 238
Defos du Rau P, Barbraud C, Mondain-Mon-val JY (2005) Incorporating uncertainty into analyses of red-crested pochard habitat selection. Biol Conserv 125:355–367
Dormann CF (2007) Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Global Ecol Biogeogr 16:129–138
Dray S, Dufour AB (2007) The ade4 package: implementing the duality diagram for ecologists. J Stat Softw 22:1–20
El Agbani MA, Qninba A, Amezian M, Cuzin F, Dakki M (2009) Le peuplement d’oiseaux d’eau du complexe des zones humides de Smir (Nord du Maroc): état actuel, intérêt patrimonial et évolution depuis les quatre dernières décennies. Bull l’Inst Sci 31:103–110
Ficetola GF, Marziali L, Rossaro B, De Bernardi F, Padoa-Schioppa E (2011) Landscape-stream interactions and habitat conservation for amphibians. Ecol Appl 21:1272–1282
Fishpool LDC, Evans MI (2001) Important bird areas in Africa and associated islands: priority sites for conservation. Birdlife International, Cambridge, p 1144
Fox AD, Jonsson JE, Aarvak T, Bregnballe T, Christensen TK, Clausen KK, Clausen P, Dalby L, Holm TE, Pavon-Jordan D, Laursen K, Lehikoinen A, Lorentsen SH, Møller AP, Nordström M, Ost M, Söderquist P, Therkilden OR (2015) Current and potential threats to Nordic duck populations—a horizon scanning exercise. Ann Zool Fenn 52:193–220
García J, Miguelez D, Astiarraga H, Zumalac arregui C (2015) Factors determining the occupation of marsh harrier Circus aeruginosus breeding grounds in a Mediterranean environment. Bird Study 62:331–338
Goovaerts P (1998) Geostatistical tools for characterizing the spatial variability of microbiological and physico-chemical soil properties. Biol Fertil Soils 27:315–334
Green AJ (1998) Habitat selection by the Marbled Teal Marmaronetta angustirostris, Ferruginous Duck Aythya nyroca and other ducks in the Göksu Delta, Turkey in late summer. Rev d’Écol (Terre Vie) 53:225–243
Green AJ, El Hamzaoui M (2000) Diurnal behaviour and habitat use of nonbreeding marbled teal, Marmaronetta angustirostris. Can J Zool 78:2112–2118
Hoffman DJ, Rice CP, Kubiak TJ (1996) PCBs and dioxins in birds. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife—interpreting tissue concentrations. CRC Press, Boca Raton, pp 165–207
Holopainen S, Arzel C, Dessborn L, Elmberg J, Gunnarsson G, Nummi P, Pöysä H, Sjöberg K (2015) Habitat use in ducks breeding in boreal freshwater wetlands: a review. Eur J Wildl Res 61:339–363
Isenmann P, Moali A (2000) Les Oiseaux d’Alge´rie [Birds of Algeria.]. Société d’Étude Ornithologiques de France, Paris
Isenmann P, Gaultier T, El Hili A, Azafzaf H, Dlensi H, Smart M (2005) Oiseaux de Tunisie [Birds of Tunisia.]. SEOF Editions, Paris
Keddy PA (2010) Wetland ecology—principles and conservation. Cambridge University Press, Cambridge
Khaffou M, Chahlaoui A (2012) La Reproduction du Tadorne Casarca Tadorna ferruginea dans la Zone Humide d’Aguelmam Sidi Ali Moyen Atlas Maroc, vol 4. No 120903, Science Lib Edition Mersenne, p 14
Korschgen CE, Dahlgren RB (1992) Human disturbances of waterfowl: causes, effects, and management. Fish Wildl Leafl 13(2):15
Lahrouz S, Dakki M, Gmira N (2012) The importance of Fouwarate marshland for wintering and breeding of the threatened ducks populations in Morocco. J Anim Plant Sci 13:1800–1810
Lant CL, Kraft E, Beaulieu J, Bennett D, Loftus T, Nicklow J (2005) Using GIS-based ecological–economic modeling to evaluate policies affecting agricultural watersheds. Ecol Eco 55:467–484
Lecis R, Norris K (2004) Habitat correlates of distribution and local population decline of the endemic Sardinian newt Euproctus platycephalus. Biol Conserv 115:303–317
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
Liang J, Hua S, Zeng G, Yuan Y, Lai X, Li X, Li F, Wu H, Huang L, Yu X (2015) Application of weight method based on canonical correspondence analysis for assessment of Anatidae habitat suitability: a case study in East Dongting Lake, Middle China. Ecol Eng 77:119–126
M’Hirit O (1999) Climats et bioclimats de la forêt. Le grand livre de la forêt marocaine. Mardaga, Liège, pp 54–60
Ma ZJ, Li B, Zhao B, Jing K, Tang SM, Chen JK (2004) Are artificial wetlands good alternatives to natural wetlands for waterbirds? A case study on Chongming Island, China. Biodivers Conserv 13:333–350
Magin C (2001) Morocco. In: Fishpool LDC and Evans MI (eds) Important bird areas in Africa and associated islands: priority sites for conservation. Pisces Publications and Birdlife International (BirdLife Conservation Series n 11), Newbury
Marone L, Lopez De Casenave J, Cueto VR (1997) Patterns of habitat selection by wintering and breeding granivorous birds in the central Monte desert, Argentina. Rev Chil Hist Nat 70:73–81
Martin TE (1993) Nest predation and nest sites. Bioscience 43:523–532
Mcgarigal K, Cishman SA, Stafford S (2000) Multivariate statistics for wildlife and ecology research. Springer Verlag, New York
McKinney RA, Raposa KB, Cournoyer RM (2011) Wetlands as habitat in urbanizing landscapes: patterns of bird abundance and occupancy. Landsc Urban Plan 100:144–152
Merendino MT, Ankney CD (1994) Habitat use by mallards and American black ducks breeding in central Ontario. Condor 96:411–421
Monfils MJ, Prince HH (2009) Breeding bird response to long-term wastewater discharge in a northern Michigan peatland. Ecol Eng 35:1357–1365
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R^2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Diaz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison MLK, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, KLeyer M, , , , Correia DLP, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HRP, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JPW, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520:45–50
Newton I (1998) Population Limitation in Birds. Academic Press, San Diego
Newton I, Campbell CRG (1975) Breeding of ducks at Loch Leven, Kinross. Wildfowl 26:83–102
Noordhuis R, Van der Molen DT, Van den Berg MS (2002) Response of herbivorous water-birds to the return of Chara in Lake Veluwemeer, The Netherlands. Aquat Bot 72:349–367
Ooi JLS, Van Niel KP, Kendrick GA, Holmes KW (2014) Spatial structure of seagrass suggests that size-dependent plant traits have a strong influence on the distribution and maintenance of tropical multispecies Meadows. PLoS One 9(1):e86782
Pebesma EJ (2006) The gstat package. www.gstat.org
Penteriani V, Gallardo M, Roche P (2002) Landscape structure and food supply affect eagle owl (Bubo bubo) density and breeding performance: a case of intra-population heterogeneity. J Zool 257:365–372
Petit S, Hayson K, Pywell R, Warman L, Allen D, Booth R, Firbank L (2003) Habitat-based models for predicting the occurrence of ground-beetles in arable landscapes: two alternative approaches. Agric Ecosyst Environ 95:19–28
Pöysä H (2001) Dynamics of habitat distribution in breeding mallards: assessing the applicability of current habitat selection models. Oikos 94:365–373
Pöysä H, Elmberg J, Nummi P, Sjöberg K (1996) Are ecomorphological associations among dabbling ducks consistent at different spatial scales? Oikos 76:608–612
Qninba A, Rguibi Idrissi H, Himmi O, Benhoussa A, El Agbani MA, Thévenot M (2008) Nouveaux cas de nidification d’oiseaux dans le complexe de zones humides du Bas Loukkos (Nord-Ouest du Maroc). Bull Inst Sci 30:45–50
Ramsar (2015) Ramsar website, Available at: [http://www.ramsar.org/cda/en/ramsar-about-history/main/ramsar/1-36-62_4000_0__]. Accessed 03 Nov 2016
Ramsar Convention Bureau (2002) What is the Ramsar convention on wetlands?. Ramsar Convention Bureau, Gland
Reich RM, Joy SM, Reynolds RT (2004) Predicting the location of goshawk nests: modeling the spatial dependency between nest locations and forest structure. Ecol Model 176:109–133
Rotella JJ, Ratti JT (1992) Mallard brood survival and wetland habitat conditions in southwestern Manitoba. J Wild Manag 56:499–507
R Development Core Team (2013) R: a language and environment for statistical computing. ISBN 3-900051-07-0, R Foundation for Statistical Computing, Vienna. http://www.R-project.org
Sánchez-Zapata JA, Anadón JD, Carrete M, Giménez A, Navarro J, Villacorta C, Botella F (2005) Breeding waterbirds in relation to artificial pond attributes: implications for the design of irrigation facilities. Biodivers Conserv 14:1627–1639
Sebastián-González E, Fuentes C, Ferrández M, Echevarrías JL, Green AJ (2013) Habitat selection of marbled teal and white-headed duck during the breeding and wintering seasons in south–eastern Spain. Bird Conserv Int 23:344–359
Thévenot M, Vernon R, Bergier P (2003) The birds of Morocco. British Ornithologists’Union/British Ornithologists’Club, Tring
Thorpe J (1997) The implications of recent serious bird strike accidents and multiple engine ingestions. Bird Strike Committee, Boston
Tucker GM, Evans MI (1997) Habitats for birds in Europe: a conservation strategy for the wider environment. BirdLife International BirdLife Conservation Series No. 6), Cambridge
Venables WN, Ripley BD (2002) Modern applied statistics with S, 2nd edn. Springer, New York
Verner J, Morrison ML, Ralph CJ (eds) (1986) Wildlife 2000: modeling habitat relationships of terrestrial vertebrates. University of Wisconsin Press, Madison
Wassens S, Hall A, Osborne W, Watts RJ (2010) Habitat characteristics predict occupancy patterns of the endangered amphibian Litoria raniformis in flow-regulated flood plain wetlands. Austral Ecol 35:944–955
Weller MW (1999) Wetland birds. Habitat resources and conservation implications. Cambridge University Press, Cambridge, p 271
Acknowledgements
We are grateful to N. Magri for helpful in realizing the map. We also thank two anonymous reviewers and the Editor of Ecological Research for their comments and advice.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cherkaoui, S.I., Selmi, S. & Hanane, S. Ecological factors affecting wetland occupancy by breeding Anatidae in the southwestern mediterranean. Ecol Res 32, 259–269 (2017). https://doi.org/10.1007/s11284-017-1436-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-017-1436-5