Abstract
Nitrogen (N) and phosphorus (P) concentrations and N:P ratios in terrestrial plants and their patterns of change along environmental gradients are important traits for plant adaptation to changes. We determined the leaf N and P concentrations of Chinese sea-buckthorn (Hippophae rhamnoides L. subsp. sinensis Rousi), a non-legume species with symbiotic N fixation (SNF), at 37 sites across northern China and explored their geographical patterns in relation to climate and soil factors. (1) The mean leaf N, P, and N:P ratio were 36.5, 2.1 mg g−1, and 17.6, respectively, higher than the mean values of most shrub species in the region. (2) Leaf N was correlated with soil mineral N in cool areas (mean annual temperature MAT <3 °C) but with temperature in warm areas (MAT >3 °C). The high leaf N and divergent leaf N–soil N relationship suggested the importance of SNF in plant N uptake; SNF increases with temperature and is probably the major N source in warm areas. (3) Leaf P was positively related to mean annual precipitation. Leaf N:P ratio was primarily driven by changes in leaf P. The high leaf P reflected the greater requirements of the N-fixing species for P. Our results represent a major advance in understanding the elemental stoichiometry of non-legume N-fixing plants, indicating high P and N requirements and a shift in N source from SNF to soil as temperature declines. This knowledge will help in assessing the habitat suitability for the species and predicting the species dynamics under environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In terrestrial ecosystems, nitrogen (N) and phosphorus (P) are the two limiting elements that most influence ecosystem function and dynamics (Chapin et al. 2002; Sterner and Elser 2002). The cycling of these two key nutrients in ecosystems globally has been greatly altered by changing factors, such as nitrogen deposition, rising atmospheric CO2 concentration, and global warming (Galloway et al. 1995; McGuire et al. 1995; Vitousek et al. 1997; Esmeijer-Liu et al. 2009). To understand how terrestrial plants will adapt to environmental change in the future, their patterns of N, P, and N:P ratio and the drivers of these parameters at global and regional scales have been increasingly studied (Elser et al. 2000; McGroddy et al. 2004; Reich and Oleksyn 2004; Han et al. 2005, 2011; He et al. 2006, 2008; Zheng and Shangguan 2007).
At the global scale, leaf N concentration was found to be negatively correlated with mean annual temperature (MAT) (Reich and Oleksyn 2004), but in another study it was positively correlated with MAT and mean annual precipitation (MAP) (Yuan and Chen 2009). Leaf P concentration was reported to be negatively correlated with MAT and positively correlated with latitude (Reich and Oleksyn 2004; Yuan and Chen 2009) and, in some cases, also positively related to MAP (Yuan and Chen 2009). These inconsistent patterns in plant nutrient concentrations in relation to environment also exist at regional scales (Sardans et al. 2011). For example, within a small geographical region of the Loess Plateau in China, leaf N and P were not correlated with latitude, MAT, nor MAP, while leaf N:P ratio increased with increasing latitude or decreasing MAP and showed no relation with MAT (Zheng and Shangguan 2007).
Overall, these studies suggest a stoichiometric pattern between leaf N and P concentrations and climate that can differ depending on the limiting factor at each scale (McGroddy et al. 2004; Reich and Oleksyn 2004; Han et al. 2005, 2011; He et al. 2006, 2008; Zheng and Shangguan 2007). Leaf chemical stoichiometry and its relationship with environmental factors also vary with species composition at the biome level (He et al. 2006, 2008), because species differ in physiology and ecology (Reich and Oleksyn 2004; He et al. 2008; Kang et al. 2011). For instance, at the global scale, leaf N in Betula and Larix species increased with MAT and decreased in Acer and Calamagrostis (Reich and Oleksyn 2004). At the regional scale, leaf N and P in Stipa and Kobresia in Chinese grasslands also showed different patterns of variation in relation to precipitation and temperature during the growing season (He et al. 2008). The relationships between leaf chemical stoichiometry and environmental factors were unique to each species, while the data on leaf chemical traits at the species level were insufficient to discern a pattern (Kang et al. 2011; Sardans et al. 2011), and the leaf chemical traits of non-legume nitrogen-fixing plant species have been poorly studied.
Chinese sea-buckthorn is a subspecies of common sea-buckthorn (Hippophae rhamnoides L. subsp. sinensis Rousi) that harbors nitrogen-fixing Frankia sp. symbionts (Stewart and Pearson 1967). This subspecies occurs only in northern China, where it is an important shrub that is widely used for soil and water conservation (Lian 2000). The species is distributed from the Loess Plateau in the east to the eastern edge of the Tibetan Plateau in the west, across an altitude range from 400 to 3300 m (a.s.l.). Across such a wide geographical range, there is a pronounced gradient of MAT, MAP, and soil substrates, providing a unique opportunity to study the chemical stoichiometry patterns of this species.
The objective of this study was to determine the leaf chemical stoichiometry (N, P, N:P ratio) of the Chinese sea-buckthorn across its distribution to discern their geographical patterns and to explore their relationships with climatic and soil factors. In addition, we also examined the variation of these stoichiometric variables from the center to the edge of the species distribution to determine whether these leaf traits increased or decreased in magnitude along this radius, because the distribution center might represent the ecologically-optimal environment for the species. This study is essential for understanding the leaf stoichiometry of non-legume nitrogen-fixing plants in relation to climate and soil conditions and for assessing the habitat suitability of this species for use in soil and water conservation under environmental changes.
Materials and methods
Study site
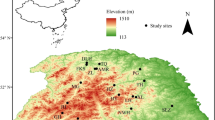
Thirty-seven natural populations of Chinese sea-buckthorn were investigated; they spanned a wide geographical range of 34°–40°N in latitude, 100°–114°E in longitude, and 1024–3209 m a.s.l. in altitude (Fig. 1). Climatic data for the past 30 years (1980–2010) were collected from the meteorological stations closest to the investigation sites in each region. In this area, MAT increases with longitude (r2 = 0.534, p < 0.0001, n = 37) but decreases with altitude (r2 = 0.850, p < 0.0001, n = 37). Soil types were identified at each site using soil maps (Soil map compilation group of Nanjing institute of soil science 1978). The soil types varied greatly and included felty soils, chernozems, loess soils, dark loess soils, cinnamon soils, and grey-cinnamon soils that differed considerably in nutrient levels.
Sampling and measurement
Leaf samples were collected according to the protocol of Cornelissen et al. (2003). Fully-expanded sun leaves were collected from 30 individual plants of each natural population during the growing season (August–September) in 2011. The plant samples were dried for 72 h at 60 °C to a constant weight. All the leaves from the same natural population were mixed, ground with a porcelain mortar into uniformly fine powder and sieved through a 100-mesh screen before chemical analysis. For both N and P determination, the samples were digested in a solution of H2SO4–H2O2. Then N concentrations were measured using a fully automatic azotometer (UDK159, VELP, Usmate Velate, Italy). Phosphorus concentrations were measured colorimetrically by molybdenum blue spectrophotometry (6505 UV spectrophotometer, Jenway, Staffordshire, UK). All the chemical determinations were repeated three times with the same samples.
In each natural population, five soil samples were collected, in the east, west, south, north and central part of a sampling area, then thoroughly mixed and air-dried. The stone and roots in the soil samples were removed by hand, and the soil was ground to pass through a 20-mesh sieve. Soil available N (AN) concentrations were measured using the alkaline hydrolysis diffusion method (Khan et al. 2001). Soil available P (AP) concentrations were extracted from 2.5 g air-dried soil with 50 mL 0.5 M NaHCO3 solution (Olsen et al. 1954).
Data analysis
We first tested the normality of the measured plant stoichiometric variables (leaf N, P, and N:P ratio) using the Kolmogorov–Smirnov test, then we explored the relationship between these variables and geographic, climatic, and soil factors using Pearson correlation and regression analysis. We conducted a principal component analysis (PCA) to define comprehensively the relationships between plant stoichiometric variables and the interrelated changes in geographic, climatic, and soil factors. We also analyzed the variation pattern of plant stoichiometric variables from the center to the edge of the species distribution. The geographic center of the distribution was identified at 36.8°N and 107.2°E (refer to Fig. 1). The figures and statistical analysis were made with Sigma Plot (2005; Systat Software, Chicago, IL, USA) and SPSS (2008; IBM, Chicago, IL, USA).
Results
Leaf N, P, and N:P ratio of Chinese sea-buckthorn in northern China
The geometric mean leaf N and P concentrations and N:P ratio of Chinese sea-buckthorn were 36.46 mg g−1 (range 32.60–43.61 mg g−1), 2.06 mg g−1 (1.50–2.69 mg g−1), and 17.64 (13.46–23.07), respectively. All variables were normally distribution with P (K–S) ranging from 0.08–0.99 (Table 1). Leaf N was positively correlated with leaf P (Fig. 2a). Leaf N:P ratio showed a negative correlation with leaf P but not with leaf N (Fig. 2b); thus, variation in N:P ratio was primarily determined by leaf P (Fig. 2c).
Relationships among leaf nitrogen (N), phosphorus (P), and N:P ratio of Chinese sea-buckthorn in northern China. The lines and curves are the significant regression between two variables with p < 0.05. a Leaf P and N (r2 = 0.149, p = 0.020, n = 36); b leaf N and N:P; c leaf P and N:P (r2 = 0.635, p < 0.0001, n = 36)
Variation in leaf N, P, and N:P ratio with geographical variables
The relationships between leaf N and geographical variables such as latitude, longitude, and altitude followed convex curves (Fig. 3a, b, c). In general, leaf N and P significantly increased as longitude increased and altitude decreased (Fig. 3b, c, e, f). The N:P ratio showed a similar trend to that of leaf N with latitude (Fig. 3a, g) but had no relationship with longitude and altitude (Fig. 3h, i).
Leaf nitrogen (N), phosphorus (P), and N:P ratio in relation to geographical variables. The lines and curves are the significant regression between two variables with p < 0.05. a Leaf N and latitude (r2 = 0.223, p = 0.014, n = 37); b leaf N and longitude (r2 = 0.519, p < 0.0001, n = 37); c leaf N and altitude (r2 = 0.308, p = 0.002, n = 37); d leaf P and latitude; e leaf P and longitude (r2 = 0.154, p = 0.018, n = 36); f leaf P and altitude (r2 = 0.13, p = 0.031, n = 36); g N:P and latitude (r2 = 0.166, p = 0.0499, n = 36); h N:P and longitude; i N:P and altitude
Variation in leaf N, P, and N:P ratio with climatic variables
Leaf N concentration increased significantly with increasing MAT (p < 0.05); leaf P concentration also showed an increasing trend as increasing MAT (r2 = 0.101; p = 0.064) (Fig. 4a, c). Leaf P concentration, but not leaf N concentration, increased as MAP increased (Fig. 4d). Nevertheless, N:P ratio displayed an opposite trend and decreased as MAP increased, but did not reach statistically significant levels (Fig. 4f).
Leaf nitrogen (N), phosphorus (P), and N:P ratio in relation to climatic variables (MAP, mean annual precipitation; MAT, mean annual temperature). The lines and curves are the significant regression between two variables with p < 0.05. a Leaf N and MAT (r2 = 0.164, p = 0.047, n = 37); b leaf N and MAP; c leaf P and MAT; d leaf P and MAP (r2 = 0.124, p = 0.035, n = 36); e N:P and MAT; f N:P and MAP
Variation in soil AN and AP with geographical and climatic variables
Soil AN significantly decreased as longitude and MAT increased and increased as altitude increased. However, soil AP concentrations showed no significant correlation with geographical and climatic variables (Tables 1, 2).
Variation patterns of plant and soil N and P in relation to the center of distribution region
We examined the relationships between leaf traits (leaf N, P, and N:P ratio), soil nutrients (AN, AP) contents and the distance of plant sampling sites to its distribution center. We found only one significant positive correlation (r2 = 0.35, p = 0.03) between leaf N and the distance to the center, with low leaf N levels at the center and higher levels at the edges of the distribution region. No other significant correlations were detected between leaf stoichiometric variables or soil nutrient contents and distance of the sampling sites from the distribution center.
Integrative relationships among leaf N, P, N:P ratio, soil AN and AP, and climatic factors MAT and MAP
A PCA was performed on the sample sites using the leaf nutrient concentrations, soil nutrient contents, and climatic factors (MAT and MAP) as variables. The loadings of all the variables in the PCA explain their interrelated changes among the sample sites (Fig. 5). The first principal component (PC1) explained 38.6 % of the variance; it showed that Chinese sea-buckthorn located at high altitude or low longitude, where soil AN was high and MAT was low (left side of PC1 in Fig. 5), had low leaf N concentrations. In contrast, Chinese sea-buckthorn located at low altitude or high longitude, where soil AN was low and MAT was high (right side along PC1), had high leaf N concentrations. This finding indicated that the changes in leaf N were not related to those in soil AN. In other words, plant N (or N-fixation by Frankia) was favored by habitats at low altitude or high MAT/longitude. PC2 explained 21.2 % of the total variance and showed that changes in plant P and soil AP were associated with variation in MAP and latitude. That is, leaf P was high in high rainfall (MAP) areas, but leaf N:P ratio was high in low rainfall areas.
Loadings of climatic factors, soil nutrient concentrations, and plant leaf stoichiometry of Chinese sea-buckthorn in a principal component analysis of the sampling sites. The first and second principal components, PC1 and PC2, explain 38.6 and 21.2 % of the variance in the data, respectively. ALT altitude, AN soil available nitrogen (N), AP soil available phosphorus (P), LAT latitude, LOG longitude, MAP mean annual precipitation, MAT mean annual temperature, N leaf N, N:P leaf N:P ratio, P leaf P, PC1 first principal component, PC2 second principal component
Discussion
Leaf N, P, and N:P ratio of Chinese sea-buckthorn in northern China
The mean leaf N, P, and N:P of Chinese sea-buckthorn were 36.46, 2.06 mg g−1, and 17.64, respectively. All the values were significantly higher than those of the 51 shrub species (means: 24.7, 1.55 mg g−1, and 16.1) in the Loess Plateau (Zheng and Shangguan 2007), where Chinese sea-buckthorn occurs (Table 3). That the leaves of Chinese sea-buckthorn, a N-fixing plant, have a higher N concentration and higher N:P ratio is not unexpected, because leaves of N-fixing plants generally contain more N but not P, and thus a higher N:P ratio, than co-occurring non N-fixing plants (Güsewell et al. 2003; Wright et al. 2004; He et al. 2008). The flora of the Loess Plateau is reported to be constrained by P (Zheng and Shangguan 2007). The above-average leaf P concentration (2.06 mg g−1) of Chinese sea-buckthorn (cf. a mean of 1.55 mg g−1 for 51 shrub species in the region; Zheng and Shangguan 2007) suggests an increased capability of this N-fixing plant to absorb P in a P-limited environment, which might be related to the greater requirement for P by N-fixing plants, as suggested by Vitousek et al. (2002).
Considerable evidence has been accumulated to indicate that a critical N:P ratio in plant leaves can be an indicator of nutrient limitation at vegetation or biome levels (Wassen et al. 1995; Aerts and Chapin 2000; Güsewell 2004). An N:P ratio <14 generally indicates N constraint, while an N:P ratio of more than 16 shows P constraint. An N:P ratio between 14 and 16 suggests limitation by either or both nutrients (Wassen et al. 1995; Koerselman and Meuleman 1996). Our results supported this conclusion at the species level. The leaf N concentration of Chinese sea-buckthorn was much higher than that other shrub species, which can most likely be attributed to symbiotic nitrogen fixation (SNF), while the high N:P ratio suggested that P limits plant growth. Leaf N and P were positively correlated.
Characteristics of variation in leaf N, P and N:P ratio of Chinese sea-buckthorn in northern China
Nitrogen-fixing plants take up N from both soil and SNF, and the proportion of N from these sources changes along an environmental gradient (Stewart and Pearson 1967). Soil nutrient levels and temperature are major factors to explain the balance of nitrogen sources for N-fixing plants (Reddell et al. 1985; Danso et al. 1992). Leaf N in Chinese sea-buckthorn reflected this fact. Our higher-altitude sample sites were mainly located in areas near the eastern edge of the Tibetan Plateau, with chernozem, felty, and gray-cinnamon soil types. Samples sites at middle and lower altitudes were mainly on the Loess Plateau, with loess, dark loess, and cinnamon soil types (Soil map compilation group of Nanjing institute of soil science 1978). The N density varies substantially with soil type in China (Tian et al. 2006). Our results demonstrated that soil AN was negatively correlated with longitude and MAT and positively with altitude (Table 2), which actually reflects the soil N changes among soil types along a temperature gradient from lower to high altitude in different geological regions. Soil N density in soils sampled in at high altitudes was 1336.2, 2469.6, and 2235.8 g m−3, respectively, for chernozems, felty soils, and gray-cinnamon soils and was much higher than that in soils sampled on the Loess plateau (loess, dark loess, and cinnamon soils with N density of 506.2, 875.5, and 928.0 g m−3, respectively).
However, our results also showed that changes in soil AN negatively correlated with changes in leaf N (Fig. 5). Leaf N of Chinese sea-buckthorn was high in habitats at low altitude or high MAT/longitude, where soil AN was relatively low. This result strongly suggested that a significant amount of leaf N in Chinese sea-buckthorn derives from SNF, especially in the warmer habitats at lower altitudes. Root nodules of sea-buckthorn have strong N-fixing ability and can fix 180 kg N ha−1 year−1, twice the amount reported for soybean nodules (Akkermans 1971; Oremus 1979). Plant N-fixation is affected markedly by soil temperatures (Sprent 1979; Reddell et al. 1985; Danso et al. 1992), as would be expected for Chinese sea-buckthorn. We concluded based on these analyses that the fraction of Chinese sea-buckthorn leaf N derived from SNF increases with decreasing altitude or increasing MAT. This view is supported by the observation of Zhang et al. (2010) that the size of Chinese sea-buckthorn root nodules decreases significantly with increasing altitude.
The proportion of leaf N in Chinese sea-buckthorn from soil versus SNF changed along the MAT gradient. Leaf N concentration reached its lowest point at about 3 °C in MAT (Fig. 4a). Thus, we concluded that 3 °C MAT is the conversion point at which Chinese sea-buckthorn derives its N from the soil versus Frankia.
Where MAT was <3 °C, leaf N concentration decreased as MAT increased, which might be related to decreasing soil AN as MAT increased. We suggest that the decrease of leaf N derived from the soil was more than the increase from SNF. Consequently, leaf N concentration decreased as MAT increased. That is, Chinese sea-buckthorn has lower SNF due to temperature limitation, thus leaf N comes mainly from the soil. Where MAT was >3 °C, leaf N concentration increased with MAT, while soil AN showed an opposite trend (Table 2). This result suggested that more leaf N derives from SNF in areas with a higher MAT, which favors the activity of Frankia. We hypothesize that the decrease in leaf N that originated from the soil was less than the increase from SNF. As a result, leaf N concentration increased as MAT increased, and thus leaf N was mainly from SNF. Thus, the leaf N of Chinese sea-buckthorn is strongly influenced by soil N availability in areas with MAT lower than 3 °C and by SNF in areas with MAT higher than 3 °C.
The lowest leaf N levels were at about 39°N along the latitudinal gradient, at about 105°E along the longitudinal gradient, and at about 2500 m a.s.l. along the altitudinal gradient (Fig. 3). These low points corresponded to where the reduction of leaf N from the soil equaled the increase from SNF.
Leaf N showed a significant positive correlation with the distance to the distribution center, with low leaf N levels at the center but high levels at the edges of the range. This pattern was most likely associated with changes in leaf N with MAT. Leaf N increases with MAT in Chinese sea-buckthorn, and MAT is low at the edges of the distribution (in the northwest, northeast, and southwest at high altitude, Fig. 1). The insignificant changes in other leaf traits from the center to the edge of the distribution might be explained by the high heterogeneity in geology and topography of the region.
Leaf P show a strong stoichiometric homeostasis with leaf N in terrestrial plants (Mcgroddy et al. 2004; Reich and Oleksyn 2004; Wright et al. 2004, 2005; Han et al. 2005; Zheng and Shangguan 2007; He et al. 2008). In our study of Chinese sea-buckthorn, leaf P also showed a consistent correlation with leaf N, increasing with longitude and decreasing with altitude. Leaf P comes from the soil, where it is mainly derived from rock weathering and P diffusivity in soil solution (Fitter et al. 1998; Lambers et al. 1998; Aerts and Chapin 2000). The soil P supply to plants is strongly influenced by climate (Chen et al. 2011). Leaf P of Chinese sea-buckthorn was positively related to MAP and showed a trend as increased with increasing MAT.
The leaf N:P ratio of Chinese sea-buckthorn increased significantly with latitude, unlike the pattern of leaf N:P ratio change along latitude in other plant species reported by Reich and Oleksyn (2004) or with that of the flora of the Loess Plateau reported by Zheng and Shangguan (2007). Our data showed that leaf N:P ratio was determined largely by leaf P, and leaf N:P showed a trend as decreased with increasing MAP, but showed no significant change with MAT. These relationships between leaf N:P ratio, temperature, and precipitation are consistent with the results reported by Zheng and Shangguan (2007) for other species in the region.
Conclusions
The geographical patterns of the N and P stoichiometry in leaves of Chinese sea-buckthorn are mainly driven by climate and soil gradients. The high leaf N concentration and N:P ratio of this species, compared with the mean values of most shrub species in the region, suggests the importance of SNF in its N uptake. Plant N uptake is primarily from soil mineral N in the cooler areas (MAT <3 °C), while it appears to be primarily from SNF in warmer areas (MAT >3 °C). The high leaf P concentration, compared with the mean leaf P of most shrub species in the region, reflects the capability of this N-fixing plant to acquire P and may be related to the greater requirements of this N-fixing species for P. Our results represent a major advance in understanding the elemental stoichiometry of non-legume N-fixing plants and the influence on stoichiometry of climate and soil factors, especially the N uptake source along a temperature gradient. This understanding will help to assess the suitability of habitats for using this species for soil and water conservation and to predict the species growth and dynamics under climate or environmental change.
References
Aerts R, Chapin FS (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Akkermans ADL (1971) Nitrogen fixation and nodulation of Alnus and Hippophae under natural condition. Thesis. Rijks University, Leiden
Chapin FS, Matson PA, Mooney HA (2002) Principles of terrestrial ecosystem ecology. Springer-Verlag, New York
Chen YH, Han WX, Tang LY, Tang ZY, Fan JY (2011) Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 34:1–7
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG, Poorter H (2003) A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Danso SKA, Bowen GD, Sanginga N (1992) Biological nitrogen fixation in trees in agro-ecosystems. Plant Soil 141:177–196
Elser JJ, Fagan WF, Denno RF, Dobberfuhl DR, Folarin A, Huberty A, Interlandi S, Kilham SS, McCauley E, Schulz KL, Siemann EH, Sterner RW (2000) Nutritional constraints in terrestrial and freshwater food webs. Nature 408:578–580
Esmeijer-Liu AJ, Aerts R, Kürschner WM, Bobbink R, Lotter AF, Verhoeven JTA (2009) Nitrogen enrichment lowers Betula pendula green and yellow leaf stoichiometry irrespective of effects of elevated carbon dioxide. Plant Soil 316:311–322
Fitter AH, Wright WJ, Williamson L, Belshaw M, Fairclough J, Meharg AA (1998) The phosphorus nutrition of wild plants and paradox of arsenate tolerance: does leaf phosphorus concentration control flowering? In: Lynch JP, Deikman J (eds) Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes. American Society of Plant Biologists, Washington D.C., Rockville, pp 39–51
Galloway JN, Schlesinger WH, Levy H II, Michaels A, Schnoor JL (1995) Nitrogen fixation: atmospheric enhancement-environmental response. Global Biogeochem Cycles 9:235–252
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Güsewell S, Koerselman W, Verhoeven JTA (2003) N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol Appl 13:372–384
Han WX, Fang JY, Guo DL, Zhang Y (2005) Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 168:377–385
Han WX, Fang JY, Reich PB, Woodward FI, Wang ZH (2011) Biogeography and variability of eleven mineral elements in plant leaves across gradients of climate, soil and plant functional type in China. Ecol Lett 14:788–796
He JS, Fang JY, Wang ZH, Guo D, Flynn DFB, Geng Z (2006) Stoichiometry and large-scale patterns of leaf carbon and nitrogen in the grassland biomes of China. Oecologia 149:115–122
He JS, Wang L, Flynn DFB, Wang X, Ma W, Fang JY (2008) Leaf nitrogen: phosphorus stoichiometry across Chinese grassland biomes. Oecologia 155:301–310
Kang HZ, Zhuang HL, Wu LL, Liu QL, Shen GR, Berg B, Man RZ, Liu CJ (2011) Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: an analysis based on local observations. For Ecol Manag 261:195–202
Khan SA, Mulvaney RL, Hoet RG (2001) A simple soil test for detecting sites that are nonresponsive to nitrogen fertilization. Soil Sci Soc Am J 65:1751–1760
Koerselman W, Meuleman AFM (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, Berlin
Lian YS (2000) Plant biology and chemistry of the genus Hippophae L. Gansu Science and Technology Press, Lanzhou
Mcgroddy ME, Daufresne T, Hedin LO (2004) Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial red field-type ratios. Ecology 85:2390–2401
McGuire AD, Mellio JM, Joyce LA (1995) The role of nitrogen in the response of forest net primary production to elevated atmospheric carbon-dioxide. Ann Rev Ecol Syst 26:473–503
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Government Print Office, Washington, DC, pp 1–19
Oremus PAI (1979) A quantitative study of nodulation in Hippophae rhamnoides L. in a coastal dune area. Plant Soil 52:59–68
Reddell P, Bowen GD, Robson AD (1985) The effects of soil temperature on plant growth, nodulation and nitrogen fixation in Casuarina cunninghamiana Miq. New Phytol 101:441–450
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci USA 101:11001–11006
Sardans J, Rivas-Ubach A, Peñuelas J (2011) Factors affecting nutrient concentration and stoichiometry of forest trees in Catalonia (NE Spain). For Ecol Manage 262:2024–2034
Soil map compilation group of Nanjing institute of soil science (1978) Soil map 1:4,000,000 People’s Republic of China. Map Publishing House, Beijing
Sprent JI (1979) The biology of nitrogen fixing organisms. McGraw-Hill, London 196 p
Sterner RW, Elser JJ (2002) Ecological Stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton
Stewart WDP, Pearson MC (1967) Nodulation and nitrogen-fixation by Hippophaë rhamnoides L. in the field. Plant Soil 26:348–360
Tian HQ, Wang SQ, Liu JY, Pan SF, Chen H, Zhang C, Shi XZ (2006) Patterns of soil nitrogen storage in China. Global Biogeochem Cycles 20:GB1001. doi:10.1029/2005GB002464
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman D (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI (2002) Toward an ecological understanding of biological nitrogen fixation. Biogeochemistry 57:1–45
Wassen MJ, Olde Venterink HGM, de Swart EOAM (1995) Nutrient concentrations in mire vegetation as a measure of nutrient limitation in mire ecosystems. J Veg Sci 6:5–16
Wright IJ, Reich PB, Westoby M et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Yuan ZY, Chen HYH (2009) Global trends in senescend-leaf nitrogen and phosphorus. Global Ecol Biogeogr 18:532–542
Zhang AM, Niu SQ, Sun K, Cao WX, Da WY (2010) Study on the root nodules features of Hippophae and Frankia isolation. Grassland and Turf 30:43–46
Zheng SX, Shangguan ZP (2007) Spatial patterns of leaf nutrient traits of the plants in the Loess Plateau of China. Trees 21:357–370
Acknowledgments
The authors thank Dahua Yu, Quan Sun, Yuxiang Zhuo, and Kun Ma for technical assistance in the analysis of plant and soil samples. This work was financially supported by National Natural Science Foundation of China (NSFC, Nos. 31160046 and 31270429).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, X., Sun, K. & Li, F.Y. Variation in leaf nitrogen and phosphorus stoichiometry in the nitrogen-fixing Chinese sea-buckthorn (Hippophae rhamnoides L. subsp. sinensis Rousi) across northern China. Ecol Res 29, 723–731 (2014). https://doi.org/10.1007/s11284-014-1165-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-014-1165-y