Abstract
Invasive exotic ants often have a mutualistic relationship with other insects excreting honeydew, and this is considered to play a key role in their invasion success. We investigated the multispecies association patterns between ants and hemipteran insects in the Yanbaru forests, Okinawa, Japan, an Asian biodiversity hotspot. We especially focused on roadside environments, which are the frontlines of invasion for exotic ants. We found that only a small number of herbaceous and pioneer plants were predominant on the roadsides. Four honeydew producers, Melanaphis formosana, Dysmicoccus sp. A, Heteropsylla cubana, and Sogata hakonensis, living on these roadside plants accounted for 94.9% of the total honeydew-producer aggregations observed. Only a few exotic ants, such as Technomyrmex brunneus and Anoplolepis gracilipes, were observed with these honeydew-producer aggregations, and densities of these ants and honeydew producers were often positively correlated. An ant exclusion experiment showed that exotic ant occurrence improved the survival of some of the hemipteran colonies. Interestingly, the abundance of native ants was not correlated with the abundance of honeydew producers, and the local density of Pheidole noda was negatively correlated with that of M. formosana. These findings, i.e., only a few ants, all exotic, tended to hemipteran honeydew producers despite the existence of many native ants, and the abundances of those exotic ants and those hemipteran insects had positive correlations, provide some insights into the mechanism of biological invasion and provide information for the management of exotic ants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasion by exotic ant species is an increasing threat to biodiversity worldwide (Holway et al. 2002; McGlynn 1999; Passera 1994). Some invasive exotic ants are known to displace native ants and other arthropods through competition and/or predation (Hoffmann et al. 1999; Holway 1998; Holway et al. 2002; O’Dowd et al. 2003). However, invasive exotic ants often have a mutualistic relationship, called trophobiosis, with other insects excreting honeydew. Honeydew contains sugary substances and amino acids, which provide nutrients to ant workers. As a reward for this carbohydrate-rich food, the exotic ants strongly protect the honeydew-producing insects from their natural enemies (Helms and Vinson 2002; Holway et al. 2002; Ness and Bronstein 2004), and this occasionally results in massive outbreaks of the honeydew producers. The consequences of ant invasion can therefore be extensive by indirectly damaging vegetation and altering local ecosystems (Hill et al. 2003; O’Dowd et al. 2003; Dunham and Mikheyev 2009) and can have important implications for agriculture (e.g., González-Hernández et al. 1999; Phillips and Sherk 1991; Reimer et al. 1990).
Trophobiosis between exotic ants and honeydew producers has been shown to have positive effects on honeydew-producer populations (e.g., Abbott and Green 2007; Bach 1991; González-Hernández et al. 1999; Grover et al. 2008; Helms and Vinson 2002; Hill et al. 2003; Kaplan and Eubanks 2002, 2005; O’Dowd et al. 2003). However, most of these studies so far have focused on mutualism between single species or at most trophobiosis between an ant species and a few particular honeydew producers. In reality, however, many areas have been invaded by multiple exotic ant species (e.g., Boer and Vierbergen 2008; Clark 1982; King and Tschinkel 2007; McClelland and Jones 2009; Pfeiffer et al. 2008; Sarty et al. 2007; Wetterer et al. 2006), and these exotic ants usually coexist with multiple species of honeydew-producing insects. Yet, few studies have tried to comprehensively describe the pattern of such multispecies mutualism in actual communities.
Okinawa Island is the main island of the Ryukyu Islands, which are located in the subtropical region of southwestern Japan. The northern part of this island, called Yanbaru, supports relatively well-conserved primary and semi-primary forests that contain many unique, endemic, and rare species and is considered a biodiversity hotspot in the world (IUCN 2000; Olson and Dinerstein 1998). A number of invasive ant species, including the yellow crazy ant, Anoplolepis gracilipes, and the big-headed ant, Pheidole megacephala, are found there, and 11 of approximately 70 ant species reported from the area so far are considered to be nonnative (Eguchi 2001; Yamauchi and Ogata 1995). In Yanbaru, exotic ants tend to be dominant in open habitats and are rare in primary forests (Yamauchi and Ogata 1995). However, even in primary and semi-primary forests, nonnative ants are often found along roadsides with native ants (Katayama and Tsuji 2010; Suwabe et al. 2009), suggesting that the forest roadside environment is a potential invasion frontline for exotic ants.
In Yanbaru, researchers have studied the relationship between exotic and native ants (Suwabe et al. 2009; Yamauchi and Ogata 1995) and between ants and a myrmecophilous plant (Katayama and Tsuji 2010), but no one has focused on trophobiosis between exotic ants and honeydew-producing insects. Indirect negative effects on local biodiversity of honeydew producers protected by A. gracilipes, one of the two worldwide invasive ants found in Yanbaru, are well documented on some tropical oceanic islands (Abbott and Green 2007; Hill et al. 2003; O’Dowd et al. 2003). However, to our knowledge, no comparable study concerning trophobiosis with A. gracilipes has been carried out in an East Asian continental island, such as Okinawa Island.
In this study, we focused on roadside environments of the forests of Yanbaru where exotic and native ants coexist (Katayama and Tsuji 2010; Suwabe et al. 2009). We carried out a comprehensive survey on the quantitative pattern of associations, namely what species of honeydew producers are attended by what species of ants at what frequencies. We then analyzed the positive effects of exotic ants on the population increase of honeydew producers. This consisted of two parts: (a) detection of any statistically significant positive correlation between the local abundance of each ant species and that of each honeydew-producer species in the field, using multivariate generalized linear models (GLMs) to screen for ant species that might have a positive influence on honeydew-producer abundance, and (b) ant exclusion experiments to test directly the positive effects of some ants on the abundance of honeydew producers.
We finally discuss that future conservation biological studies should assess exotic ants’ indirect impacts on plant communities and biodiversity through honeydew producers in Yanbaru.
Materials and methods
Preliminary census on vegetation as host communities for honeydew producers
We surveyed plant species composition and identified the dominant plants of roadside environments of the forests of Yanbaru. We randomly chose 97 points (except points in coastal regions where forests are scarce) from 203 permanent study points that had been established for another long-term study. These 203 permanent points were located on the ground surface of roadsides along paved roads at 1-km intervals so as to roughly cover the entire Yanbaru region (26°37′–52′N, 128°06′–19′E). For each of the 97 study points, we set two quadrats (20 m × 0.5 m for each side of the road). Plant species within the quadrats and each species’ abundance, which was assessed in terms of ground cover (0–100% with a 5% interval), were determined by viewing from directly above. These censuses were carried out from April 10 to April 24, 2010.
From the 97 study points, we randomly surveyed 36 points from October 25 to October 31, 2008 to determine which plant species were used by honeydew producers. Vegetation in each quadrat was observed for 15 min (30 min for each point) by standing adjacent to the quadrat so as not to disturb the vegetation and recording plant species hosting honeydew producers. The number of aggregations (colonies) of honeydew producers on each plant species was also recorded. In this census, we did not distinguish between honeydew-producer species.
Field census
From the above preliminary censuses we found that two herbaceous plants, Japanese silver grass (Miscanthus sinensis) and Spanish needle (Bidens pilosa), were dominant, together covering 36% of the roadside ground surface (Table 1). We also noticed that 84% of the honeydew-producer aggregations were found on these two species of plants (Table 2). For this reason, in the main census described below, we used only sites where either of these two plants existed. We also excluded sites that were not surrounded by primary or semi-primary forests, such as sites surrounded by orchards, because exotic ants are known to be dominant in these artificial environments in Yanbaru (Yamauchi and Ogata 1995). In addition, we excluded sites that were weeded by local road managers during the study period.
From eight paved roads built in the forests (Benoki Forest Road, Hiji Forest Road, Iji Forest Road, Janagusuku Forest Road, Ôkuni Forest Road, Okuyona Forest Road, Uka Forest Road, and Yona Forest Road), we established 40 study points, with each point located at 500-m intervals (or longer if the regular point did not satisfy the above three environmental requirements) (Fig. 1). At each point, we set a pair of linear quadrats (20 × 0.5 m), one on the ground at each side of the road, as in the preliminary census for honeydew producers. To estimate ant abundance from workers seen on the ground, we also set ten subquadrats (0.5 × 0.5 m, each located at 2-m intervals) on the road surface at the edge of each linear quadrat in order to avoid disturbing vegetation and honeydew producers in the quadrats when counting ants. Details of a study point are schematically shown in Fig. 2.
From October 27 to November 20, 2009, we observed the vegetation once in each linear quadrat for 30 min (60 min for each study point). We recorded the species (identified later in the laboratory) of honeydew producers found on the aerial parts of the vegetation, their host plant species, the number of aggregations, and their attending ant species (an "attending ant" was defined as an ant that was completely motionless for 1 s or longer within a 10-mm distance from a honeydew producer). Each subquadrat was also observed for 30 s, and all ant workers that were found were collected by an aspirator and later identified in the laboratory. We also recorded the abundance of each honeydew producer’s host plant as described previously for the preliminary census of vegetation.
The study periods for the field censuses and the subsequent ant exclusion experiment were chosen because exotic ants are most active from autumn to early spring in Yanbaru (Suwabe et al. 2009).
Ant exclusion experiments
To experimentally examine the effect of ants on the population density of honeydew producers, we focused on three ant species, A. gracilipes, Ph. megacephala, and Technomyrmex brunneus, because their abundance had a positive correlation with the abundance of some honeydew producers (see “Results”). For each ant species, we selected two to four study plots where the focal ant species was most dominant and almost exclusively occupied honeydew producers there. We also focused on two dominant honeydew producers, Melanaphis formosana (aphid) and Heteropsylla cubana (psyllid).
Ant exclusion experiments for M. formosana
We selected a total of 48 ramets of the Japanese silver grass infested by the aphid M. formosana that was exclusively occupied by A. gracilipes from four study plots, a total of 41 ramets of the grass infested by the aphid that was exclusively occupied by Ph. megacephala from two study plots, and a total of 39 ramets of the grass infested by the aphid that was exclusively occupied by Tec. brunneus from four study plots. In each selected ramet, we haphazardly chose two paired shoots infested by the aphid. Before ant exclusion, all but one aggregation of aphids (size of each aggregation was approximately 5–15 individuals) were removed by the soft blush. After removal, we smeared a strong sticky substance (Fuji-Tangle®) on the base of one of the two paired shoots to exclude ants but to allow access to winged predators. We left the other shoot intact. We arbitrarily clipped neighboring shoots of the focal two shoots to prevent ant workers from accessing the treated shoot via untreated shoots. These treatments were carried out from December 1–2, 2009, and we then observed and recorded the survival of aphid aggregations on these shoots every 2–8 days for approximately 1 month.
Ant exclusion experiments for H. cubana
We selected four or five shrubs of the white leadtree (Leucaena leucocephala) infested by the psyllid H. cubana in each of two plots in which A. gracilipes was the most dominant ant. Similarly, we chose two or three L. leucocephala shrubs infested by the psyllid in each of two plots in which Tec. brunneus was the most dominant ant. In each selected shrub, we haphazardly chose 2–14 shoots infested by the psyllid. We assigned half of the selected shoots to the ant exclusion treatment and the rest were controls. The ant exclusion treatment was carried out as described for M. formosana using Fuji-Tangle from December 3–4, 2009, and observations of survivorship of psyllid aggregations were carried out as previously mentioned. We found no H. cubana aggregation occupied by Ph. megacephala and therefore did not use Ph. megacephala for the exclusion experiments.
During observations of honeydew-producer aggregation survival, we noted any activity by natural enemies of the honeydew producers and recorded the species and the situations in which they were found, including predating, staying near the prey’s aggregation, and being attacked by ants.
Data analysis
To screen the ant species that might positively influence the abundance of honeydew producers, field census data were analyzed by the "glm.nb" function in R 2.10.1(R Developmental Core Team 2009) to fit multivariate GLMs with a negative binomial distribution (which is ideal for count data of organisms showing a clumped distribution). Each study plot was regarded as the independent unit, the abundance of each honeydew producer was assigned as the response variable, and the abundances of the host plant and that of the nine ant species that were observed to attend the honeydew producers were assigned as explanatory variables. In these analyses, we defined the abundance of each honeydew producer as the total number of aggregations in two 20 × 0.5 m quadrats in each study plot (approximate size of each aggregation, M. formosana: ca. 5–15 individuals; Dysmicoccus sp. A: ca. 5–20 individuals; S. hakonensis: ca. 5–20 individuals; H. cubana: ca. 15–50 individuals), the abundance of each ant species as the total number of its workers collected in the 20 subquadrats of each study plot, and the abundance of each host plant as the percentage ground cover in the two quadrats (which was transformed to a binarized dummy variables on the basis of whether the score was larger or smaller than the average before analyzing). We assumed that effects of explanatory variables were independent statistically, and therefore their interaction terms were not included in the model.
To ensure robustness, we also ran a generalized liner mixed model (GLMM) with a Poisson distribution in which the study point identification number was assigned as a random factor using the "glmmML" function in R. These two models yielded similar results, but Akaike’s Information Criteria value (Akaike 1974) of the GLMM models was much higher than the values of the GLM models and we have judged the GLM models are more suitable for our analyses. Therefore, only the results of the GLM with a negative binomial distribution are presented below.
To test the effect of attending ants on the persistence of honeydew-producer aggregations, the survival rates of ant-excluded and control aggregations were compared using the log-rank test of survival analyses with the "survdiff" function in R. The effect of different natural enemies of hemipterans on the proportion remaining at the end of the study was tested using Fisher’s exact tests, using only groups that had these enemies.
Results
Species occurrence and frequency
We found only a small number of dominant herbaceous and pioneer plant species along the roadsides (Table 1). Of these pioneer plants, B. pilosa, Erianthus formosanus, and L. leucocephala are considered to be exotic plants by Ikehara (1979). These plants had frequent associations with honeydew producers (Table 2).
The frequencies of occurrence of honeydew producers and ants are shown in Table 3. We recorded 11 species of honeydew producers and 20 species of ants in the study plots. Of the honeydew producers, Melanaphis formosana (Aphididae), Dysmicoccus sp. A (undescribed species of Pseudococcidae, identified by H. Tanaka), H. cubana (Psyllidae), and Sogata hakonensis (Delphacidae) were the major dominant species and accounted for 94.9% of total honeydew-producer aggregations. All study plots had at least one of these four species. Other honeydew producers were observed in a few study plots, but their abundance was small (Table 3) and we excluded them in the subsequent analyses.
Of the ant species collected in the subquadrats, A. gracilipes, Ph. megacephala, and Tec. brunneus were found in large numbers and accounted for 82.1% of the total number of ant workers collected. Among these three, Tec. brunneus appeared in 70.0% of the study plots, whereas Ph. megacephala and A. gracilipes each appeared in ca. 10.0%.
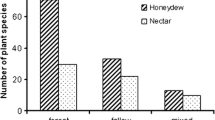
The species composition of ants attending the four most abundant honeydew producers are shown in Fig. 3. We recognized nine species of ants, A. gracilipes, Camponotus bishamon, Monomorium chinense, Pheidole fervens, Ph. megacephala, Pheidole noda, Pristomyrmex punctatus, Tec. brunneus, and Tetramorium bicarinatum that attended honeydew producers. Among them, eight (A. gracilipes, M. chinense, Ph. fervens, Ph. megacephala, Ph. noda, Pr. punctatus, Tec. brunneus, and Tet. bicarinatum) attended to M. formosana, seven (C. bishamon, M. chinense, Ph. fervens, Ph. megacephala, Ph. noda, Pr. punctatus, and Tec. brunneus) to Dysmicoccus sp. A, five (A. gracilipes, M. chinense, Ph. fervens, Ph. megacephala, and Tec. brunneus) to S. hakonensis, and two (A. gracilipes and Tec. brunneus) to H. cubana.
Species composition of ants attending the top four most abundant honeydew-producer aggregations on roadside vegetation in the forests of Yanbaru. Exotic ant species regarded by Yamauchi and Ogata (1995) are indicated by asterisks
Among the nine ants, three exotic ants, A. gracilipes, Ph. megacephala, and Tec. brunneus, attended honeydew producers most frequently. Indeed, together they exclusively tended 89% of the total honeydew-producer aggregations. These three ants were also the three most dominant ant species on the ground surface and also on the vegetation of the study plots. Furthermore, the abundances of each ant species on the ground and on the vegetation were positively and significantly correlated (A. gracilipes: r s = 0.995, p < 0.0001; Ph. megacephala: r s = 0.997, p < 0.0001; Tec. brunneus: r s = 0.922, p < 0.0001). However, the species composition of attending ants differed among species of honeydew producers (Fig. 3). Tec. brunneus attended to Dysmicoccus sp. A and S. hakonensis at high frequencies; however, A. gracilipes and Ph. megacephala attended to these species at low frequencies, whereas all three exotic ant species attended to M. formosana at high frequencies. Furthermore, these three ant species rarely attended H. cubana.
Results of GLM analyses of the abundance of honeydew producers
Multivariate GLMs (Table 4) showed that the abundances of four exotic ants, A. gracilipes, Ph. megacephala, Tec. brunneus, and Tet. bicarinatum, were positively and significantly correlated to the abundance of M. formosana, while a statistically significant negative correlation was detected between the abundance of Ph. noda and M. formosana. There was also a statistically significant positive correlation between the abundance of Tec. brunneus and Dysmicoccus sp. A. Furthermore, the abundance of Ph. fervens had a significant positive correlation with S. hakonensis. In contrast, H. cubana had no significant correlation with any ant species. The abundance of host plants generally did not explain the abundance of honeydew producers (Table 4).
These results are consistent with the results of another GLM model in which the abundance of each ant species on "vegetation" and the abundance of the host plant were used as the explanatory variables (results not shown).
Results of ant exclusion experiments
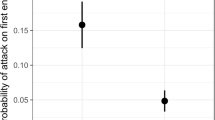
Exclusion of all three ant species led to a statistically significant reduction in the survival rates of M. formosana aggregations compared to the control (log-rank test, p < 0.0001) (Fig. 4). In contrast, the survival rate of H. cubana aggregations did not change by exclusion of either Tec. brunneus or A. gracilipes (Fig. 5). Throughout the experimental period, we never observed a turnover of ant species on a Japanese silver grass shoot (control) infested by M. formosana.
The natural enemies of honeydew producers
Figures 6 and 7 show the percentage of honeydew-producer aggregations in which invasion by natural enemies occurred during the ant exclusion experiments. We observed attacks of hoverfly larvae (n = 4) and parasitoid wasps (n = 37) on M. formosana. Hoverfly (Syrphidae spp.) invasion of M. formosana aggregations was more frequently observed on ant-excluded shoots than control shoots (Fisher’s exact test, p < 0.05 or <0.01). Parasitoid wasps (Chalcidoidea spp.) showed a similar trend, although it was statistically nonsignificant (Fig. 6). Furthermore, the proportion of aggregations invaded by any of the natural enemies was significantly higher in ant-excluded shoots than control shoots, regardless of the ant species excluded (Fig. 6).
Proportion of Melanaphis formosana aggregations invaded by natural enemies in the ant-excluded and ant-attended treatments. Differences in the invasion rate between ant-excluded shoots and ant-attended shoots were statistically significant at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) or nonsignificant (n.s.) (Fisher’s exact test). Sample size of each treatment is the same as Fig. 4
Proportion of Heteropsylla cubana aggregations invaded by natural enemies in the ant-excluded and ant-attended treatments; n.s., nonsignificant difference. Sample size of each treatment is the same as Fig. 5
The only natural enemy that was directly observed to attack the psyllid H. cubana was a hoverfly larva (n = 1), and the invasion ratios of hoverflies to aggregations were not significantly different between treatment and control shoots (Fig. 7).
Discussion
So far, few studies have described the pattern of multispecies mutualism between honeydew producers and ants, including exotic ants, in the field as comprehensively as our study. We revealed that most of the honeydew-producer aggregations occurring in the roadside environment of the Yanbaru forests had trophobiotic relationships with ants, and surprisingly, their honeydew seemed to be almost exclusively used by a few exotic ants (Fig. 3). These exotic ants were shown to contribute to the population increase of the honeydew producer, M. formosana, through their defense against natural enemies (Figs. 4, 6). The local abundance of workers of exotic ants is often positively correlated with the local abundance of honeydew producers, with some species specificity in the associations (Table 4).
Trophobiosis of ants in roadside environments of Yanbaru forests and its possible relationship with biological invasion
Our results showed that three species of exotic ants, A. gracilipes, Ph. megacephala, and Tec. brunneus, were dominant, both in terms of abundance (Table 3) and in attendance of honeydew producers (Fig. 3), and that these three species positively affected survivorship of the honeydew producer, M. formosana (Figs. 4, 6). Positive effects on survivorship of the aphid was clearly demonstrated, whereas no effect was shown on survivorship of H. cubana (Figs. 5, 7), suggesting that H. cubana is not a myrmecophilous honeydew producer.
Our results suggest that in the Yanbaru region, A. gracilipes and Ph. megacephala attend nearly exclusively to M. formosana (Fig. 3). We know that invasions of these two exotic ants are limited to open disturbed habitats and the forest edges in Yanbaru (Suwabe 2009; Yamauchi and Ogata 1995), and we therefore consider that in Yanbaru their impact on the overall community structure of honeydew producers is small and limited. In contrast, Tec. brunneus is more abundant in Yanbaru (Table 3) and seems to be able to use many honeydew-producer species (Fig. 3). It can also invade the forest interior in some situations (Suwabe 2009). Furthermore, some Technomyrmex species, including Tec. brunneus (syn. Tec. albipes; Bolton 2007), have been considered as invasive or problematic exotic ants in some parts of the world (Hansen and Müller 2008; Holway et al. 2002; Samways et al. 1982; Sulaiman 1997). The occurrence of this species, therefore, could cause an outbreak of honeydew producers, not only on roadside vegetation but also in the forest interior. We have occasionally observed a severe occurrence of the coccid-like aphid Metanipponaphis cuspidatae on the chinquapin tree, Castanopsis sieboldii, attended by the ant (H. Tanaka pers. obs.). This tree is an important primary producer and a useful tree for the timber industry in Yanbaru.
Further empirical information is required to determine if Tec. brunneus causes serious outbreaks of honeydew producers in Yanbaru forests. The abundances of two other exotic ants, Ph. fervens and Tet. bicarinatum, also had positive correlations with the abundance of some honeydew producers (Table 4); however, their occurrence in honeydew-producer colonies was relatively infrequent (Fig. 3; Table 3), suggesting that their impact on environments in Yanbaru forests is small. Further studies are needed to confirm this.
In striking contrast to the exotic ants, native ants rarely attended honeydew producers (Fig. 3) and had no positive correlation with the abundance of honeydew producers (Table 4). In fact, the abundance of Ph. noda was significantly negatively correlated with M. formosana (Table 4). These results suggest that trophobiosis between native ants and honeydew producers in Yanbaru is weak and that Ph. noda, the most common native ant in Yanbaru (Suwabe 2009; Suwabe et al. 2009), suppresses the population growth of honeydew producers, possibly through predation.
Two interpretations might be possible for the differences between native and exotic ants with regard to trophobiosis in Yanbaru. First, native ants do not depend on honeydew and thus had only a weak or no mutualistic relationship with honeydew producers. Second, native ants can use honeydew but were excluded by exotic ants particularly in disturbed habitats, such as roadsides, where exotic ants were most populous. The fact that there was no statistically significant negative correlation in the abundance between any pair of a native and an exotic ant (GLM with negative binomial distribution, p > 0.05) might suggest the first hypothesis is more plausible. However, experimental studies in the field are needed to critically test these hypotheses. If the first hypothesis is proved to be true, the question arises as to why native ants did not evolve trophobiosis with honeydew producers in the insect community of Yanbaru.
Biological invasion and road construction
The primary forests of Yanbaru were considered to be nearly free from the threat of exotic ant invasions (Yamauchi and Ogata 1995). However, as suggested by recent studies (Suwabe 2009; Suwabe et al. 2009), we also revealed that exotic ants are common in the roadside environments of Yanbaru forests. Moreover, our study showed that exotic ants are even the dominant occupants of honeydew-producer colonies there.
Exotic ants are known to dominate over indigenous ants in disturbed environments (Bolger 2007; Forys et al. 2002; Le Breton et al. 2003; Stiles and Jones 1998; Suarez et al. 1998). Nakamaru et al. (2007) theoretically demonstrated that nest founding by budding, a characteristic shared by the majority of exotic ants, is advantageous in frequently disturbed habitats. By budding, ants can occupy empty patches created by disturbances more quickly than by independent founding (see also Tsuji 2010; Tsuji and Tsuji 1996). Our results, however, provide an alternative explanation. It is known that the ecological dominance of exotic ants often depends on the presence of honeydew producers, that is, they become strongly invasive when nectar resources are available (Davidson 1998; Lach 2003). In addition, hemipteran insects, the major honeydew producers, tend to have increased numbers in disturbed or fragmented vegetation (Braschler et al. 2003; Fowler et al. 1993). Furthermore, extrafloral nectar-bearing pioneer plants, such as Macaranga tanarius and Mallotus japonicus, that are known to attract ants (Katayama and Tsuji 2010) are often well established in disturbed habitats in Yanbaru (H. Tanaka, pers. obs.). Therefore, the observed dominance of exotic ants in this study might be because roadsides in Yanbaru forests provide a suitable habitat for hemipteran honeydew producers and nectar-producing pioneer plants and thus their symbionts, exotic ants, also prosper. In other words, there may be a positive feedback between the abundance of honeydew producers and that of exotic ants. Experimental removal of honeydew producers would help determine whether the disturbance itself or increased availability of honeydew causes the domination of exotic ants in disturbed habitats.
Why do hemipteran honeydew producers become abundant in disturbed habitats such as roads within Yanbaru forests? Soon after deforestation, the environment is often occupied by the Japanese silver grass, which is a well-known pioneer plant and very rare inside intact semi-primary forests in Yanbaru (H. Tanaka, pers. obs.). This is also the main host plant of dominant honeydew producers, especially M. formosana (Table 3). In our present study, the abundance of Japanese silver grass was not correlated with the abundances of any honeydew-producing insect in the roadsides (Table 4). However, when a certain requirement—it might be the existence of an ant with a high ability of symbiosis with the hemipteran insects—was fulfilled, honeydew producers would occur frequently. Such indirect effect of the plant’s prevalence might partially explain the invasion of exotic ants in Yanbaru.
To prevent the invasion of exotic ants in Yanbaru, we argue that road construction, which is one of the main artificial disturbances, should be kept to a minimum. Otherwise, measures to limit the availability of sugar along roadsides, including controlling Japanese silver grass and extrafloral nectar-bearing pioneer plants, could be needed.
References
Abbott KL, Green PT (2007) Collapse of an ant-scale mutualism in a rainforest on Christmas Island. Oikos 116:1238–1246
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Bach CE (1991) Direct and indirect interactions between ants (Pheidole megacephala), scales (Coccus viridis) and plants (Pluchea indica). Oecologia 87:233–239
Boer P, Vierbergen B (2008) Exotic ants in The Netherlands (Hymenoptera: Formicidae). Entomol Ber 68:121–129
Bolger DT (2007) Spatial and temporal variation in the Argentine ant edge effect: implications for the mechanism of edge limitation. Biol Conserv 136:295–305
Bolton B (2007) Taxonomy of the dolichoderine ant genus Technomyrmex Mayr (Hymenoptera: Formicidae) based on the worker cast. Contrib Am Entomol Inst 35:1–150
Braschler B, Lampel G, Baur B (2003) Experimental small-scale grassland fragmentation alters aphid population dynamics. Oikos 100:581–591
Clark DB (1982) The exotic ant Wasmannia auropunctata: autecology and effect on ant diversity and distribution on Santa Cruz Island, Galapagos. Biotropica 14:196–207
Davidson DW (1998) Resource discovery versus resource domination: a functional mechanism for breaking the trade-off. Ecol Entomol 23:484–490
Dunham AE, Mikheyev AS (2009) Influence of an invasive ant on grazing and detrital communities and nutrient fluxes in a tropical forest. Divers Distrib 16:33–42
Eguchi K (2001) A revision of the Bornean species of the ant genus Pheidole, Insecta: Hymenoptera: Formicidae: Myrmicinae. Tropics Monogr Ser 2:1–154
Forys EA, Allen CR, Wojcik DP (2002) Influence of proximity and amount of human development and roads on the occurrence of the red imported fire ant in the lower Florida Keys. Biol Conserv 108:27–33
Fowler HG, Silva CA, Venticinque E (1993) Size, taxonomic and biomass distributions of flying insects in Central Amazonia: forest edge vs understory. Rev Biol Trop 41:755–760
González-Hernández H, Johnson MW, Reimer NJ (1999) Impact of Pheidole megacephala (F) (Hymenoptera: Formicidae) on the biological control of Dysmicoccus brevipes (Cockerell) (Homoptera: Pseudococcidae). Biol Control 15:145–152
Grover CD, Dayton KC, Menke SB, Holway DA (2008) Effects of aphids on foliar foraging by Argentine ants and the resulting effects on other arthropods. Ecol Entomol 33:101–106
Hansen DM, Müller CB (2008) Invasive ants disrupt Gecko pollination and seed dispersal of the endangered plant Roussea simplex in Mauritius. Biotropica 41:202–208
Helms KR, Vinson SB (2002) Widespread association of the invasive ant Solenopsis invicta with an invasive mealybug. Ecology 83:2425–2438
Hill M, Holm K, Terence V, Shah NJ, Matyot P (2003) Impact of the introduced yellow crazy ant Anoplolepis gracilipes on Bird Island, Seychelles. Biol Conserv 12:1969–1984
Hoffmann BD, Andersen AN, Hill GJE (1999) Impact of an introduced ant on native rain forest invertebrates: Pheidole megacephala in monsoonal Australia. Oecologia 120:595–604
Holway DA (1998) Effect of Argentine ant invasions on ground-dwelling arthropods in northern California riparian woodlands. Oecologia 116:252–258
Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ (2002) The causes and consequences of ant invasions. Annu Rev Ecol Syst 33:181–233
Ikehara N (1979) Okinawa Syokubutsu Yagai Katuyou Zukan (Pictorial book of plants in Okinawa for field utilization), vol 3 (in Japanese). Shinsei Tosyo Syuppan, Naha
IUCN (2000) Conservation of Dugong (Dugong dugon), Okinawa Woodpecker (Sapheopipo noguchii) and Okinawa Rail (Gallirallus okinawae) in and around the Okinawa Island. The World Conservation Congress at its 2nd Session in Amman, Jordan, 4–11 October 2000
Kaplan I, Eubanks MD (2002) Disruption of cotton aphid (Homoptera: Aphididae)—natural enemy dynamics by red imported fire ants (Hymenoptera: Formicidae). Environ Entomol 31:1175–1183
Kaplan I, Eubanks MD (2005) Aphids alter the community-wide impact of fire ants. Ecology 86:1640–1649
Katayama M, Tsuji K (2010) Habitat differences and occurrence of native and exotic ants on Okinawa Island. Entomol Sci 13:425–429
King JR, Tschinkel WR (2007) Range expansion and local population increase of the exotic ant, Pheidole obscurithorax, in the southwestern United States (Hymenoptera: Formicidae). Fla Entomol 90:435–439
Lach L (2003) Invasive ants: unwanted partners in ant–plant interactions? Ann Missouri Bot Card 90:91–108
Le Breton J, Chazeau J, Jourdan H (2003) Immediate impacts of invasion by Wasmannia auropunctata (Hymenoptera: Formicidae) on native litter ant fauna in a New Caledonian rainforest. Austr Ecol 28:204–209
McClelland GTW, Jones IL (2009) The invasive ant fauna (Hymenoptera, Formicidae) of Laysan Island, Hawaiian Islands National Wildlife Refuge. Proc Hawaii Entomol Soc 41:37–46
McGlynn TP (1999) The worldwide transport of ants: geographic distribution and ecological invasions. J Biogeogr 26:535–548
Nakamaru M, Beppu Y, Tsuji K (2007) Does disturbance favor dispersal? An analysis of ant migration using the colony-based lattice model. J Theor Biol 248:288–300
Ness JH, Bronstein JL (2004) The effects of invasive ants on prospective ant mutualists. Biol Invasions 6:445–461
O’Dowd DJ, Green PT, Lake PS (2003) Invasion ‘meltdown’ on an oceanic island. Ecol Lett 6:812–817
Olson DM, Dinerstein E (1998) The global 200: a representation approach to conserving the earth’s most biologically valuable ecoregions. Conserv Biol 12:502–515
Passera L (1994) Characteristics of tramp species. In: Williams DF (ed) Exotic ants: impact and control of introduced species. Westview Press, Boulder, pp 23–43
Pfeiffer M, Tuck HC, Lay TC (2008) Exploring arboreal ant community composition and co-occurrence patterns in plantations of oil palm Elaeis guineensis in Borneo and Peninsular Malaysia. Ecography 31:21–32
Phillips PA, Sherk CJ (1991) To control mealybugs, stop honeydew-seeking ants. Calif Agric 45:26–28
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org. Accessed 20 December 2009
Reimer N, Beardsley JW, Jahn G (1990) Pest ants in the Hawaiian Islands. In: Vander Meer RK, Jaffe K, Cedeno A (eds) Applied myrmecology. A world perspective. West View Press, Boulder, pp 40–50
Samways MJ, Nel M, Prins AJ (1982) Ants (Hymenoptera: Formicidae) foraging in citrus trees and attending honeydew-producing Homoptera. Phytophylactica 14:155–157
Sarty M, Abbott KL, Lester PJ (2007) Community level impacts of an ant invader and food mediated coexistence. Insect Soc 54:166–173
Stiles JH, Jones RH (1998) Distribution of the red imported fire ant, Solenopsis invicta, in road and powerline habitats. Landsc Ecol 335:335–346
Suarez AV, Bolger DT, Case TJ (1998) Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology 19:2041–2056
Sulaiman SFM (1997) Impact of weed management on ant density and fruit yield in the control of pineapple wilt disease. Acta Hortic 425:475–484
Suwabe M (2009) Okinawa-hontou ni Okeru Ari no Gunsyû-Seitaigaku, Jiniteki Kakuran ga Gairai-Ari no Sinryaku ni Ataeru Eikyou (Community ecology of ants in the Okinawa Island. Effects of the anthropogenic disturbances on invansions by non-native tramp ants and on native ant community). PhD thesis, The United Graduate School of Agricultural Sciences, Kagoshima University (In Japanese)
Suwabe M, Ohnishi H, Kikuchi T, Kawara K, Tsuji K (2009) Difference in seasonal activity pattern between non-native and native ants in subtropical forest of Okinawa Island, Japan. Ecol Res 24:637–643
Tsuji K (2010) Unicolonial ants: loss of colony identity. In: Breed M, Moore J (eds) Encyclopedia of animal behavior. Academic Press, Oxford (in press)
Tsuji K, Tsuji N (1996) Evolution of life history strategies in ants: variation in queen number and mode of colony founding. Oikos 76:82–92
Wetterer JK, Espadaler X, Wetterer AL, Aguin-Pombo D, Franquinho-Aguiar AM (2006) Long-term impact of exotic ants on the native ants of Madeira. Ecol Entomol 31:358–368
Yamauchi K, Ogata K (1995) Social structure and reproductive systems of exotic versus endemic ants (Hymenoptera: Formicidae) of the Ryukyu Islands. Pacif Sci 49:55–68
Acknowledgments
We thank Masami Hayashi for identification of Sogata hakonensis and Masakazu Sano for identifications of Aphis nasturtii, Metanipponaphis cuspidatae, and Melanaphis formosana. We also thank Alexander S. Mikheyev for his comments on our earlier manuscript. This study was supported in part by KAENHI (no. 21247006), a Young Investigator Research Grant from the University of the Ryukyus, and Mitsui and Co., Ltd. Environment Fund (no. 08R-B047). The Subtropical Field Science Center (Yona Field) of the University of the Ryukyus and the Yanbaru Wildlife Conservation Field Center of the Ministry of the Environment, Japan, also supported our work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tanaka, H., Ohnishi, H., Tatsuta, H. et al. An analysis of mutualistic interactions between exotic ants and honeydew producers in the Yanbaru district of Okinawa Island, Japan. Ecol Res 26, 931–941 (2011). https://doi.org/10.1007/s11284-011-0851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-011-0851-2