Abstract
Prescribed burning is a common site preparation practice for forest plantation in southern China. However, the effects of prescribed burning on soil microbial communities are poorly understood. This study examined changes in microbial community structure, measured by phospholipid fatty acids (PLFAs), after a single prescribed burning in two paired vegetation sites in southern China. The results showed that the total amount of PLFA (totPLFA) was similar under two vegetation types in the wet season but differed among vegetation type in the dry season, and was affected significantly by burning treatment only in the wet season. Bacterial PLFA (bactPLFA) and fungal PLFA (fungPLFA) in burned plots all decreased compared to the unburned plots in both seasons (P = 0.059). Fungi appeared more sensitive to prescribed burning than bacteria. Both G+ bacterial PLFA and G− bacterial PLFA were decreased by the burning treatment in both dry and wet seasons. Principal component analysis of PLFAs showed that the burning treatment induced a shift in soil microbial community structure. The variation in soil microbial community structure was correlated significantly to soil organic carbon, total nitrogen, available phosphorus and exchangeable potassium. Our results suggest that prescribed burning results in short-term changes in soil microbial communities but the long-term effects of prescribed burning on soil microbial community remain unknown and merit further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prescribed burning is used widely as a forest management strategy to prepare sites for seeding or planting, control tree diseases, improve wildlife habitat, and reduce the thickness of forest floor and thereby reduce the risk of stand-replacing wildfire (Schoch and Binkley 1986; Neary et al. 1999; Carter and Foster 2004). Moderate frequency and intensity fires, such as those prescribed in forest management, can improve above- and below-ground ecosystem structure and function, and help maintain the biodiversity and ecological balance of forest ecosystem, but prescribed burning may have important impacts on belowground ecosystem structure, functions and processes (Neary et al. 1999; Certini 2005). Besides the reduction or elimination of aboveground biomass, soil physical, chemical and biological properties are affected to a greater or lesser extent depending on the severity and duration of fire (Certini 2005). In general, soil structure and texture are destroyed, and soil organic matter, nitrogen and soil microbial activity are decreased (Gundale et al. 2005; Swallow et al. 2009).
Eucalyptus, a fast-growing species, is planted widely for wood production in southern China. However, the planting of Eucalyptus is a controversial issue regarding soil fertility, soil biodiversity and understory plant diversity (Cao et al. 2010). Prescribed burning is a common management practice to prepare sites for Eucalyptus planting in southern China. Prescribed burning has been shown to affect soil microbial activity and function (Pietikäinen and Fritze 1993; Bååth et al. 1995; Staddon et al. 1998; Hamman et al. 2008; Ponder et al. 2009). It is suggested that the short-term effect of prescribed fire on soil microorganisms is a result of direct heating to soil, while long-term effects might be caused indirectly through changes to soil physical and chemical properties and the alteration of post-fire plant community composition (Neary et al. 1999; Hart et al. 2005). Burning effects on soil microbes are greatest in the top soil layers where microbial communities are most abundant (Neary et al. 1999). High temperature through soil heating can kill most microorganisms immediately. For instance, immediately after a fire in a stand of Pinus spp, microbial biomass was decreased drastically in the surface soil (0–5 cm) and reduced by 50% in the subsurface zone (5–10 cm) (Prieto-Fernández et al. 1998). Other literature also reports the direct effects of prescribed burning on soil microbial communities (DeBano et al. 1998; Certini 2005; Ponder et al. 2009), but the indirect effects of prescribed fire on soil microorganisms through changes in vegetation community composition and soil environmental conditions are poorly understood.
In the present study, we examined the effects of prescribed burning on soil microbial community structure in two paired vegetation sites (Shrubland vs Eucalyptus urophylla plantation) in the dry and wet season, southern China. Our objectives were: (1) to evaluate the indirect effects of prescribed burning on soil microbial community through alterations in soil chemical properties; and (2) to determine whether changes in microbial community structure are related to fire-induced alterations in soil chemical properties.
Materials and methods
Site description
The experimental site is located at the Heshan Hilly Land Interdisciplinary Experimental Station (112°54′E, 22°41′N), Chinese Academy of Sciences (CAS) in Guangdong province, China, which is one of the core stations of the Chinese Ecosystem Research Network (CERN). The site was established in March 2005 and occupies an area of 50 ha, and has 14 different forest types (monoculture, mixed, native plantations) or management strategies (burning, clear-cutting and fertilizing) with three replicates each. The average elevation of the area is 80 m. The climate is subtropical monsoon with an annual mean rainfall of 1,700 mm and evaporation of 1,600 mm. This area has distinct seasonal differences, with a wet season from April to September and a dry season from October to March. The average rainfall in wet season and dry season are about 1,400 mm and 300 mm, respectively. The mean annual temperature is 21.7°C, with a mean monthly maximum temperature of 29.2°C in July and a mean monthly minimum temperature of 12.6°C in January. The soil type is acrisol developed from sandstone with a pH of around 4.0.
Field design and soil sampling
Prescribed burning of moderate intensity was conducted to prepare sites for planting in March 2005. The prior vegetation type is shrubland after logging all trees (Pinus elliotti). All the vegetation in the experimental site was slashed. Logging residues were left on the stand in the unburned plots but were burned in the burned plots. Eucalyptus urophylla seedlings were planted in both treatment plots at a spacing of 2 × 3 m, at a density of about 1,600 trees in each hectare, but plant community in shrubland restored naturally. Two paired vegetation sites (shrubland vs Eucalyptus plantation) were selected for this study about 3 years after burning. Thus, our study has the following four treatments: burned shrubland and unburned shrubland, burned Eucalyptus urophylla plantation, unburned Eucalyptus urophylla plantation. Each treatment has three replicate plots with an area of 1 ha for each plot. The plots were arranged in a randomized block design. The average height and diameter at breast height of E urophylla trees were about 11.9 m and 9.1 cm, respectively, during the study period (Chen et al. 2009). The understory vegetation was similar before the Eucalyptus was planted in the plantation sites. The dominant species of understory vegetation were Dicranopteris dichotoma, Rhodomyrtus tomentosa, Baeckea frutescens, Clerodendron fortunatum, Trema tomentosa. However, the composition of understory vegetation in the plantation sites changed 3 years after planting as it became dominated by Dicranopteris dichotoma (>90% coverage) with other species fading out.
Soil samples from a depth of 0–10 cm were collected from burned and unburned plots in shrubland and Eucalyptus urophylla plantation using a soil corer (50 cm in diameter). In each plot, three composite soil samples were collected from three blocks (upper, middle and lower slope) in December 2007 (dry season) and July 2008 (wet season), respectively. Each composite sample was bulked from five random soil cores of the same block. All soils were sieved immediately with a sieve of 2 mm diameter and stored at −20°C until microbial community analysis. Basic soil physical and chemical data of each treatment plots were reported by Sun et al. (2009) and are summarized in Table 1.
Microbial community analysis
Soil microbial community structure was determined by phospholipid fatty acids (PLFAs) analysis. The lipid extraction procedure followed the method described by Bossio and Scow (1998). Briefly, 8 g dry-weight-equivalent of fresh soil subsamples was extracted in 23 ml extraction mixture containing chloroform:methanol:phosphate buffer (1:2:0.8, v/v/v). The extraction was transferred to a separatory funnel after centrifugation and allowed to separate overnight. The chloroform layer was collected and dried under N2 at 32°C. Lipids were redissolved in chloroform and fractionated on a 0.5 g silica gel solid-phase extraction column (Supelco, Bellefonte, PA). Neutral and glycol lipids were eluted by 5 ml chloroform, followed with 10 ml acetone. Polar lipids were eluted by 5 ml methanol, dried under N2 at 32°C and then subjected to a mild-alkali methanolysis to recover the PLFA as methyl esters. Fatty acid methyl esters were dissolved in 200 μl hexane solvent containing nonadecanoic acid methyl ester (19:0) as an internal standard, and were analyzed with an Agilent 6890 Gas Chromatograph (Agilent Technologies, Palo Alto, CA) equipped with a flame ionization detector, using N2 as the carrier gas, H2 and air to support the flame. The GC capillary column was an Ultra 2 (25 m × 0.2 mm id × 0.33 μm film thickness), crosslinked 5% phenyl methyl silicone. A 2 μl injection of the above dilution with a 1:50 split was employed at 250°C for the injector and 300°C for the detector. The oven temperature ramped from 170°C to 300°C at 5°C min−1 and held for 12 min. Peaks were identified using bacterial fatty acid standards and MIDI peak identification software (MIDI, Newark, DE). The fatty acid nomenclature used was described by Zelles (1999). Total microbial biomass was estimated from the sum of all the extracted PLFAs. Bacterial biomass was estimated from the summed concentrations of the following PLFAs: i15:0, a15:0, 15:0, i16:0, 16:1ω9, 16:1ω7, 16:1ω5c, i17:0, a17:0, cy17:0, 16:1 2OH, 18:1ω7, 18:0, cy19:0 (Frostegård and Bååth 1996; Bossio and Scow 1998; Myers et al. 2001; Smithwick et al. 2005; Hamman et al. 2007). Fungal biomass was estimated from the concentrations of the biomarkers 18:2ω6c (Bååth et al. 1995), 18:3ω6c (Hamman et al. 2007), 18:1ω9c (Myers et al. 2001; Smithwick et al. 2005), 20:1ω9c (Swallow et al. 2009). Gram-positive bacteria were represented by the sum of PLFAs: i15:0, a15:0, i16:0, i17:0, a17:0 (Zogg et al. 1997; Klamer and Bååth 1998; Fraterrigo et al. 2006). Gram-negative bacteria included 16:1ω7c, cy17:0, 18:1ω7t, cy19:0 (Myers et al. 2001; Wilkinson et al. 2002). The ratios of fungi/bacteria and G+/G− were calculated from the above PLFAs.
Statistical analysis
Microbial biomass calculated from PLFA and ratios were subjected to a two-way analysis of variance (ANOVA), with treatment, vegetation type and their interaction as factors. Thirty PLFAs indicative of soil microorganisms were detected and identified to perform principal component analysis (PCA) and data (percent) were log transformed. All data were analyzed with SPSS Version16.0 (SPSS, Chicago, IL). Redundancy analysis (RDA) was used to test the relationship between soil microbial community (30 PLFAs) and environmental variables. The RDA was performed using Canoco, version 4.5. Significance was tested using Monte Carlo permutation tests (999 permutations). All statistical significance was accepted at P < 0.05.
Results
Responses of soil microbial community to prescribed burning
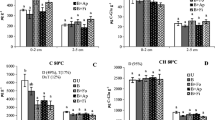
The burning treatment decreased totPLFA in shrubland and Eucalyptus plantation in both seasons (P = 0.053). The vegetation type showed significant effects on totPLFA in the dry season (P < 0.05), but no effects in the wet reason (Fig. 1; Table 3). BactPLFA was significantly higher in Eucalyptus plantation than in shrubland in the dry season regardless of burning (P < 0.05), but no difference was found in the wet season (Fig. 2; Table 3). Both bactPLFA and fungPLFA were decreased by burning regardless of season (Fig. 2). Compared to unburned treatment, bactPLFA and fungPLFA were reduced by 18–36 and 38–55%, respectively (Fig. 2). In all cases, bactPLFA was the main component of totPLFA. G+ bacterial PLFA was significantly lower in shrubland than in Eucalyptus plantation in the dry season regardless of burning (P < 0.05), but no difference was found in wet season. Both G+ PLFA and G− PLFA were decreased by burning regardless of season; but G− PLFA showed a more pronounced response (Fig. 3, Table 3). Prescribed burning significantly decreased the fungal-to-bacterial ratio in the wet season (P < 0.05) but had no significant effect in the dry season. The G+: G− ratio tended to increase after burning in both seasons except for Eucalyptus plantation in the wet season (Table 2).
Analysis of main factors affecting soil microbial community
PCA of the PLFA data from shrubland and Eucalyptus plantation revealed that the shifts in microbial community structure were due either to the burning treatment or to the season of sampling (Fig. 4a). The first two principal components accounted for 59% of the total variation in PLFA patterns. PC1 explained 39% of the variation and discriminated the samples in burned plots from those in unburned plots (along PC1, P < 0.01 from AVOVA). The soils in burned plots were clustered to the left and the soils in unburned plots to the right of the origin (Fig. 4a). The loadings of individual PLFAs showed that the samples in unburned plots were characterized mainly by high concentrations of the iso-branching PLFAs (i16:0, i17:0, i15:0) normally correlated with Gram-positive bacteria, while the samples in burned plots were represented by monounsaturated PLFAs (19:1ω9c, 17:1ω8c, 15:1ω7c) (Fig. 4b). Thus, lower values in burned plots with regard to unburned plots were found for the PLFAs 18:0, i16:0, 16:1 ω5c, i17:0, 18:1ω9c and i15:0, while relatively higher values were found in the burned plots compared to unburned plots for the PLFAs 19:1ω9c, 17:1ω8c and 15:1ω7c (Fig. 4b). PC2, separating wet season from dry season (along PC2, P < 0.01), accounted for only 20% of the variation. The samples from the dry season were located mainly at the lower section of the second axis, while samples from the wet season were mainly on the upper section of the second axis (Fig. 4a). Higher values in the wet season along PC2 were the hydroxyl PLFAs (11:0 3OH, 11:0 2OH, 13:0 3OH). Anteiso branching PLFAs (a14:0, a13:0, a17:0, a15:0) received high negative weights on PC2 (Fig. 4b).
Principal component analysis (PCA) of PLFAs showing score plot and loading plot for the individual PLFA of soil samples in shrubland and Eucalyptus plantation. Red wet season, black dry season, open triangles unburned shrubland, closed triangles burned shrubland, open circles unburned Eucalyptus plantation, filled circles burned Eucalyptus plantation
Correlations between soil microbial community and soil chemical properties
The correlations between soil microbial community and soil chemical properties were analyzed by RDA (Fig. 5). Nine soil variables, including pH, soil organic carbon (SOC), total nitrogen (TN), soil C:N ration (C/N), total phosphorus (TP), available phosphorus (AP), exchangeable potassium (K); magnesium (Mg) and calcium (Ca), explained 39.2% of the variation in soil microbial community structure (eigenvalue = 0.392, F = 1.861, P = 0.002). The variations in PLFA profiles were closely correlated to TN (F = 3.648, P = 0.002), K (F = 3.533, P = 0.003), SOC (F = 2.746, P = 0.019), and AP (F = 2.287, P = 0.038). Most variation was explained by axis 1 (eigenvalue = 0.207, F = 6.791, P = 0.008). Axis 1 was highly correlated with AK and AP. Axis 2 illustrated 67% of the variation in PLFA profiles, and correlated strongly to TN and SOC.
Redundancy analysis (RDA) of PLFA profiles for soil samples used 30 PLFAs as species and nine environmental parameters. Vectors represent environmental variables. SOC Soil organic carbon, TN total nitrogen, C/N C:N ratio, TP total phosphorus, AP available phosphorus. Open triangles Unburned shrubland, closed triangles burned shrubland, open circles unburned Eucalyptus plantation, filled circles burned Eucalyptus plantation
Discussion
Prescribed burning decreased soil microbial biomass and altered microbial community structure in both shrubland and Eucalyptus plantation in the present study (Figs. 1, 2, 3, 4; Table 3), which was consistent with findings from other studies (Bååth et al. 1995; Campbell et al. 2008; Swallow et al. 2009). Bååth et al. (1995) observed that totPLFA was decreased by 35% in a Scots pine stand in Central Finland 2 years after prescribed burning compared to the control. Similarly, Campbell et al. (2008) found that totPLFA was reduced by 50% 2 years after burning compared to the control in a wet sclerophyll forest. Swallow et al. (2009) found that soil microbial biomass in burned sites was reduced significantly compared to unburned sites in a boreal mixedwood forest in northwestern Alberta. However, Ponder et al. (2009) found totPLFA was not affected by burning treatment in an upland Missouri Ozark forest after two annual prescribed burnings. Hamman et al. (2007) observed that soil microbial biomass estimated by total concentration of EL-FAMEs did not change with either low- or high-severity burning, but there was a shift in total community structure in a ponderosa pine/slimstem muhly forest in central Colorado over a period of 3 weeks after burning. It was reported that burning could directly change the soil microbial biomass by transferring heat into soil, and that process was transient (Neary et al. 1999; Certini 2005), but in our study soil microbes were determined 3 years after prescribed burning and the direct heat-induced effect may have gone. It was also suggested that the decrease in microbial biomass by burning may be associated with an increase of pH (Bååth et al. 1995); however, this would not explain our results since pH was decreased by burning in the present study. We considered that the lower quality of soil substrates in our burned plots was the main reason why there was less microbial biomass in burned plots than in unburned plots. PLFA analysis showed that prescribed burning caused a more pronounced reduction in fungal biomass than in bacterial biomass (Fig. 2), indicating a shift in soil microbial community structure. This shift became evident when the PLFAs from different treatments were compared using PCA (Fig. 4). This response may have been associated with soil nutrients and substrate availability.
In our study, bactPLFA were lower in burned plots than in unburned plots. This response may have correlated with poor nutrient availability due to burning. Joergensen and Wichern (2008) reported that bacteria have low substrate use efficiency and occur mainly in nutrient-rich and alkaline/saline soils. In general, soil fungi are higher in forest A horizons and litter layers, and are favored by recalcitrant organic materials and high C/N ratio in soil (Blagodatskaya and Anderson 1998; Högberg et al. 2007). Prescribed burning reduced the thickness of the forest floor and caused a low C/N ratio. This may partly explain the lower fungPLFA in burned plots. In addition, ectomycorrhizal fungi, an important component of the fungal community, can be destroyed easily by burning with a subsequent slow recolonization rate (Bååth et al. 1995). The burning treatment caused a more pronounced reduction in fungal biomass than in bacterial biomass (Fig. 2). This showed that soil fungi were more sensitive to burning than bacteria (Campbell et al. 2008). The ratio of fungPLFA to bactPLFA was commonly used as an index to show the relative content of fungi and bacteria in the microbial community (Cao et al. 2010). In the present study, the ratios of fungPLFA to bactPLFA were low in the burned plots, further indicating that the burning treatment affected soil fungi more than bacteria. This was consistent with the findings of other studies (Bååth et al. 1995; Díaz-Raviña et al. 2006; Campbell et al. 2008).
Prescribed burning caused a reduction in both G+ and G− bacterial biomass when compared to unburned soils in the present study. In general, the biomass of G+ bacteria was higher than that of G− bacteria in both shrubland and Eucalyptus plantation whether they were burned or not (Fig. 3). Some other studies also found greater abundance of G+ bacterial biomass in burned soils, and considered high G+ bacterial biomass to be associated with the high availability of substrate from dead microorganisms or organic matter solubilized by burning (Yeager et al. 2005; Díaz-Raviña et al. 2006). In contrast, the reasons for the reduction in our study might be reduced soil organic matter and soil nutrients as discussed below.
PCA analysis showed that the season of sampling also affects the observed soil microbial community structure, as indicated by PLFA pattern (Fig. 4). BactPLFA, fungPLFA, G+ and G− bacterial PLFA were higher in the wet season than in the dry season for both vegetation types (Figs. 2, 3). In southern China, the wet period (from April to September) is characterized by rain and high temperature. The litter layer was directly exposed to these environmental conditions due to understory reduction by burning. More nutrients returned to mineral soils through the rapid decomposition of litter, which can affect the microbial community. By altering rates of transpiration and evaporation and eliminating interception of precipitation due to burning, soil water regimes may directly change the composition and the activity of soil microbes (Boyle et al. 2005).
Jiménez Esquilín et al. (2007) reported that soil microbial communities were similar between edge and center pile soils 15 months after burning, but were different from those in unburned soils. They considered that the changes in microbial community structure were probably the post-fire effects of vegetative dynamics, altered C and nutrient availability or other soil physico-chemical properties. Changes in vegetative community structure after fire have the potential to become the dominate driver of soil microflora (Hart et al. 2005). The decrease in organic matter input by changing plant productivity and vegetative composition after prescribed burning can alter microbial community structure (Kaye and Hart 1998; Boyle et al. 2005). Fernández et al. (1997) found the carbon content in burned soils to be significantly lower than in unburned soils. The remaining organic matter in the burned soils contained more humin and consequently provided a poor substrate for microbial growth. Campbell et al. (2008) also observed that burning treatment caused a significant reduction in soil carbon sources and therefore altered microbial community structure. In our previous study, we found that prescribed burning resulted in changes in soil nutrient status (Sun et al. 2009); in the present study, we found that prescribed burning altered the soil microbial community structure as indicated by PLFA pattern (Fig. 4). We believe the changes in soil microbial biomass and community structure may be attributed partly to the decrease in nutrient availability. RDA indicated that soil chemical properties could explain the variation in PLFA pattern, supporting the view that the decreases in soil organic matter and soil nutrients are probably the reasons for the observed reductions in soil fungPLFA and bactPLFA.
References
Bååth E, Frostegård Å, Pennanen T, Hannu F (1995) Microbial community structure and pH response in relation to soil organic matter quality in wood-ash fertilized, clear-cut or burned coniferous forest soils. Soil Biol Biochem 27:229–240
Blagodatskaya EV, Anderson TH (1998) Interactive effects of pH and substrate quality on the fungal-to-bacterial ration and qCO2 of microbial communities in forest soils. Soil Biol Biochem 30:1269–1274
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Boyle SI, Hart SC, Kaye JP, Waldrop MP (2005) Restoration and canopy type influence soil microflora in a ponderosa forest. Soil Sci Soc Am J 69:1627–1638
Campbell CD, Cameron CM, Bastias BA, Chen CG, Cairney JWG (2008) Long term repeated burning in a wet sclerophyll forest reduces fungal and bacterial biomass and responses to carbon substrates. Soil Biol Biochem 40:2246–2252
Cao YS, Fu SL, Zou XM, Cao HL, Shao YH, Zou LX (2010) Soil microbial community composition under Eucalyptus plantation of different age in subtropical China. Eur J Soil Biol 46:128–135
Carter MC, Foster CD (2004) Prescribed burning and productivity in southern pine forests: a review. For Ecol Manag 191:93–109
Certini G (2005) Effects of fire on properties of forest soils: a review. Oecologia 143:1–10
Chen DM, Zhang Y, Lin YB, Chen H, Fu SL (2009) Stand level estimation of root respiration for two subtropical plantations based on in situ measurement of specific root respiration. For Ecol Manag 257:2088–2097
DeBano LF, Neary DG, Ffolliott FF (1998) Fire effects on ecosystems. Wiley, New York
Díaz-Raviña M, Bååth E, Martín A, Carballas T (2006) Microbial community structure in forest soils treated with a fire retardant. Biol Fertil Soils 42:465–471
Fernández I, Cabaneiro A, Carballas T (1997) Organic matter changes immediately after a wildfire in an Atlantic forest soil and comparison with laboratory soil heating. Soil Biol Biochem 29:1–11
Fraterrigo JM, Balser TC, Turner MG (2006) Microbial community variation and relationship with nitrogen mineralization in historically altered forests. Ecology 87:570–579
Frostegård Å, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65
Gundale MJ, Deluca TH, Fiedler CE, Ramsey PW, Harrington MG, Gannon JE (2005) Restoration treatments in a Montana ponderosa pine forest: effects on soil physical, chemical and biological properties. For Ecol Manag 213:25–38
Hamman ST, Burke IC, Stromberger ME (2007) Relationships between microbial community structure and soil environmental conditions in a recently burned system. Soil Biol Biochem 39:1703–1711
Hamman ST, Burke IC, Knapp EE (2008) Soil nutrients and microbial activity after early and late season prescribed burns in a Sierra Nevada mixed conifer forest. For Ecol Manag 256:367–374
Hart SC, DeLuca TH, Newman GS, MacKenzie MD, Boyle SI (2005) Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For Ecol Manag 220:166–184
Högberg MN, Högberg P, Myrold DD (2007) Is microbial composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150:590–601
Jiménez esquilín AE, Stromberger ME, Massman WJ, Frank JM, Shepperd WD (2007) Microbial community structure and activity in a Colorado Rocky Mountain forest soil scarred by slash pile burning. Soil Biol Biochem 39:1111–1120
Joergensen RG, Wichern F (2008) Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol Biochem 40:2977–2991
Kaye JP, Hart SC (1998) Ecological restoration alters N transformations in a ponderosa pine-bunchgrass ecosystem. Ecol Appl 8:1052–1060
Klamer M, Bååth E (1998) Microbial community dynamics during composting of straw material studied using phospholipid fatty acid analysis. FEMS Microbiol Ecol 27:9–20
Myers RT, Zak DR, White DC, Peacock A (2001) Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci Soc Am J 65:359–367
Neary DG, Klopatek CC, DeBano LF, Ffolliott PF (1999) Fire effects on belowground sustainability: a review and synthesis. For Ecol Manag 122:51–71
Pietikäinen J, Fritze H (1993) Microbial biomass activity in the humus layer following burning: short-term effects of two different fires. Can J For Res 23:1275–1285
Ponder F Jr, Tadros M, Loewenstein EF (2009) Microbial properties and litter and soil nutrients after two prescribed fires in developing savannas in an upland Missouri Ozark Forest. For Ecol Manag 257:755–763
Prieto-Fernández A, Acea MJ, Carballas T (1998) Soil microbial and extractable C and N after wildfire. Biol Fertil Soils 27:132–142
Schoch P, Binkley D (1986) Prescribed burning increased nitrogen availability in a mature loblolly pine stand. For Ecol Manag 14:13–22
Smithwick EAH, Turner MG, Metzger KL, Balser TC (2005) Variation in NH4 + mineralization and microbial communities with stand age in lodgepole pine (Pinus contorta) forests, Yellowstone National Park (USA). Soil Biol Biochem 37:1546–1559
Staddon WJ, Duchesne LC, Trevors JT (1998) Impact of clear-cutting and prescribed burning on microbial diversity and community structure in a Jack pine (Pinus banksiana Lamb) clear-cut using Biolog gram-negative microplates. World J Microbiol Biotechnol 14:119–123
Sun YX, Wu JP, Zhou LX, Lin YB, Fu SL (2009) Changes of soil nutrient after prescribed burning of forestland in Heshan City, Guangdong Province (in Chinese). Chin J Appl Ecol 20:513–517
Swallow M, Quideau SA, MacKenzie MD, Kishchuk BE (2009) Microbial community structure and function: the effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol Biochem 41:770–777
Wilkinson SC, Anderson JM, Scardelis SP, Tisiafouli M, Taylor A, Wolters V (2002) PLFA profiles of microbial communities in decomposing conifer litters subject to moisture stress. Soil Biol Biochem 34:189–200
Yeager CM, Northuop DE, Grow CC, Barns SM, Kuske CR (2005) Changes in nitrogen-fixing and ammonia-oxidizing bacterial communities in soil of a mixed conifer forest after wildfire. Appl Environ Microbiol 71:2713–2722
Zelles L (1999) Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol Fertil Soils 29:111–129
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61:475–481
Acknowledgments
This study was supported financially by the National Natural Science Foundation for Distinguished Young Scholars (No. 30925010), the National Basic Research Program of China (No. 2009CB421101) and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-EW-Z-6), Special thanks to CAS Heshan Hilly Land Interdisciplinary Experimental Station, and the National Field Research Station of Forest Ecosystems, China.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sun, Y., Wu, J., Shao, Y. et al. Responses of soil microbial communities to prescribed burning in two paired vegetation sites in southern China. Ecol Res 26, 669–677 (2011). https://doi.org/10.1007/s11284-011-0827-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-011-0827-2