Abstract
Myrmecochory (seed dispersal by ants) is an evolutionarily and ecologically common mutualism. Since the first study of the phenomenon, ecologists have sought to develop techniques to track ant-dispersed seeds. Often, thick leaf litter and the potential burial of seeds by ants make tracking of seeds difficult. Here we describe a seed-tracking technique for small seeds, which uses magnetic tags, developed for mark–recapture studies of fish, to track seeds after dispersal into the ant nest in temperate deciduous forests. We discuss our use of the technique as well as suggestions for improvement and other possible applications of the technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seeds dispersal by ants, myrmecochory, is common in many lineages of plants across diverse geographic regions (Lengyel et al. 2009). In temperate deciduous forests in eastern North America, ants disperse 20–70% of the total herbaceous flora (Handel et al. 1981; Zelikova et al. 2008). Remarkably, dispersal of most of these species appears to be by a single species complex of ants, Aphaenogaster rudis (Ness et al. 2009). Though A. rudis is the keystone disperser, there are still aspects of its relationship with myrmecochores that remain unexplored. We first began our study of myrmecochory as we sought to observe the fate of seeds after initial dispersal to the nest by A. rudis. Our task presented us with several difficulties. We could observe the initial dispersal of seeds into the nest, but it was difficult to find the seeds within the nest as it appeared that ants redispersed the seeds into the surrounding litter. The small size of the seeds (length 3–5 mm, weight 4–5 mg) of our plant species of interest, Asarum canadense combined with the thick leaf litter of the mixed hardwood forests, made it nearly impossible to find seeds in the leaf litter without aid. Other methods used to track ant-dispersed seeds were ineffective because of the potential burial of seeds underground and placement among the leaf litter (e.g., fluorescent dye and UV light; Bullock et al. 2006) or because they were expensive and highly regulated (e.g., radiolabels; Kalisz et al. 1999). Therefore, we adapted a technique originally developed for mark–recapture studies of salmonid fishes in order to track the fate of ant-dispersed seeds after dispersal to the nest. Here we report on the details of this method, offer suggestions for improvement, and briefly mention other contexts in which the new technique may prove useful.
Materials and methods
Study site and species
We conducted our study in Lake Raleigh Woods, a mixed-pine hardwood forest located on Centennial Campus of North Carolina State University, Raleigh, NC. We tracked the dispersal of a myrmecochore in the family Aristolochiaceae (Birthwort), Asarum canadense L. The seeds are small (3–5 mm) and ovate with a fleshy appendage, called an elaiosome, attached to the length of the seed. We fed all seeds to colonies of Aphaenogaster rudis, the keystone seed disperser in eastern temperate forests (Ness et al. 2009).
Seed preparation
We used a small (0.25-mm diameter; 1.1-mm length; 0.4-mg weight) magnetized stainless-steel Coded Wire Tag (CWT) (Northwest Marine Technologies, Inc., Shaw Island, WA), to mark each seed (Fig. 1a). We loaded the CWTs into the Single Shot Tag Injector (NMT, Inc.), which is a syringe with a small hypodermic needle (Fig. 1b). We held the seeds with a pair of small forceps so the elaiosome faced down in order to prepare for the injection. We inserted the injector tip into the broader side of the top of the seed, with care not to angle the injector too far up or down to prevent the tag from exiting the seed upon injection. After injecting the CWT into the seed, we marked the tops of the seeds with yellow enamel paint to increase their visibility in the leaf litter after the initial detection by the Handheld Wand Detector (HWD; NMT, Inc.; Fig. 1c).
Pilot study
We conducted a pilot study in the spring of 2008 to test the limitations of the technique. We found that the HWD could locate seeds buried up to 4 cm underground. We were also concerned that the addition of a tag to the seed might change the behavior of A. rudis towards the seeds. In preference studies, we provided both tagged and untagged seeds simultaneously and recorded the removal rate and behavior of A. rudis towards each seed. A. rudis showed no preference for tagged versus untagged seeds and removed both within equal time and in equal quantities. In addition, we conducted a study in the summer of 2007 that allowed us to observe the redispersal of untagged seeds within a barrier surrounding A. rudis nests. The distance and redispersal rates for tagged seeds are comparable to the distance and redispersal rates of untagged seeds, given the restrictions of the barrier. Therefore, we are confident that the movement and treatment of seeds by ants is the same independent of the presence of the tag.
Field study
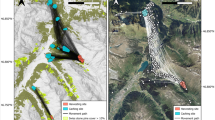
We fed 20 A. rudis colonies up to 50 seeds between late May and early July 2008, between the hours of 09:00 and 14:00. Each nest was at least 15 m from any other individual nest. We observed the removal of the seeds to ensure that only a selected A. rudis colony dispersed all seeds into the nest in each trial. We assumed redispersal distances would be less than 150 cm, a distance well above the reported seed dispersal distances for A. rudis (Gómez and Espadaler 1998; Zelikova et al. 2008). After 7 days, we used the HWD to scan systematically for tagged seeds within a 150-cm radius surrounding the nest. We began at 150 cm from the nest, scanned the first 10 cm of the outer perimeter, and marked detections with a flag. We continued in a like manner moving toward the nest in the center of the search area. We then excavated the leaf litter in a 2.5-cm radius around each detection, placed the litter into a plastic bin, and searched the litter until we located the seed or, in some cases, loose tag. We then marked positive detections with a new flag and measured the distance from the nest entrance to the seed location (Fig. 2). We also excavated the nest in 1–2 cm layers until no ants or nest cavities remained in the nest site and no positive seed detections occurred. We scanned sites twice to ensure that we recovered as many seeds as possible. On average, a complete scan and excavation of a nest site (~7 m2) required 5 h of search effort by two people, though the search effort varied with the condition of the site and the presence of magnetic materials embedded in the soil that led to false detections. We conducted all statistical analysis of the data with R Statistical Software (R Development Core Team 2009).
a Locations of individual seeds (dark circles) found in relation to the nest (triangle) we fed the seeds, and b the excavated nest site (dark flags denote positive seed detections, and the light flag is the nest location). We measured the radial distance from the nest to the seed for all nests. For this nest, the mean redispersal distance was 44.67 cm, with a standard deviation of 19.05 cm
Results
We recovered 63.3% (539 tagged seeds recovered of 864 total tagged seeds) of the seeds that were fed to A. rudis colonies, and the recovery rates ranged from 26.5 to 98% (mean = 61.3%, SE = 4.5%) for the 20 individual nest sites. The low recovery rates tended to occur at nest locations that contained metallic material in the soils, which lead to false detections. We calculated a nonparametric test for correlation (Spearman rank correlation) and found that there is no significant relationship between recovery rate and mean redispersal distance (S = 1,611.21, p = 0.37). Therefore, the recovery rate did not affect the mean redispersal distance measured for each nest.
We found 37 of the 539 tagged seeds buried within the nests, none deeper than 2 cm in the ground. In addition, we found 48 tags unattached to seeds. Though we took precautions, we may have injected some tags too far into the seed and that exposed the tag after the ants removed the elaiosome. Of the 48 tags recovered, we found 41 outside of the nest. Therefore, it is possible that the tags fell out of the seed during redispersal, which may cause a slight underestimate of those individual redispersal distances.
Discussion
One of the chief difficulties in ecology is visualizing ecological processes and seed dispersal is often a difficult process to measure. The use of CWTs was an effective method of seed detection and retrieval in our study of short-distance dispersal of A. canadense by A. rudis in mixed hardwood forests with dense leaf litter. There are two important factors to consider prior to the use of our tracking technique. First, one must consider the depth at which the dispersal vector deposits the seeds. If the depth is greater than 4 cm, then decreased recovery is possible. Second, one must consider the size of the search area. We scanned an area of ~7 m2, which required an average of 5 h of search effort between two people. For larger search areas, the technique may become impractical due to the small size of the wand detector. However, with sites slightly larger than or smaller than our established search area, the tracking technique may be a useful tool for seed recovery.
Comparison to other seed-tracking techniques
The use of a tracking technique always depends on the question of interest (see Bullock et al. 2006) and the size and dispersal vector of the seed (Forget and Wenny 2005). Though internal labeling methods similar to our method exist (reviewed in Forget and Wenny 2005), they are not suitable for small seeds with a small dispersal vector. Therefore, the only other seed-tracking technique comparable to the method we describe here is the use of radiolabels to mark and track seeds within the leaf litter (Kalisz et al. 1999). The radiolabel method offers the capability of searching large areas, but requires the use of expensive (~$2 per radiolabeled seed versus $0.36 per seed for CWTs (http://www.nmt-inc.com); price quote for radiolabel from PerkinElmer 3/24/2010) and heavily regulated equipment (Bullock et al. 2006). In contrast, our technique is not regulated and is less costly, especially since equipment is often available to rent or borrow and recovered tags are reusable. Both methods may have false detections associated with search effort either because of the presence of metallic materials during the use of our method or because of traces of the radioisotope coming off the radiolabeled seeds. In addition, the recovery rates for both techniques are similar, 63% for our study (within 1 year) and 63 and 73% for radiolabels (over 2 years; Kalisz et al. 1999). Therefore, for studies of short-distance dispersal, we contend that the method we developed is a less expensive and a less regulated method than radiolabeling methods and a useful alternative to other tracking techniques.
Improvement of the technique
We offer several suggestions for improvement of the technique for future studies. First, we experienced a high volume of false detections if there were high concentrations of metallic materials in the soil, a common problem with magnetic tagging techniques for large seeds (Alverson and Díaz 1989). The frequency of false detections increased the search effort necessary to fully scan and excavate a site (upwards of 8–10 h total for two people). More importantly, the presence of the metallic materials may decrease the recovery rate of tagged seeds, as indicated by the lower recovery rates at those sites. We recommend an initial scan of the potential search area in order to minimize the potential for false detections, to minimize search effort, and to increase the recovery rate of tagged seeds.
Our second suggestion is to employ multiple wand detectors per site. The increase in searchers and detectors may allow for a larger search area and decrease search effort. Third, we did encounter some tag loss (8.9%). We could reduce this number with extra care in proper tag injection. We often observed exposed tags at the former location of the removed elaiosome. Therefore, it may be useful to seal the injection site, or even coat the tag prior to injection with a clear, odorless form of glue to minimize the possibility of tag loss.
Further applications
We suggest that the best use of the technique is for studies of short-distance dispersal, because of the restrictions on the search range possible with the required equipment. We do offer the following suggestions for other possible applications. The use of anticides on baits as a method of eradication requires the poison reaches the brood and queen. If researchers tagged baits, they could observe the location of the majority of the tags upon excavation of the nest after ant consumption of the bait to be sure the bate choice reached the necessary location. Tagged baits may also be useful to track which ant species exploit different types of bait. A scan of the area around the baits would detect which species took the baits by the presence of the tags in the nest. In addition, each tag has an individual code on it. The individual code on each tag would also allow researchers to determine which type of bait different ant species exploited or the original location of the bait. Other uses for the technique are up to the imagination of the user.
References
Alverson WS, Díaz AG (1989) Measurement of the dispersal of large seeds and fruits with a magnetic locator. Biotropica 21(1):61–63
Bullock JM, Shea K, Skarpaas O (2006) Measuring plant dispersal: an introduction to field methods and experimental design. Plant Ecol 186(2):217–234. doi:10.1007/s11258-006-9124-5
Forget PM, Wenny D (2005) How to elucidate seed fate? A review of methods used to study seed removal and secondary seed dispersal. In: Forget PM, Lambert JE, Hulme PE, Vander Wall SB (eds) Seed fate: predation, dispersal and seedling establishment. CABI Publishing, Wallingford, pp 379–394
Gómez C, Espadaler X (1998) Myrmecochorous dispersal distances: a world survey. J Biogeogr 25(3):573–580. doi:0.1046/j.1365-2699.1998.2530573.x
Handel SN, Fisch SB, Schatz GE (1981) Ants disperse a majority of herbs in a mesic forest community in New York State. Bull Torrey Bot Club 108(4):430–437
Kalisz S, Hanzawa FM, Tonsor SJ, Thiede DA, Voigt S (1999) Ant-mediated seed dispersal alters pattern of relatedness in a population of Trillium grandiflorum. Ecology 80(8):2620–2634. doi:10.1890/0012-9658(1999)080[2620:AMSDAP]2.0.CO;2
Lengyel S, Gove AD, Latimer AM, Majer JD, Dunn RR (2009) Ants sow the seeds of global diversification in flowering plants. PLoS One 4(5):e5480. doi:10.1371/journal.pone.0005480
Ness JH, Morin DF, Giladi I (2009) Uncommon specialization in a mutualism between a temperate herbaceous plant guild and an ant: are Aphaenogaster ants keystone mutualists? Oikos 118(12):1793–1804. doi:10.1111/j.1600-0706.2009.17430.x
Zelikova TJ, Dunn RR, Sanders NJ (2008) Variation in seed dispersal along an elevational gradient in Great Smoky Mountains national park. Acta Oecol 34(2):155–162. doi:10.1016/j.actao.2008.05.002
Acknowledgments
We thank J. Rice for the suggestion to track seeds with coded wire tags and the use of his equipment. We thank K. Gross, R. R. Dunn and two anonymous reviewers for their reviews of the manuscript. Funding for this research was provided by NSF grant EF-0434298 and DEB-0842101 to K. Gross and a DOE NICCR grant to R. R. Dunn and DOE PER grant, DE-FG02-08ER64510 and NC State University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Canner, J.E., Spence, M. A new technique using metal tags to track small seeds over short distances. Ecol Res 26, 233–236 (2011). https://doi.org/10.1007/s11284-010-0761-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-010-0761-8