Abstract
We investigated predictions concerning the competitive relationships between tigers Panthera tigris and leopards Panthera pardus in Bardia National Park, Nepal, based on spatial distributions of scats and territorial markings (sign), analyses of scat content and census of wild ungulate prey. Medium-sized ungulates, in particular chital Axis axis, was the main food of both predators, but leopards consumed significantly larger proportions of domestic animals, small mammals, and birds than tigers. Tiger sign were never found outside the park, while leopard sign occurred both inside and outside, and were significantly closer to the park border than tiger sign. Significantly higher prey densities at locations of tiger sign than that of leopards were mainly due to a preference of the latter species for the park border areas. Our results imply that interference competition––and not competition for food––was a limiting factor for the leopard population, whose distribution was restricted to the margins of the tiger territories. We suggest that the composition of the prey base is a key factor in understanding the different results and interpretations reported in studies on tiger/leopard coexistence. There are two potential mechanisms that link interference competition and prey: (1) low abundance of large ungulate prey decreases foraging efficiency of tigers, leading to increased energetic stress and aggression towards leopards; and (2) increased diet overlap due to scarcity of large prey leads to increased encounter rates and increased levels of interference competition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost all prey are consumed by multiple predator species, and, accordingly, most predators are prone to interact with other species of the same guild. The combined effect of multiple predators on shared prey is often additive, and may produce a top-down mode of population regulation of prey (Sinclair et al. 2003). As a result, coexisting carnivores may adversely influence each other due to their limiting effect on shared resources. This form of interaction is termed exploitative competition (Begon et al. 1990).
During the last few decades, much attention has been aimed at competitive relationships that may occur when the shared resources are not necessarily limited in terms of abundance. Instead, one species imposes a cost on an inferior competitor by limiting its foraging ability through a process termed interference competition (Goss-Custard 1980; Creel et al. 2001). Interference competition involves kleptoparasitism, intraguild predation, and avoidance behavior (Creel et al. 2001). Because intraguild predation is a major factor promoting avoidance and, due to its severe consequences, it has been considered as interference competition in its most extreme form (Polis et al. 1989).
Tigers and leopards are sympatric in several parts of Asia, and reports concerning the nature of their coexistence are conflicting. Karanth and Sunquist (1995, 2000) studied prey selection and interactions between these solitary felids in Nagarahole, India, where the densities of both species are high (Karanth 1993, 1995), and their prey base is rich and diverse in terms of species and sizes (Karanth and Sunquist 1992). The authors observed similar patterns between the species in space use and activity patterns (Karanth and Sunquist 2000), but differences in prey selection with respect to prey size (Karanth and Sunquist 1995). The authors thus concluded that behavioral factors play a minor role in shaping predator communities in tropical forests, and that availability of an appropriate range of prey sizes and high tree cover and density were key factors for their coexistence. This conclusion is in accordance with classical competition theory, which states that animals that are subject to exploitative competition can only coexist if each species has access to some distinct resource that they are better able to utilize than the other species (MacArthur and Levins 1967; Tilman 1982). If both competitors depend on a single resource, then the species that is able to subsist on the lowest resource density will suppress the other. Among predators, the upper limit to prey size is typically determined by the ability of the predator to kill large prey and avoid being injured in the process, whereas the other end of the prey size continuum is more diffuse, consisting of less profitable and opportunistically killed prey (Gittleman 1985; Radloff and du Toit 2004). Hence, competition between tigers and leopards may be minimized when the former have access to larger prey items that the latter are unable to kill. Furthermore, the size difference between tigers and leopards entails a different energetic gain from killing smaller prey. Due to their smaller size, leopards should be able to escape exploitative competition by focusing their foraging efforts on prey items that are too small to be profitable for tigers.
Theory and empirical data suggest that behavioral factors may play a role in tiger–leopard coexistence in certain circumstances. Indications of tiger avoidance were observed among leopards in Chitwan National Park, Nepal, by Seidensticker (1976) and McDougal (1988). Furthermore, the size difference between tigers and leopards, their similar feeding habits, and their close taxonomic relatedness are strong indicators of a high risk of intraguild predation (Polis et al. 1989; Palomares and Caro 1999; Donadio and Buskirk 2006). Empirical studies have demonstrated that an inferior competitor may avoid interference competition by inhabiting “competition refuges”, areas where encounters with the superior opponent are less frequent (Durant 1998; Woodroffe and Ginsberg 2005). Two main types of refuges are reported in the literature; some species avoid conflicts by inhabiting the margins of their competitors’ home ranges, whereas others seek out areas within the ranges of their opponents that are low in resource density, thereby reducing the probability of interspecific encounters (Woodroffe and Ginsberg 2005). According to Creel et al. (2001), a sound approach of detecting avoidance due to interference competition among carnivores requires objective methods of mapping habitat quality for the competitors and of recording their spatial distributions. In our study, we used non-invasive methods for recording these two aspects of tiger and leopard ecology. Based on analyzes of scat contents, ungulate prey census data and distributions of scats and territorial markings, we investigated predictions concerning the competition and coexistence in Bardia National Park, Nepal.
The tiger density in our study area in Bardia was among the highest ever recorded (Wegge et al. 2004, 2009: 13.3–19.3 tigers per 100 km2), whereas the leopard density was much lower (Heinen and Kattel 1992; Støen and Wegge 1996; Wegge et al. 2009: five leopards per km2). Tiger and leopard competition would probably not be exploitative, firstly, because the study area sustained very high densities of medium-sized ungulates (Wegge et al. 2009; Støen and Wegge 1996), which are suitable prey for leopards (Odden and Wegge 2005, 2009; Hayward et al. 2006; Wegge et al. 2009). Secondly, food consumption of radio-collared leopards in our study area was high (ca. 4 kg per day, Odden and Wegge 2009). Hence, if competition occurs, it is more likely to be due to direct behavioral interactions between the two species, and not due to limited food resources. According to the theory and empirical evidence outlined above, we predict that tigers and leopards in Bardia exhibit spatial segregation. Being the behaviorally dominant species, the spatial distribution of tiger sign, i.e., scats and territorial markings, should correspond positively with the density distribution of their main prey, whereas leopard sign should be most common in areas that are less frequently used by tigers, i.e., areas with a lower direct correspondence to the distribution of their prey.
Methods
Study area
Our research activities were conducted within a 105-km2 area in the southwestern part of the Bardia National Park (986 km2). The study area is delineated by the Geruwa River in the west, human settlements and agricultural fields in the south and east, and the Amreni–Chisapani highway in the north. The Geruwa River consists of numerous larger and smaller water channels interspersed with islets with tall grass (Saccharum spp.) and patches of early forest successional stages, dominated by sissoo (Dalbergia sissoo) and khair (Acacia catechu). Bordering the river are broken patches of riverine forest (Trewia nudiflora, Ficus spp., Mallotus phillippinensis, Syzigium cumini), and wooded grasslands consisting of a mixture of hardwood trees (including Adina cordifolia, Schleichera trijuga, Lagerstroemia spp.) and species of short grasses (including Vetiveria zizanoides, Imperata cylindrica). Sal Shorea robusta forest grows on slightly elevated and drier soils away from the main river course.
Ungulate densities
The methods of estimating ungulate densities and distributions were explained in detail in Wegge et al. (2009), and will be briefly described here. Ungulates were counted by flushing animals out from patches of vegetation surrounded by either roads or dry riverbeds. During 1997–1999, 169 counts were conducted in 46 different habitat blocks with an average size of 33.7 ha. To ensure that we counted all animals within the patch, we employed a large number of beaters (≈65 people/block), walking in a line 10–20 m apart. We modeled the influence of habitat on the densities of each ungulate species by using generalized linear models (GLM). The most parsimonious models describing ungulate densities were selected by comparing the Akaike information criterion corrected for small samples (AICc, Burnham and Anderson 2002).
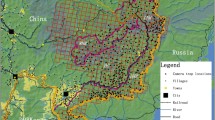
Chital was the most abundant ungulate [Weight (W) ~ 50–60 kg, Density (D) = 84.7 ± 7.9 (SE) animals per km2], followed by hog deer Axis porcinus (W ~ 30–40 kg, D = 13.2 ± 1.5), muntjac Muntiacus muntjak (W ~ 15–20 kg, D = 2.3 ± 0.4) and wild boar Sus scrofa (W ~ 35–40 kg, D = 2.2 ± 0.4, Wegge et al. 2009). Larger ungulates occurred in very low densities. These were barasingha Cervus duvauceli (W > 150 kg, D = 0.4 ± 0.1), nilgai Boselaphus tragocamelus (W > 150 kg, D = 0.3 ± 0.1) and sambar Cervus unicolor (Wegge et al. 2009). The latter species was encountered sporadically, but their density was too low to obtain reliable density estimates. Models of ungulate density showed that the different species were associated with different vegetation types (Wegge et al. 2009). Wild boar and muntjac were most common in forested vegetation types in the eastern part of study area, whereas the more abundant species, chital and hog deer, were associated with grasslands that were distributed mainly along the river in the western part. Barasingha and nilgai were weakly associated with forested vegetation. The differences in density and habitat use among the ungulate species produced a clear gradient in total ungulate density from west to east (Fig. 1). Accordingly, we defined two main habitat types based on the variation in total density of ungulates; the patchwork of grasslands and successional forest in the west is hereafter termed “successional forest”, whereas the eastern part is termed “sal forest”.
Distribution of habitat types (a) and total estimated densities of axis deer, hog deer, muntjac, wild boar, barasingha, and nilgai (b) in a 105-km2 study area in the southwestern part of Bardia National Park, Nepal, in 1997–1999. The successional forest (a) included various types of younger forest successions and grasslands
Scat collection and analyses
We analyzed the content of 364 tiger scats and 96 leopard scats that were collected during surveys of an extensive network of roads and trails in the study area that were conducted from 1999 to 2001. Scats of tigers were distinguished from those of leopards by their larger size, and by examining the size and shape of associated tracks and markings. Scats were omitted when the species was uncertain. This method of separating tiger and leopard scats was shown to be appropriate by Andheria et al. (2007).
We collected 20–30 hairs from each scat sample (Mukherjee et al. 1994a, 1994b), and compared them with a reference collection (Karanth and Sunquist 1995; Støen and Wegge 1996). We excluded the scats from further analysis if they contained too few intact hairs to make a reliable identification of the prey species. The reference collection included hair from all potential wild and domestic ungulate, primate, and carnivore prey species.
We investigated the relative differences in selection of prey by the following method: First, we grouped the prey species in the following categories; medium-sized ungulates (chital, hog deer, wild boar, and muntjac, ca. 15–60 kg), large ungulates (barasingha, nilgai, and sambar <150 kg), domestic animals (cows, buffalos, sheep, goats, pigs, and dogs) and smaller wild mammals and birds. Next, we ran a series of binomial logistic regression models, where predator species, i.e., tiger or leopard, was the response variable, and the presence of each of the prey categories in the scats were explanatory variables. We compared 15 models based on the Akaike information criterion corrected for small samples (AICc; Burnham and Anderson 2002). The full model included all four prey categories as main effects, whereas the other 14 models represented all the simpler combinations of these prey categories, excluding interaction terms.
Predator distribution
During 2000/2001, we plotted positions of tiger and leopard scats and territorial markings (scrapes), and mapped the routes that were followed. We did not include pugmarks, as the occurrence of this sign may vary markedly among different habitats according to the ground substratum. On foot, we surveyed a route of 142 km of roads and trails inside the national park, and 64 km in buffer zone outside the park border, which consisted mainly of degraded sal forest. The whole route of 206 km was surveyed on two occasions at an interval of 2 months. The analyses of these data were conducted in three steps: (1) We compared the relative use of the areas inside and outside the national park by the two species; (2) We investigated the differences in distributions between tiger and leopard sign within the park boundaries with binomial logistic regression models. We used predator species (tiger or leopard) as a binary response variable and the different characteristics of the sign locations as explanatory variables: habitat type (ST), the densities of all wild ungulates (TT), of medium-sized ungulates (MD: chital, hog deer, muntjac, and wild boar) and large ungulates (LG: barasingha, sambar, and nilgai). We overlaid a grid with cells of 33 ha on a digitized habitat map of the study area, and used ungulate density models that were developed in a recent study in the same area to predict the densities of each prey category in each cell (Wegge et al. 2009). For each predator sign, we assigned ungulate density values from the grid cell where the sign was located. The predictors were correlated and, thus, we did not allow them to appear in the same models. Accordingly, we compared four models based on the AICc; (3) Lastly, we measured the distances between the sign and the park border, and compared the data from each of the two predator species.
Results
Predator diets
Medium-sized ungulates were the most common prey for both tigers and leopards (Table 1). However, the prey categories small mammals/birds (SM) and domestic animals (DM) were more common in leopard scats than in tiger scats, and these categories were included in all the best models of interspecific differences in scat contents (Tables 1, 2), suggesting that the occurrence of these prey items were the best predictors of whether a scat stemmed from a tiger or a leopard. Larger ungulates (LG) were rare in scats from both tiger and leopard (Table 1).
Predator sign distribution
The best model of predator sign distribution inside the national park performed far better than any of the other candidate models (model 1, Table 3). This model included only one term, habitat type (ST). Tiger sign were found on 218 occasions in successional forest and on 252 occasions in sal forest (χ 2 = 0.2, df = 1, p = 0.65). In contrast, leopard sign were found only 3 occasions in successional forest and on 36 occasions in sal forest (χ 2 = 14.2, df = 1, p < 0.001). All three models that included the predicted densities of ungulates (ΔAICc ~ 13–15) performed considerably better than the null model (ΔAICc = 24.51). The models predicted that tiger sign were more likely to occur than leopard sign in areas with high total densities of ungulates (M3), high densities of medium-sized ungulates (M2), and with low density of large ungulates (M4).
We found sign of tigers (n = 470) exclusively inside the national park, whereas leopard sign were distributed both inside (n = 39) and outside the park (n = 12). Hence, tiger sign occurred nearly ten times more frequently than did leopard sign, and there was evidence that their relative distribution inside and outside the park differed from that of the leopard (Fisher's exact test, p < 0.001). Also, the distribution of tiger sign differed from a random distribution (Fisher's exact test, p < 0.001), whereas leopard sign did not (χ2 = 0.4, df = 1, p = 0.506). The sign found within the habitat that was used by both predators, i.e., sal forest, was distributed differently between the two species. Tiger sign were further away from the park border than were leopard sign (tiger: 1.47 ± 0.06 km SE, leopard: 0.8 ± 0.14 km SE; t test, t = 3.43, df = 261, p = 0.001).
Discussion
A characteristic feature of leopards is their ability to dwell near human settlements. In contrast to tigers, which need relatively large and undisturbed areas with a healthy natural prey base (Karanth and Stith 1999), leopards often live alongside humans, sometimes in areas where wild prey are scarce (Singh 2005; Athreya 2006). In the vicinity of human settlements, domestic animals may dominate leopard diets (Edgaonkar and Chellam 2002), resulting in a high potential for conflicts with their human neighbors (Athreya 2006). These general interspecific differences were confirmed in our study; leopards included a far larger proportion of domestic animals in their diet than tigers, and leopard sign were evenly distributed on both sides of the park border and close to human settlements, whereas tigers clearly avoided the human-dominated areas.
The avoidance of human-dominated areas by tigers created a potential competition refuge for leopards. Hence, this competition refuge was situated in an area with a high likelihood of human conflict and persecution. Our data indicated that the leopards responded to this opportunity, using the buffer zone forest outside the park and, when in the park, using areas closer to the park border than tigers. Hence, the leopards seemed to avoid tigers by using the periphery of the tiger range instead of using areas of low quality within the home ranges of the tigers. The latter way of avoiding tigers was probably not feasible, as we did not detect any significant difference among tigers in the distribution of sign with respect to areas of rich and poor prey density.
The line of reasoning above rests on the assumptions that the distribution of leopard sign was a result of avoidance of tigers rather than a preference for human-dominated areas. Both theory and empirical data support these assumptions. Medium-sized ungulates were the main food source of both species, and the fact that leopard sign within the park were mainly located in areas with a relatively low availability of this resource indicated that the leopards were subject to some form of constraint. We previously argued that interspecific competition for food was an implausible limiting factor for the leopards based on recent reports from the same area that showed a high food consumption of leopards (Odden and Wegge 2009) and a high availability of potential prey (Wegge et al. 2009). Indeed, if any of the two species was affected by limited food availability it should be tigers, as they would be less able to subsist on a shared and limited food resource due to their larger size. Furthermore, Wegge et al. (2009) showed that annual predation rates were relatively low among the most abundant medium-sized ungulates, chital (~7–8%) and hog deer (~11%), thus, rendering further evidence that exploitative competition was an insignificant determinant factor affecting the coexistence of the two predator species.
The prey-rich successional forests that were rarely used by leopards contained a higher proportion of open grasslands than the sal forest (Fig. 1). These areas may thus have been less attractive for leopards due to lower availability of suitable trees for food caching. However, we do not believe that tree density was the most important factor affecting the space use of leopards in Bardia. The successional forests were fine-grained mosaics of grasslands and forests (Fig. 1) and food-caching trees were therefore available within relatively short distances. Secondly, radio-collared leopards in the study area did not avoid grasslands that were available in their territories (Odden and Wegge 2005). The location data from the collared leopards showed a somewhat similar pattern as the distribution of sign. Leopard territories were situated along the park border and leopards were regularly found outside of the park.
The results of this study indicate that leopards avoid tigers. This contrasts with the inference made by Karanth and Sunquist (1995, 2000) from their studies in Nagarhole National Park, India, but concurs with observations in Chitwan National Park, Nepal (Seidensticker 1976; McDougal 1988; Seidensticker et al. 1990). The differing results imply that the behavior of the two species differs according to the ecological settings of the study areas, and that there is no single “key to coexistence” between tigers and leopards. Prey were abundant both in our study area in Bardia and in Nagarahole, but the composition of the prey base differed in one essential aspect, large ungulate prey were far more abundant in Nagarahole. Camera trapping resulted in 5.44 leopard photographs per 100 trap nights in Nagarahole (Karanth and Nichols 1998), versus only 0.25 per 100 trap nights in our study area (Wegge et al. 2009). This suggests that leopards were far more abundant in Nagarahole. We suggest three alternative explanations to the limited leopard abundance in Bardia. Firstly, the density of tigers was higher in Bardia (Wegge et al. 2004, 2009: 13.3–19.3 tigers per 100 km2) than in Nagarahole (Karanth and Nichols 1998: 11.5 per 100 km2). Secondly, with a deficient availability of large prey, tigers must kill medium-sized prey at much higher rates in order to obtain sufficient amounts of food (Sunquist et al. 1999). Hence, the benefit/cost ratio of foraging may be relatively low for tigers in our study area even though the total prey density is high. Interspecific aggression has been shown to be positively correlated with the level of energetic stress (Palomares and Caro 1999). Despite the high abundance of wild ungulates in Bardia, the tigers may be more hungry, and aggressive, than in Nagarahole. Furthermore, small prey is quickly consumed. Thus, when subsisting on small prey, tigers must spend a larger part of their activity budget on hunting, increasing the probability of encounters with leopards. Hence, interference competition may be more pronounced in Bardia than in Nagarahole due to more energetic stress and higher frequency of hunting. Thirdly, previous analyses of prey density and distribution showed that the different ungulate prey species were, to some extent, spatially segregated due to different habitat preferences (Wegge et al. 2009). In areas where prey species have a clumped distribution, interspecific encounters should be positively correlated with the degree of diet overlap. Consequently, when large prey is absent and the main food species are shared, as in our study area in Bardia, the level of interference competition would be increased due to the higher probability of encountering the opponent.
References
Andheria AP, Karanth KU, Kumar NS (2007) Diet and prey profiles of three sympatric large carnivores in Bandipur Tiger Reserve, India. J Zool 273:169–175
Athreya V (2006) Is relocation a viable management option for unwanted animals? The case of the leopard in India. Conserv Soc 4:419–423
Begon M, Harper JL, Townsend CR (1990) Ecology: individuals, populations and communities, 2nd edn. Blackwell, Cambridge
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, Berlin
Creel S, Spong G, Creel NM (2001) Interspecific competition and the population biology of extinction-prone carnivores. In: Gittleman J, Macdonald D, Funk S, Wayne R (eds) Conservation of carnivores. Cambridge University Press, Cambridge, pp 35–60
Donadio E, Buskirk SW (2006) Diet, morphology and interspecific killing in carnivora. Am Nat 167:524–536
Durant SM (1998) Competition refuges and coexistence: an example from Serengeti carnivores. J Anim Ecol 67:370–386
Edgaonkar A, Chellam R (2002) Food habit of the leopard, Panthera pardus, in the Sanjay Gandhi National Park, Maharashtra, India. Mammalia 66:353–360
Gittleman JL (1985) Carnivore body size––ecological and taxonomic correlates. Oecologia 67:540–554
Goss-Custard JD (1980) Competition for food and interference amongst waders. Ardea 68:31–52
Hayward MW, Henschel P, O’Brien J, Hofmeyr M, Balme G, Kerley GIH (2006) Prey preferences of the leopard (Panthera pardus). J Zool 270:298–313
Heinen JT, Kattel B (1992) Parks, people and conservation: a review of management issues in Nepal’s protected areas. Popul Environ 14:49–84
Karanth KU (1993) Predator–prey relationships among large mammals of Nagarahole National Park, India. PhD Dissertation, Mangalore University, Mangalore, India
Karanth KU (1995) Estimating tiger Panthera tigris populations from camera-trap data using capture–recapture models. Biol Cons 71:333–338
Karanth KU, Nichols JD (1998) Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79:2852–2862
Karanth KU, Stith BM (1999) Prey depletion as a critical determinant of tiger population viability. In: Seidensticker J, Christie S, Jackson P (eds) Riding the tiger: tiger conservation in human dominated landscapes. Cambridge University Press, Cambridge, pp 100–113
Karanth KU, Sunquist ME (1992) Population structure, density and biomass of large herbivores in the tropical forests of Nagarahole, India. J Trop Ecol 8:21–35
Karanth KU, Sunquist ME (1995) Prey selection by tiger, leopard and dhole in tropical forests. J Anim Ecol 64:439–450
Karanth KU, Sunquist ME (2000) Behavioural correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarahole, India. J Zool 250:255–265
Liao JG, McGee D (2003) Adjusted coefficients of determination for logistic regression. Am Stat 57:161–165
MacArthur RH, Levins R (1967) The limiting similarity, convergence and divergence of coexisting species. Am Nat 101:377–385
McDougal C (1988) Leopard and tiger interactions at Royal Chitwan National Park, Nepal. J Bombay Nat Hist Soc 85:609–610
Mukherjee SP, Goyal SP, Chellam R (1994a) Refined techniques for the analysis of Asiatic lion Panthera leo persica scats. Acta Theriol 39:425–430
Mukherjee SP, Goyal SP, Chellam R (1994b) Standardisation of scat techniques for leopard (Panthera pardus) in Gir National Park, Western India. Mammalia 58:139–142
Odden M, Wegge P (2005) Spacing and activity patterns of leopards Panthera pardus in Royal Bardia National Park, Nepal. Wildl Biol 11:145–152
Odden M, Wegge P (2009) Kill rates and food consumption of leopards in Bardia National Park, Nepal. Acta Theriol 54:23–30
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153:492–508
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Radloff FGT, du Toit JT (2004) Large predators and their prey in a southern African savannah: a predator’s size determines its prey size range. J Anim Ecol 73:410–423
Seidensticker J (1976) On the ecological separation between tigers and leopards. Biotropica 8:225–234
Seidensticker J, Sunquist ME, McDougal C (1990) Leopards living at the edge of the Royal Chitwan National Park, Nepal. In: Daniel JC, Serrao JS (eds) Conservation in developing countries: problems and prospects. Bombay Natural History Society and Oxford University Press, Bombay, pp 415–423
Sinclair ARE, Mduma S, Brashares JS (2003) Patterns of predation in a diverse predator–prey system. Nature 425:288–290
Singh HS (2005) Status of the Leopard Panthera pardus in India. Cat News 42:15–17
Støen OG, Wegge P (1996) Prey selection and prey removal by tiger (Panthera tigris) during the dry season in lowland Nepal. Mammalia 60:363–373
Sunquist M, Karanth KU, Sunquist F (1999) Ecology, behaviour and resilience of the tiger and its conservation needs. In: Seidensticker J, Christie S, Jackson P (eds) Riding the tiger: tiger conservation in human dominated landscapes. Cambridge University Press, Cambridge, pp 5–18
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton
Wegge P, Pokharel CP, Jnawali SR (2004) Effects of trapping effort and trap shyness on estimates of tiger abundance from camera trap studies. Anim Conserv 7:251–256
Wegge P, Odden M, Pokharel CP, Storaas T (2009) Predator–prey relationships and responses of ungulates and their predators to the establishment of protected areas: a case study of tigers, leopards and their prey in Bardia National Park, Nepal. Biol Conserv 142:189–202
Woodroffe R, Ginsberg JG (2005) King of the beasts? Evidence for guild redundancy among large mammalian carnivores. In: Ray J, Berger J, Redford KH, Steneck R (eds) Large carnivores and biodiversity: does saving one conserve the other? Island Press, New York, pp 154–176
Acknowledgments
This study was funded by the Royal Norwegian Agency for Development Cooperation (NORAD) as part of a collaborative research program between the Department of National Parks and Wildlife Conservation/Nepal and the Norwegian University of Life Sciences. Bardia National Park (BNP) and Nepal Trust for Nature Conservation (NTNC) provided technical support. In particular, we want to thank Narayan Choudary and Tanku Choudary (BNP), and Shanta Raj Jnawali, Man Bahadur Lama, Man Singh Lama, and Suk Bahadur (NTNC) for assistance during fieldwork. Manuela Panzacchi, Jon E. Swenson, Ullas Karanth, and Atle Mysterud provided constructive input to the manuscript. All field activities on which this research was based comply with the laws of Nepal.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Odden, M., Wegge, P. & Fredriksen, T. Do tigers displace leopards? If so, why?. Ecol Res 25, 875–881 (2010). https://doi.org/10.1007/s11284-010-0723-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-010-0723-1