Abstract
A one-male group (BE-Group) of proboscis monkeys was studied along the Menanggul River, a tributary of the Kinabatangan River, Sabah, Malaysia, from May 2005 to 2006. It has generally been assumed that proboscis monkeys only set up their sleeping sites along the riverbank; however, when more than 1 m of water covered the forest floor for more than 700 m inland from the riverbank during the seasonal flood, the BE-Group slept inside the forest. It seems that the sleeping-site selection of the BE-Group was not influenced by food availability during the flooded months because the food availability by the vegetational survey did not vary much between flooded and non-flooded months. In addition, feeding behaviors of the focal monkey in the BE-Group also did not vary much between flooded and non-flooded days. On the other hand, the water level statistically influenced the sleeping-site selection. The proboscis monkeys remained in inland forest during the flooded days because of the reduced predation threat, as terrestrial predators such as clouded leopards are prevented from foraging by deep water covering the forest floor. On non-flooded days when the BE-Group slept at the riverbank, they frequently slept close to other one-male groups on the riverside trees. Contrastingly, when the group slept inside the forest on flooded days when the water level was high, they slept away from other groups. This difference in the need for one-male groups to sleep close to each other might be attributed to the decreased predation threat during high water level in the flooded days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral strategy against predation is a common topic across various mammalian taxa. Ranging behaviors, especially sleeping-site selections, reflect the predation avoidance strategy in mammals though food abundance and distribution and are also the major influence on their ranging (e.g., primates: van Schaik et al. 1996; Koenig 2000; Artiodactyla: Teng et al. 2004; Brodie and Brockelman 2009; Carnivora: Buskirk and Powell 1994; Miyoshi and Higashi 2005). However, compared to the studies about the effects of availability and distribution of food sources on their ranging behaviors, the studies about effects of predation/predation threat seem to be difficult because of a lack of predation evidence, especially in wild mammals. Non-human primates are also one of the mammalian taxa that have been intensively discussed regarding the effects of predation threats and food sources on their ranging behaviors. It is still difficult to clearly determine the relationship between predation threat and behavioral adaptations although reports of predation on non-human primates have increased in recent years (Cheney and Wrangham 1987; Miller and Treves 2007). Some studies in non-human primate species conclude that predation avoidance is the major basis for use and choice of particular sleeping sites (e.g., Hamilton 1982; Reichard 1998; Fan and Jlang 2008) though many other effects, including food abundance and distribution, have also been reported, e.g., food condition (Oates 1987), water resources (Altmann and Altmann 1970), and the weather (Makey and Waterman 1982). Further, in group-living primates, predation/predation threat might influence not only ranging patterns but also their social systems (Alexander 1974; Clutton-Brock and Harvey 1977; van Schaik and van Hooff 1983).
The proboscis monkey (Nasalis larvatus), a member of the subfamily Colobinae, is endemic to Borneo Island and primarily inhabits mangroves, peat swamps, and riverine forests. Since some of their favorite habitats are swampy, observation and tracking of the monkeys in inland forests is difficult, and hence, knowledge about the ecology of proboscis monkeys is incomplete. Nonetheless, since proboscis monkeys typically return to the riverbank for sleeping (Bennett and Sebastian 1988; Yeager 1991a; Murai 2004a), most previous studies have been conducted by observation from a boat on the river when the monkeys are on the riverbank in the early morning and late afternoon. The typical social unit of proboscis monkeys is a one-male group consisting of one adult male, several females and their immatures, as well as all-male groups consisting only of young males. Recent long-term studies suggest that the proboscis monkeys have a multi-level society, in which one-male groups regularly associate with each other at the riverbank (Yeager 1991a; Murai 2004b).
The proboscis monkeys are at a risk of predation even in trees by terrestrial predators such as clouded leopards, and to reduce the predation threat, they typically sleep at the riverside trees where predators can only approach them from the land side (Matsuda et al. 2008a). In addition, these monkeys select sleeping sites where the branch-to-bank distance between the trees located on opposite sides of the river is narrow, because these sleeping locations may provide good escape routes from the terrestrial predators at night (Matsuda et al. 2008b). Although no detailed data exist confirming the selection of inland forest for sleeping sites by proboscis monkeys, Yeager (1993) speculates that the monkeys sleep inland when water levels are high because of the increased number of trees falling along the river due to flooding. Apart from the effects of predation threats and the water level, fruit availability may be an important factor for their sleeping-site selection. Proboscis monkeys prefer to feed on fruits (when these are available) (Yeager 1989; Matsuda et al. 2009a), and recently a few direct tracking studies in inland forests have demonstrated that ranging patterns of proboscis monkeys are influenced by the availability of these fruits (Boonratana 2000; Matsuda et al. 2009b).
Predation threats and river water level also seem to be important factors influencing the multi-level society in proboscis monkeys (Yeager 1993; Murai 2004b). Temporary associations may allow groups to coordinate river crossings, thereby reducing individual risk of predation by aquatic predators such as crocodilians (Yeager 1991b), and Yeager (1993) and Murai (2004b) demonstrate that the water level is negatively correlated with the association degree among one-male groups in their multi-level society.
In the present study, during the flooded days when the forest floor was covered by water, the predation threat to proboscis monkeys by terrestrial predators was absent because the predators cannot forage for prey in deep water, and aquatic predators were able to almost ignore because the monkeys did not cross the river and usually traveled in trees. At that time, the focal one-male group of proboscis monkeys dramatically changed their ranging patterns and set up their sleeping sites in the inland forest in contrast to former studies, where it has been reported that the monkeys generally return to the riverbank for sleeping. In addition, less association was observed between the one-male groups at the sleeping site in the inland forest on flooded days compared to that of non-flooded days. Therefore, we sought (1) to describe the changes of sleeping sites in proboscis monkeys from riverbank to inland during flooded months in detail, (2) to compare the feeding behaviors and food availabilities among pre-flooded, flooded, and post-flooded days, and (3) to discuss the most important factors (water level of river, predation threats, food availability, and/or tree falls) for why the study group set up their sleeping sites in the inland forest and why the association degree among the groups was lower during the flooded months.
Methods
Study site and group
This study was conducted in the riverine forest along the Menanggul River, a tributary of the Kinabatangan River in Sabah, Malaysia (118°30′E, 5°30′N), from May 2005 to 2006. The southern area of the Menanggul River is extensively covered by natural forest while the northern area has been deforested for oil palm plantations, except for a protected zone along the river (cf. Matsuda et al. 2009). The mean minimum and maximum temperatures were approximately 24 and 32°C, respectively, and total precipitation at the site was 2,510 mm (June 2005 to May 2006). The water level of the river was always measured at 18:00–19:00 whenever we went out to the Menanggul River after finishing research. Monthly mean water level fluctuated from 66 cm in August 2005 to 345 cm in February 2006 (Fig. 1).
The focal one-male group (BE-Group) of proboscis monkey was habituated to observers and each individual was identifiable. At the end of the study, the group comprised 16 individuals: one adult male, six adult females, five juveniles and four infants. Sleeping sites of the BE-Group were distributed in a wide range, overlapping with those of 10–12 other one-male groups and 2–3 all-male groups (Matsuda 2008). The home range of the group was 138.3 ha and the group used both riverbanks situated at a distance of 1,400–5,250 m from the river mouth (Matsuda et al. 2009b). As reported by Matsuda et al. (2008a), clouded leopards (Neofelis diardi) and estuarine crocodiles (Crocodylus porosus) are potential predators of proboscis monkeys at the study site.
Behavioral data collection
All behavioral data were collected using a focal animal sampling method (Altmann 1974). The observers (I. M. plus two research assistants) were separated into two groups in order to simultaneously follow an adult male and an arbitrarily selected female. We followed the focal adults in the BE-Group from 6:00–6:30 to 18:30–19:00 and recorded the time spent for each behavior and the food type being eaten. Behaviors were divided into four categories: resting, moving, feeding, and others. Each month, focal following was conducted for 11–17 days, except in February 2006, when the study area was flooded. During this period, the location of the BE-Group was unknown. In March 2006, the group was located; however, the forest floor was still flooded and the focal monkeys were followed by boat throughout the day. Since all the members of the BE-Group traveled almost the same route and the members rarely moved more than 50 m away from the alpha male (Bejita), the location of Bejita was recorded by a GPS unit at 10-min intervals during the daily observation period.
It has been suggested that proboscis monkeys set up their sleeping sites on riverside trees (Bennett and Sebastian 1988; Yeager 1991a; Murai et al. 2007). To confirm BE-Group sleeping-site locations when the group was not being followed in the forest, boat-based surveys were conducted along the Menanggul River (from the river mouth to a point 6,000 m upstream) during the late afternoon for 6–13 days/month (total 138 times). An exception was during the month of February in 2006, when evening boat surveys were conducted for 22 days, most of which were spent searching for the missing BE-Group.
In both foot- and boat-based surveys, we almost always verified by boat the presence of other one-male groups within 500 m of the BE-Group at the riverbank after confirming the sleeping site of the BE-Group.
Vegetation survey and food availability
Sixteen 200 to 500-m-long and 1-m-wide trails (trail code: TR1–TR16) were set up in the study site at 500-m intervals from the river mouth to 4,000 m upstream for phenological survey. Along the trails, trees ≥10 cm diameter breast height (DBH) and vines ≥5 cm diameter located on the trail or within 1 m from the trail edge were labeled, i.e., surveyed width = 3 m. The length of most of the 16 trails was 500 m; however, some trails measured less than that because the oil palm plantation expanded its range to within 500 m of the riverbank (TR2: 200 m, TR4 and TR6: 400 m, TR8: 250 m, and TR10: 400 m). At the end of each monthly survey, except February 2006 when the study area was flooded to a level higher than the DBH labels, the phenology of the 2,142 labeled plants along the 16 trails was recorded by examining each plant for the presence or absence of young leaves (including leaf buds), fruits (both ripe and unripe), and flowers (including floral buds).
Out of the 2,142 trees and vines along the trails, 1,902 were plant species that we observed proboscis monkeys to use as a food source during the study period (Matsuda et al. 2009a). Food availability is determined as the number of young leafing, fruiting, and flowering potential food plants in the monthly phenological survey.
Data analysis
Estimating the frequency of sleeping at riverbank
Before the forest flooded, we were always able to detect the BE-Group at the riverbank by boat-based surveys in the late afternoon. This indicates that the detection by boat-based surveys is 100%, as long as they sleep at the riverbank. Therefore, we can presume that they slept away from the riverbank during the flooded days when we were unable to locate them by boat-based surveys. Based on this presumption, we calculated the rate of the use of riverbank as their sleeping-site each month as follows: (number of days the group was confirmed to be sleeping at the riverbank by full-day follow-ups + number of days they were located at the riverbank by boat-based surveys)/(number of days full-day follow-ups were conducted + number of days boat surveys were conducted).
Term definition for comparing behavior on flooded days with that on non-flooded days
To examine the flooding-induced behavioral changes in proboscis monkeys in January 2006, we analyzed the behavioral data in each of the three 3-day periods, which were determined by the condition of the water level of the river: period I: 7th–9th (days before flood and BE-Group slept at the riverbank), period II: 12th, 14th, and 15th (flooded days and the group slept in the inland forest) and period III: 17th–19th (days after flood and the group slept at the riverbank). Since no difference was observed in the time budget of each behavior among individuals, excluding the minor time allocation to other behaviors (Matsuda et al. 2009a), only Bejita’s behaviors were analyzed in this paper.
Dietary overlap of Bejita among the three periods in January for all possible pairs of periods was calculated using the Holmes-Pitelka index, D i = ΣS i, where D i is the total percent overlap and S i is the percent overlap between shared food items (Holmes and Pitelka 1968; Struhsaker 1975). To evaluate whether the degree of dietary variability within a month was different between January and other months, other months were also divided by each of the three 3-day periods and the dietary overlap was calculated. In each month, period I was selected from 4th to 13th, period II was from 9th to 16th, and period III was from 15th to the 21st, except November 2005, in which period III was selected from the 26th to the 28th. Each day in periods and each period in months was selected to be similar to those in January.
Results
Ranging pattern changes
Sleeping sites of the BE-Group were studied for 295 nights with full-day follow-ups and boat-based surveys. Of these 295 nights, 261 sleeping sites of the BE-Group were located on the riverbank and 34 sites were considered to be in the inland forest (nine sites were directly observed). The group did not return to the riverbank to sleep throughout February. The water level at the river mouth ranged from 8 to 433 cm with a median of 108 cm when the sleeping sites were located on riverbank, and from 220 to 455 cm with a median of 350 cm when the sites were located inside the forest. The ratio of sleeping at the riverbank was less when the water level was higher (Fig. 1; Spearman’s rank correlation test; n = 13, r s = −0.31 and p = 0.01).
The group returned to the riverbank every day for sleeping until the 10th of January. However, from the 11th of January, when the water level reached 238 cm at the mouth of the Menanggul River and the forest floor around TR10 was covered by about 1-m-deep water, the BE-Group started wandering in the forest without returning to the riverbank until the 17th of January (Fig. 2a). During wandering in the forest, the BE-Group had sleeping sites at small open gaps or near former tractor roads. From the 18th of January, when the water level was 185 cm at the river mouth and the water depth at TR10 decreased to about 40 cm, the BE-Group began returning to the riverbank again for sleeping (Fig. 2b). Although the BE-Group could not be located in February, we happened to rediscover it on the 3rd of March and resumed following the group by boat. During the second flood, the water covered a wide range of the forest, including the southern area of the Menanggul River and the oil palm plantation (Fig. 2c).
The difference in daily path length between the days when the group slept in the inland forest and at the riverbank was insignificant (U-test: U = 584 and p = 0.71); inland forest: 830 m (n = 7, SD = ± 223 m) and riverbank: 798 m (n = 154, SD = ± 318 m). However, BE-Group’s maximum distance away from the riverbank was significantly different between the days when the group slept in the inland forest and when the group slept at the riverbank (U-test: U = 1093 and p < 0.0001); inland forest: 564 m (n = 7, SD = ±150 m and range = 356–800 m) and riverbank: 159 m (n = 154, SD = ±97 m and range = 23–575 m).
Not only the BE-Group but also other one-male and all-male groups were less detected when more than 1 m of water covered the forest floor in January (11–17th), February (5–28th), and March (1–10th) compared to other days with lower water level (<220 cm): mean number of detected groups per boat-based survey on high water days (N = 30; Mean = 2.7; SD = ±1.6) and low water days (n = 108; Mean = 7.0; SD = ±2.9) were significantly different (U-test: U = 317 and p < 0.00001). Low detectability on high water days indicates that proboscis monkey groups inhabiting our study site slept along other parts of river or in inland forest.
Spatiotemporal distribution of food availability
During the flooded days when the BE-Group slept in the inland forest, the group always traveled around the northern part of TR10 (Fig. 2a, c). In addition, availability of fruits is a significant factor influencing the ranging behavior of the BE-Group (Matsuda et al. 2009b), and thus, spatiotemporal distribution of fruit availability was only analyzed on TR10. Since BE-Group’s mean maximum distance away from the riverbank on non-flooded days was 159 m, TR10, which was 400 m long, was divided into two segments (from 0 to 150 m and from 150 to 400 m from the riverbank). The mean monthly number of fruiting trees and vines in each segment per survey area during the flooded (January and March) and non-flooded months (May 2005–December and April–May 2006) is shown in Fig. 3. Fruit availability was higher in the 150–400 m segment than in the 0–150 m segment during both flooded and non-flooded months, denying the possibility that food availability in the inland forest, compared to the riverbank, increased in the flooded months.
Feeding behavior during three periods
In January, Bejita was followed for 35.9 h in period I, 31.3 h in period II and 36.4 h in period III. Since feeding behavior was the most important factor controlling the activity budget of proboscis monkeys in the BE-Group, and was mostly influenced by their fruit-eating (cf. Matsuda et al. 2009a), the activity budget of feeding and the amount of fruit consumption was analyzed among the three periods. Time budget variation in the feeding behavior among the three periods was not significant (Kruskal–Wallis test: H = 1.87, p = 0.39); period I: 28.8%, period II: 23.4% and period III: 24.6%. The difference in consumption of fruits among the three periods was also insignificant (H = 1.87, p = 0.39); period I: 0.6%, period II: 4.8% and period III: 6.2%.
In January, the amount of dietary overlap was 58.5% (period I–II), 50.7% (period II–III) and 45.5% (period I–III). The mean overlap in January was 51.5% (SD ± 6.5) and was second lowest compared to the other months. Nonetheless, the mean overlap in March, including the flooded period (period I), was 76.5% (±4.3) and was third highest (Table 1).
Changes in association with other one-male groups
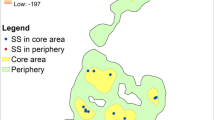
When the water level was high and the BE-Group slept inside the forest, other one-male groups were never observed within a 200-m radius from the BE-Group. However, a one-male group sleeping in the inland forest approximately 300 m away from the BE-Group was incidentally observed on the 13th of January. On the other hand, when the water level was low and the BE-Group slept on the riverside trees, there were 101 instances (40.1% of all sleeping site confirmed at the riverbank) when the sleeping sites of other one-male groups were located within 200 m from the BE-Group’s sleeping sites (Fig. 4). Thirty-seven sleeping sites were located within 50 m, with occasional occurrence of sharing the same tree. In addition, the nearest sleeping sites of other one-male groups were frequently situated on the same riverside as that of the BE-Group’s sleeping site.
Discussion
The study group rapidly changed their sleeping site from the riverbank to the inland forest in January 2006, when the water level of the river rose and the forest floor was flooded. Not only in January but also in February and March during the flooded days the group was observed or considered to sleep in the inland forest. Water level of river, predation threats, food availability, and/or other environmental factors such as increased tree falls due to flooding might have been the reasons for the relocation of the sleeping site of the study group from riverbank to inland forest. In addition, the influence of setting up sleeping sites in the inland forest on multi-level social organization of proboscis monkeys is discussed.
It has been suggested that proboscis monkeys typically sleep adjacent to riverbanks to protect themselves effectively from terrestrial predators, especially clouded leopards, which are their major terrestrial predators in our study site, since sleeping on riverbanks allows the predators to attack only from the land side (Matsuda et al. 2008a). In this study, the water level significantly influenced the selection of sleeping sites of proboscis monkeys, i.e., whether they slept at the riverbank or in the inland forest. This result suggests that the monkeys do not need to return to the riverbank when the water level is high because of the reduced predation threat, as the terrestrial predators are prevented from foraging by deep water even in the inland forest. During the flooded days, the maximum distance of the study group from the riverbank was significantly longer than that during the non-flooded days, evidently because the group is not restricted to return to the riverside for sleeping before sunset during the flooded days.
One of the major factors influencing ranging behaviors of primates is the distribution and abundance of food (Oates 1987; van Schaik et al. 1996; Koenig 2000). Generally, the ranging behavior of primate species that prefer to feed on limited and seasonal food sources such as fruits or flowers is more influenced by the food distribution and abundance compared to primate species that prefer to feed on equally distributed food such as leaves. Ranging of proboscis monkeys is influenced by the availability of fruits, despite their main food being young leaves that are equally distributed in the forests (Boonratana 2000; Matsuda et al. 2009a). In the whole vegetational transects (2.15 ha) of the study site, the availability of fruits for proboscis monkeys during the flooded months (January and March 2006) was not different from that in non-flooded months (Matsuda et al. 2009a). Even though we focused on the availability of fruits on TR10 where the study group frequently traveled on flooded days, the availability of fruits in the inland forest did not increase in the flooded months. Actually, the amount of fruit consumption by Bejita increased (though it was insignificant) when the group slept in the inland forest in January compared to the amount before flooded days, but the higher fruit consumption continued even after the flood ended and the group slept at the riverbank again. In addition, the dietary overlap percentage showed that the monkeys did not change their diet considerably among the periods in January even though the mean dietary overlap percentage in January was relatively low. These results suggest that the distribution and abundance of fruits and feeding behaviors are not major factors in determining the sleeping sites of the study group in the inland forest.
In a previous study, Yeager (1993) speculated that one-male groups of proboscis monkeys sleep inside the forest during the high water season because the monkeys are scared of tree falls and streambank erosion, which undermines vegetation and breaks off land masses from the river’s edge. Of 21 trees falls along the river from the mouth to 6,000 m upstream, however, only three trees fell along the river in the study site during the flooded months (January–March) (I. Matsuda, unpublished data). In addition, even though five trees fell along the river after a windy day in September, the study group was confirmed to be sleeping at the riverbank the next morning, suggesting that the reason proposed by Yeager (1993) is not plausible in explaining the sleeping-site changes in our study group.
Kummer (1971) and Dunbar (1986) consider that the multi-level society reported in Theropithecus gelada and Papio hamadryas might be adaptations employed by the species for effective predation avoidance. The proboscis monkey is one of the species that has been reported to form a multi-level society while sleeping at a riverbank (Yeager 1991a; Murai 2004b), and Yeager (1991a) and Murai (2004b) suggest that this multi-level society might also be related to predation avoidance, especially when the monkeys cross the river. On the other hand, Yeager (1993) reports that the degree of association between one-male groups in a multi-level society at the riverbank shows a negative correlation with water level, since it is speculated that the monkeys sleep in the inland forest during the flooded months. According to Murai (2004b), who studied at the same site as the present study, the water level also negatively correlates with the degree of association between the one-male groups. In this study, the BE-Group slept alone inside the forest on flooded days, although the group often slept close to other one-male groups at riverside trees on non-flooded days, supporting the idea that water level influenced the association degree between one-male groups. The low degree of association, which was observed during high water level, might have also been a result of reduced predation threats. In other words, one-male groups in multi-level society might not need to aggregate to avoid the predators because of reduced predation threats by deep water forest on flooded days. Thus, water level and/or predation threat might influence not only the sleeping-site selection of proboscis monkeys but also their multi-level social organization.
This study presented a novel observation of proboscis monkeys setting sleeping sites inland as it has generally been assumed that they set up sites only along riverbanks. We concluded that the reduced predation threats by terrestrial predators were the most probable reason why the study group set up their sleeping sites in the inland forest and the association degree among groups was lower during the flooded months. Although our results focused on a single group inhabiting riverine forests, and no clear evidence exists that other proboscis monkeys also frequently slept inland in response to water-level condition, low detectability of proboscis monkey groups by boat-based survey during high-water periods in this study and other studies (Yeager 1993) suggest that other groups slept inland rather than sleeping along the river in other places when the water levels were high. There may be the potential to build upon our new findings for more detailed investigations which will have major implications for re-examining the social organization of proboscis monkeys and the ecological factors influencing their behavioral adaptation. However, since our results might not be representative of the entire proboscis monkey populations, especially those inhabiting other forest types (mangrove and peat swamp forests), further detailed studies on their ranging behaviors in various vegetation types during flooded months will be helpful in clarifying how predation threats influence their ranging behavior and social structure. Lastly, this study, which examined the possibility of the effects of food availability, dietary shift, and predation threats on the ranging patterns in proboscis monkeys under a kind of natural experiment, will provide an interesting example to consider the behavioral strategy against predation not only in non-human primates but also other mammalian taxa.
References
Alexander RD (1974) The evolution of social behaviour. Ann Rev Ecol Syst 5:325–383
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 69:227–267
Altmann SA, Altmann J (1970) Baboon ecology: African field research. Karger, Basel
Bennett EL, Sebastian AC (1988) Social organization and ecology of proboscis monkeys (Nasalis larvatus) in mixed coastal forest in Sarawak. Int J Primatol 9:233–256
Boonratana R (2000) Ranging behavior of proboscis monkeys (Nasalis larvatus) in the lower Kinabatangan, Northern Borneo. Int J Primatol 21:497–518
Brodie JF, Brockelman (2009) Bed site selection of red muntjac (Muntiacus muntjak) and sambar (Rusa unicolor) in a tropical seasonal forest. Ecol Res (in press)
Buskirk SW, Powell RA (1994) Habitat ecology of fishers and American martens. In: Buskirk SW, Harestad AS, Raphael MG, Powell RA (eds) Martens, sables, and fishers: biology and conservation. Cornell University Press, New York, pp 283–296
Cheney DL, Wrangham RW (1987) Predation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 227–239
Clutton-Brock TH, Harvey PH (1977) Primate ecology and social organization. J Zool Lond 183:1–39
Dunbar RIM (1986) The social ecology of gelada baboons. In: Rubenstein DI, Wrangham RW (eds) Ecological aspects of social evolution. Princeton University Press, Princeton, pp 332–351
Fan PF, Jlang XL (2008) Sleeping sites, sleeping trees, and sleeping-related behaviors of black crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. Am J Primatol 70:153–160
Hamilton WJ (1982) Baboon sleeping site preference and relationship to primates grouping patterns. Am J Primatol 3:41–53
Holmes RJ, Pitelka FA (1968) Food overlap among coexisting sandpipers on northern Alaskan tundra. Syst Zool 17:305–318
Koenig A (2000) Competitive regimes in forest-dwelling Hanuman langur females (Semnopithecus entellus). Behav Ecol Sociobiol 48:93–109
Kummer H (1971) Primate Societies: Group Techniques of Ecological Adaptation. University of Victoria, Aldine/Atherton Inc., Chicago
Makey D, Waterman PG (1982) Ranging behaviour of a group of black colobus (Colobus satanas) in the Douala-Edea Reserve, Cameroon. Folia Primatol 39:264–304
Matsuda I (2008) Feeding and ranging behaviors of proboscis monkey Nasalis larvatus in Sabah, Malaysia [Dissertation]. Graduate School of Environmental Earth Science, Hokkaido University
Matsuda I, Tuuga A, Higashi S (2008a) Clouded leopard (Neofelis diardi) predation on proboscis monkeys (Nasalis larvatus) in Sabah, Malaysia. Primates 49:227–231
Matsuda I, Tuuga A, Akiyama Y, Higashi S (2008b) Selection of river crossing location and sleeping site by proboscis monkeys (Nasalis larvatus) in Sabah, Malaysia. Am J Primatol 70:1097–1101
Matsuda I, Tuuga A, Higashi S (2009a) The feeding ecology and activity budget of proboscis monkeys. Am J Primatol 71:478–492
Matsuda I, Tuuga A, Higashi S (2009b) Ranging behavior of proboscis monkey in a riverine forest with special reference to ranging in inland forest. Int J Primatol 30:313–325
Matsuda I, Kubo T, Tuuga A, Higashi S (2009) A Bayesian analysis of the temporal change of local density of proboscis monkeys: implications for environmental effects on a fission–fusion society. Am J Phys Anthropol (in press)
Miller LE, Treves A (2007) Predation on primates. In: Campbell CJ, Fuentes A, Mackinnon KC, Panger M, Bearder SK (eds) Primates in perspective. Oxford University Press, New York, pp 525–543
Miyoshi K, Higashi S (2005) Home range and habitat use by the sable Martes zibellina brachyuran in Japanese cool-temperate mixed forest. Ecol Res 20:95–101
Murai T (2004a) Social behaviors of all-male proboscis monkeys when joined by females. Ecol Res 19:451–454
Murai T (2004b) Social structure and mating behavior of proboscis monkey Nsalis larvatus (Primates; Colobinae) [Dissertation]. Graduate School of Environmental Earth Science, Hokkaido University
Murai T, Mohamed M, Bernard H, Mahedi PA, Saburi R, Higashi S (2007) Female transfer between one-male groups of proboscis monkey (Nsalis larvatus). Primates 48:117–121
Oates JF (1987) Food distribution and foraging behavior. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 197–209
Reichard U (1998) Sleeping sites, sleeping places, and pre-sleep behavior of gibbons (Hylobates lar). Am J Primatol 46:35–62
Struhsaker TW (1975) The red colobus monkey. Chicago University Press, Chicago
Tenaza RR (1975) Territory and monogamy among Kloss’ gibbons (Hylobates klossii) in Siberut Island, Indonesia. Folia Primatol 24:60–80
Teng L, Liu Z, Song YL, Zeng Z (2004) Forage and bed sites characteristics of Indian muntjac (Muntiacus muntiak) in Hainan Island, China. Ecol Res 19:675–681
van Schaik CP, van Hooff J (1983) On the ultimate causes of primate social systems. Behaviour 85:91–117
van Schaik CP, van Amerongen A, van Noordwijk MA (1996) Riverine refuging by wild Sumatran long-tailed macaques. In: Fa JA, Lindburg DG (eds) Evolution and ecology of macaque societies. Cambridge University Press, Cambridge, pp 160–181
Yeager CP (1989) Feeding ecology of the proboscis monkey (Nasalis larvatus). Int J Primatol 10:497–530
Yeager CP (1991a) Proboscis monkey (Nasalis larvatus) social organization: intergroup patterns of association. Am J Primatol 23:73–86
Yeager CP (1991b) Possible antipredator behavior associated with river crossings by proboscis monkeys (Nasalis larvatus). Am J Primatol 24:61–66
Yeager CP (1993) Ecological constraints on intergroup associations in the proboscis monkey (Nasalis larvatus). Trop Biodivers 1:89–100
Acknowledgments
We express our sincere thanks to the Economic Planning Unit of the Malaysian Government, especially M. Bt. A. Manan and G. Vu, for permission to conduct our research in Malaysia. We particularly thank the staff of the Sabah Wildlife Department for their permission to conduct our research in Lower Kinabatangan Wildlife Sanctuary. We also thank the staff of Forestry Department, Sabah, especially Haji H. Tukiman, L. Ruki, J. Sugau, J. T. Pereira, and P. Miun for arranging our base camp and supporting the identification of all plants species. We are grateful to I. Lackman-Ancrenaz, M. Ancrenaz, Z. A. Jaffer, A. B. Etin, and all members of the Kinabatangan Orangutan Conservation Project for their help. We thank our research assistants, especially A. B. Arsih and M. S. B. A. Karim, for their support. Advice and support has been generously supplied by K. Watanabe, J. Yamagiwa, T. Iwakuma, N. Agetsuma and G. Hanya. We are certainly indebted to Y. Matsuda who helped us overcome many difficulties throughout our study. Finally, we are grateful to T. Sekijima and anonymous reviewers for their fruitful comments that improved this manuscript. This study includes data collection and analysis compiled using protocols approved by the Economic Planning Unit of the Malaysian Government and the Sabah Wildlife Department of Malaysia, and they adhered to the legal requirements of Malaysia.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Matsuda, I., Tuuga, A. & Higashi, S. Effects of water level on sleeping-site selection and inter-group association in proboscis monkeys: why do they sleep alone inland on flooded days?. Ecol Res 25, 475–482 (2010). https://doi.org/10.1007/s11284-009-0677-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-009-0677-3