Abstract
Diverse populations of invertebrates constitute the food web in detritus layers of a forest floor. Heterogeneity in trophic interactions within such a species-rich community food web may affect the dynamic properties of biological communities such as stability. To examine the vertical heterogeneity in trophic interactions among invertebrates in litter and humus layers, we studied differences in species composition and variations in carbon and nitrogen stable-isotope ratios (δ13C and δ15N) using community-wide metrics of the forest floors of temperate broadleaf forests in Japan. The species composition differed between the two layers, and the invertebrates in the litter layer were generally larger than those in the humus layer, suggesting that these layers harbored separate food webs based on different basal resources. However, the δ13C of invertebrates, an indicator of differences in the basal resources of community food webs, did not provide evidence for separate food webs between layers even though plant-derived organic matter showed differences in stable-isotope ratios according to decomposition state. The minimum δ15N of invertebrates also did not differ between layers, suggesting sharing of food by detritivores from the two layers at lower trophic levels. The maximum and range of δ15N were greater in the humus layer, suggesting more trophic transfers (probably involving microorganisms) than in the litter layer and providing circumstantial evidence for weak trophic interactions between layers at higher trophic levels. Thus, the invertebrate community food web was not clearly compartmentalized between the detrital layers but still showed a conspicuous spatial (vertical) heterogeneity in trophic interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the structural heterogeneity within a community food web is important for predicting the dynamic properties of the community, such as a stability of species richness and composition (Paine 1980; McCann et al. 1998). May (1972, 1973) predicted that a community should be more stable when trophic interactions (consumer-resource interactions) are arranged into blocks or compartments rather than a random pattern in the food web. Subsequent studies showed that the interactions between food chains and/or the coupling of distinct energy channels by mobile consumers are important for the stability of the community (Pimm 1979; Post et al. 2000; Rooney et al. 2006). Thus, it is important to examine substructuring, such as compartmentalization, of a food web (Pimm and Lawton 1980; Raffaelli and Hall 1992). The causal factors of the compartmentalized structure of a food web may vary with the scale of the species assemblage. Within the web of a functional group, compartments can result from high-frequency interactions between particular pairs of species (Fonseca and Ganade 1996; Dicks et al. 2002; Van Veen et al. 2008). Within a whole community, the web may be compartmentalized due to spatial heterogeneity within an environment, such as the division into adjacent freshwater and terrestrial habitats (Pimm and Lawton 1980). Compartmentalization may also occur in association with differences in microhabitats such as between pelagic and benthic layers of an aquatic system (Krause et al. 2003).
The forest floor may host a food web with a compartmentalized structure. Large amounts of plant-derived organic matter accumulate, forming a stratified structure within the forest floor with a coarse litter layer and a finer humus layer resulting from decomposition in the food web (Moore et al. 1988, Hättenschwiler et al. 2005). Such stratified microhabitats may cause compartmentalization of the forest floor food web. Setälä and Aarnio (2002) compared food webs between the litter and humus layers using 15N-labeling and suggested that small-sized decomposers tend to be restricted to the humus layer while larger decomposers exploit the overlying litter layer (see also Caner et al. 2004).
Diverse invertebrate species of various sizes inhabit a forest floor and are involved in the detrital food web (Petersen and Luxton 1982). A detrital food web is often depicted as different energy channels unified by a single group of top predators in the humus layer (e.g., De Ruiter et al. 1995; Scheu 2002; Rooney et al. 2006). The ecological roles of microdecomposers (e.g., fungi and bacteria) and microarthropods (e.g., springtails and mites) in the humus layer have been studied extensively (Ingham et al. 1989; De Ruiter et al. 1998; Schröter et al. 2003). However, the roles of macroinvertebrates moving throughout the litter layer, particularly predators such as ground beetles, ants, and spiders, are not well known because they are mobile and easily omitted from sampling. Therefore, for a comprehensive understanding of forest-floor food webs, it is necessary to identify the trophic positions of these higher-order consumers more precisely.
Most studies on food webs have been based on networks of trophic interactions among species, but it is usually difficult to reconstruct a network using feeding observations or gut-content analysis for a very large number of species. Stable-isotope analyses using carbon and nitrogen stable isotopes (13C and 15N) provide convenient tools with which to study complex trophic interactions within a food web (Peterson and Fry 1987; Tiunov 2007). These have been used extensively to examine the food webs of forest floors (Ponsard and Arditi 2000; Scheu and Falca 2000; Scheu 2002; Halaj et al. 2005). The carbon stable-isotope ratio (δ13C) is only slightly enriched in the process of consumption and assimilation, whereas the nitrogen stable-isotope ratio (δ15N) of a consumer’s tissues is more enriched than that of its diet (Vander Zanden and Rasmussen 2001; Post 2002). Therefore, the δ13C of consumers indicates the basal resource of a food chain, while δ15N indicates the trophic level of consumers, and these two stable-isotope ratios in combination can be used to simultaneously determine the trophic position of a large number of component species in a food web. Recently, community-wide metrics have been proposed to evaluate the trophic structure of a food web, which include multiple functional groups, based on the positional variation of consumers in a δ13C-δ15N bi-plot (Layman et al. 2007). Such metrics can be used to compare among community food webs of different systems or different sites, although empirical studies remain scarce.
We aimed to determine the heterogeneity of the invertebrate community food web structure in the floors of temperate forests in Japan. Focusing on the layered structure, we discriminated between invertebrates living on and within the litter layer (ground invertebrates) and those living within the humus layer (soil invertebrates) and compared the variation of carbon and nitrogen stable-isotope ratios between ground and soil invertebrates using community-wide metrics of the stable-isotope ratios to detect heterogeneities of food-web structure between the litter and humus layers. The stable-isotope ratios of plant-derived organic matter (basal resource) increase with soil depth due to progressive decomposition (Balesdent et al. 1993; Högberg 1997; Ehleringer et al. 2000; Ponsard and Arditi 2000; Billing and Richter 2006), and these increases are reflected in the stable-isotope ratios of the detritivores (Tayasu et al. 1997; Schneider et al. 2004; Uchida et al. 2004; Chahartaghi et al. 2005; Hishi et al. 2007; Hyodo et al. 2008). Variations of isotope ratios in consumers at lower trophic levels (lower-order consumers) such as herbivores and detritivores may further be reflected in those at higher trophic levels (higher-order consumers) such as carnivores. Thus, the difference of decomposition states in basal resources of a food chain may be indicated not only by δ13C values of consumers but also by their δ15N values in detrital food web (Scheu 2002). We expected that if the invertebrate community food web was compartmentalized between layers, corresponding differences would appear in the stable isotope ratios, which would be summarized by community-wide metrics.
Materials and methods
Sampling
The study sites were secondary forests in the warm temperate zone with evergreen and deciduous broadleaf trees, located in the eastern part of the Kyoto Basin, central Japan: Yoshida-yama (35°01′47′N, 135°47′14′E; altitude 121 m) and Uryu-yama (35°02′21′N, 135°48′09′E; altitude 301 m). Yoshida-yama (hereafter Yoshida) is located on a small hill, and Uryu-yama (hereafter Uryu) is on a mountainside. The forest floors of both sites were covered with mull-type humic soil (Ponge 2003), consisting of litter and humus layers. The litter layer exhibited little segmentation due to rapid decomposition. Sampling quadrats of 400 m2 (20 × 20 m and 10 × 40 m) were established at Yoshida and Uryu.
Invertebrate sampling was conducted every 2 weeks from May to July (summer) and from October to November (autumn), 2006. We defined ground invertebrates as those occurring in the litter layer and soil invertebrates as those occurring in the humic soil layer up to 3 cm depth. We used pitfall traps (7 cm diameter by 8 cm deep plastic cups) and our hands to collect ground invertebrates and Tullgren apparatuses to collect soil invertebrates. An array of 25 pitfall traps was placed so that the rims were level with the soil surface in each quadrat at noon and were collected after 24 h. Each cup contained 50 ml of 20% ethanol to prevent trapped invertebrates from rotting or being eaten by carnivorous species until the collection. Together with the pitfall sampling, the collection by hand of ground invertebrates and the Tullgren apparatuses sampling of soil invertebrates were performed in 16 subquadrats (50 × 50 cm) placed arbitrarily within each quadrat. In each subquadrat, the ground invertebrates (Megascolecidae, lepidopteran larvae, and dipteran larvae) were collected by hand while carefully removing litter. Thereafter, to collect soil invertebrates, surface humus (25 × 25 × 3 cm deep) was brought back to the laboratory and placed in the Tullgren apparatuses. Samples were placed on a 3-mm mesh sieve and heated for 48 h by electric light bulbs; animals that fell through the sieve were immediately fixed in 70% ethanol to prevent desiccation and rotting. These collected invertebrates were identified by species as accurately as possible and kept at −30°C for stable-isotope analysis. Although ethanol preservation may alter δ13C in animal tissue by removing lipid content, any such changes are small and insignificant in soil animals (Sticht et al. 2006).
Plant-derived organic matter is the basal resource of forest-floor food webs. Leaves and litter (ground basal resources) and humic soil (soil basal resource) were collected from the sampling quadrats at both Yoshida and Uryu. Five samples of each type of organic matter were prepared for stable isotope analysis per monthly sampling. Leaves were collected in May and June, and litter and humus were collected in May, June, July, and October. The leaf species sampled as foods of lepidopteran larvae were those grazed by lepidopteran larvae; individual leaves were treated as separate samples. Litter was collected from the litter layer and prepared as a bulk sample for stable isotope analysis. Humus was collected from the external soil layer in which plant tissue was completely broken down, and a cupful (9 cm in diameter, 4 cm deep) of humus was treated as a separate sample. These samples were dried at 60°C for 24 h in the laboratory immediately after collection. After desiccation, the leaf and litter samples were cut into fine pieces with scissors, and the humus was filtered through a 1-mm mesh sieve. Samples were kept at −30°C until stable-isotope analysis.
Stable-isotope analysis
We determined the carbon and nitrogen stable-isotope ratios (δ13C and δ15N) for all invertebrate species for which we had collected approximately 1 mg dry weight (the measurable amount) at the two sites. The sample size for stable isotope measurements per species was 1–15 (see Electronic supplementary material). All invertebrate and organic matter samples were dried at 60°C for 48 h prior to stable-isotope analysis. Individual large invertebrates (>3 mm body length; body length was defined as maximum distance between the distal ends of a body) and a number of small invertebrates (<3 mm body length) were ground into a fine powder using an agate mortar and pestle. Small invertebrate taxa, of which we collected only a few individuals, were used directly for analysis. When both larvae and adults were sampled for a species, only adults were analyzed for hemimetabolous insects, whereas both larvae and adult holometabolous insects were analyzed separately (e.g., Coleoptera, Lepidoptera, and Diptera). For social insects in the groups Formicidae and Isoptera, only workers were analyzed. For Oligochaeta (Enchytraeidae and Megascolecidae) and lepidopteran larvae (Geometridae and Noctuidae), the gut contents were surgically removed before desiccation. Samples of approximately 1 and 4 mg of invertebrate and organic matter, respectively, were placed in a tin cup for combustion.
The δ13C and δ15N values were measured using a mass spectrometer (Finnigan MAT Delta S, Bremen, Germany) coupled with an elemental analyzer (Fisons EA1108, Milan, Italy) at the Center for Ecological Research, Kyoto University. Their values are expressed as the per mil (‰) deviation from international standards, calculated as follows:
where R for δ13C is 13C/12C and for δ15N is 15N/14N. The international standards were Pee Dee Belemnite for δ13C and atmospheric nitrogen for δ15N. DL-Alanine (δ13C: −23.47‰, δ15N: −1.66‰) was also analyzed as a working standard. The analytical precision (standard error of the values measurements for the working standard) was ±0.2‰ for δ13C and ±0.2‰ for δ15N.
Comparison of community and trophic structures
To compare the species compositions between sites, seasons, and microhabitats, non-metric multidimensional scaling (NMDS; Clarke 1993) was performed for the presence/absence data of trophic species using MASS packages (sammon) of R version 2.5.1. Adults and larvae of the same species were treated as separate trophic species if appropriate (Briand and Cohen 1984).
Differences in invertebrate communities and the trophic structures between sites (Yoshida and Uryu), seasons (summer and autumn), and microhabitat (litter and humus layer) were examined in terms of ten metrics with species richness and stable-isotope ratios. Invertebrate species richness was evaluated as the number of trophic species per microhabitat. Layman et al. (2007) proposed several metrics of trophic diversity applicable to bi-plot data with mean δ13C and δ15N values of each trophic species in a community: δ15N range (NR), δ13C range (CR) and total area (TA). NR and CR are the distances between the two species with the most enriched and most depleted δ15N and δ13C values, respectively; TA is the convex hull area encompassed by all species in the δ13C-δ15N bi-plot space (see Layman et al. 2007 for details). We also compared the mean, maximum, and minimum values of δ13C and δ15N (Cmean, Cmax, Cmin, Nmean, Nmax, and Nmin) of the component communities to evaluate their relative positions on the bi-plot. To examine the effects of microhabitat, site, and season on the ten community-wide metrics, a three-way analysis of variance (ANOVA) was performed using JMP version 5 (SAS Institute Inc., Cary, NC, USA). In addition, the overlap of trophic niche of invertebrate community between microhabitats was assessed by the percentage of overlapping area in the total area of convex hulls of the two microhabitats, i.e., 100 × [overlapping area of convex hulls]/([TA of litter layer] + [TA of humus layer] − [overlapping area of convex hulls]). If the food web was compartmentalized between litter and humus layers, the values of community-wide metrics would differ between two layers and the overlap of trophic niche between two layers would be small.
Results
In total, 212 trophic species (187 species, including 124 and 147 species from Yoshida and Uryu, respectively) were collected from both sites and analyzed for stable-isotope analysis (see ESM for detail). The species composition differed greatly between the litter and humus layers. NMDS ordination showed that species compositions of invertebrate communities were clearly distinguished between litter and humus layers by axis 1 (Fig. 1). Only 17 trophic species (8.0%) were collected from both layers. Of these, we estimated three ant species to be foragers in both layers, and the other species were classified as either ground or soil invertebrates according to the layer in which their respective adults were collected, because adults were used in the stable-isotope analysis. For all trophic species, body length (log10-transformed) was significantly greater for ground invertebrates than soil invertebrates, although body length did not differ between seasons or between sites (three-way ANOVA: microhabitat, F 1,399 = 274.0, p < 0.0001; season, F 1,399 = 0.94, p = 0.33; site, F 1,399 = 1.0, p = 0.32; interactions among three factors were excluded because they were not significant; necrophagous and coprophagous consumers were excluded as is mentioned below; ESM).
Non-metric multidimensional scaling ordination for species compositions of eight invertebrate communities in forest floor. Final stress of this ordination was 0.043. Invertebrate communities are distinguished by site (circle Yoshida, square Uryu), season (open summer, closed autumn) and microhabitat (L litter layer, H humus layer)
The δ13C and δ15N values of plant-derived organic matter differed significantly among three types of organic matter (leaves, litter, and humus) and did not change by seasons or site (three-way ANOVA for δ13C: type, F 2,94 = 96.2, p < 0.0001; season, F 1,94 = 0.005, p = 0.95; site, F 1,94 = 1.7, p = 0.20: three-way ANOVA for δ15N: type, F 2,94 = 43.5, p < 0.0001; season, F 1,94 = 2.3, p = 0.13; site, F 1,94 = 1.4, p = 0.24; interactions among three factors were excluded because they were not significant: Figs 2 and 3). The stable-isotope ratios increased progressively according to the decomposition process (i.e., in the order of leaves, litter, and humus). The least square means (±SE) of leaves, litter, and humus were −31.0 ± 0.2, −29.6 ± 0.1, and −28.2 ± 0.1‰, respectively, for δ13C, and −4.4 ± 0.2, −4.0 ± 0.1, and −2.5 ± 0.1‰, respectively, for δ15N.
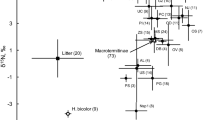
δ13C and δ15N values of invertebrate communities at Yoshida in summer (a ground invertebrates collected from the litter layer; b soil invertebrates collected from the humus layer) and in autumn (c ground invertebrates; d soil invertebrates). Each point represents mean δ13C and mean δ15N of each trophic species. Filled diamonds, squares, and triangles represent Carabidae, Formicidae, and carnivores (Chilopoda, Araneae, Opiliones, Pseudoscorpiones, and Gamasida), respectively. Open circles represent other species. Species of Formicidae collected from both layers are represented by half-filled squares
δ13C and δ15N values of invertebrate communities at Uryu in summer (a ground invertebrates, b soil invertebrates) and in autumn (c ground invertebrates, d soil invertebrates). See legend of Fig. 1 for explanation
Figures 2 and 3 show the bi-plots of δ13C and δ15N for trophic species of ground and soil invertebrate communities in summer and autumn at Yoshida and Uryu. Not shown are species of Nicrophorinae, Scarabaeidae (except Melolonthinae), Histeridae, and Staphylinidae (except soil species) collected in summer, most of which had much higher δ15N values than carnivores (4.1–13.5‰ at Yoshida; 2.6–11.0‰ at Uryu; see ESM for details). These were considered necrophagous and coprophagous consumers that scavenge vertebrate carcasses or excrement (Ikeda et al. 2007) and were excluded from the statistical analyses.
Of the ten community-wide metrics of food-web structure by site, season, and microhabitat, only the number of trophic species was significantly different between sites (Table 1), and the isotopic profile of invertebrate communities did not differ between sites. The number of trophic species, NR, and TA decreased significantly from summer to autumn, indicating a seasonal decrease in trophic diversity (Table 1). The number of trophic species in summer was approximately twice that in autumn at both sites.
The microhabitat was the main factor responsible for heterogeneities of food web structure. The number of trophic species, NR, Nmean, Nmax, Cmean and TA differed significantly between litter and humus layers, although the overlap of trophic niche as indicated by the proportion of overlap between convex hulls was large (Table 1). The difference in Nmean and Cmean indicated that soil invertebrates had higher overall δ13C and δ15N values than ground invertebrates. Trophic diversity was higher in soil invertebrates than in ground invertebrates as was indicated by the larger number of trophic species and the higher NR and TA values in soil invertebrates than in ground invertebrates (Table 1).
The difference in trophic diversity (NR and TA) between litter and humus layers was attributed to the difference in the higher trophic level, because Nmax was significantly larger in soil than ground invertebrates, whereas Nmin did not differ between microhabitats (Table 1, Figs. 2 and 3). Based on δ15N values, Carabidae, Formicidae, and carnivores (mainly Araneae) were higher-order consumers in the litter layer, although some ant species (Formicidae: Camponotus) exhibited low δ15N values (approximately −1‰). In the humus layer, the representative higher-order consumers were Formicidae and carnivores (mainly Chilopoda and Gamasida). For these consumers at higher trophic levels, soil invertebrates tended to have higher δ15N values than ground species (Figs. 2 and 3). The most enriched δ15N values were approximately 3‰ for most of ground higher-order consumers (mainly Carabidae and Formicidae), although some ground carnivores (Araneae) showed higher δ15N values similar to soil higher-order consumers. In contrast, the most enriched δ15N values were approximately 6‰ for soil higher-order consumers. On the other hand, lower-order consumers consisted of lepidopteran larvae, Magascolecidae, Diplopoda, and Crustacea in the litter layer, and Diplopoda, Crustacea, and a portion of Collembola in the humus layer. No clear difference in δ15N between litter and humus layers was apparent for these lower-order consumers at lower trophic levels (Figs. 2 and 3).
Discussion
We found that species composition differed with only a slight overlap between the ground and soil invertebrate communities inhabiting litter and humus layers (Fig. 1). The humus layer harbored more species than the litter layer (Table 1), but we need a caution about the effect of different sampling methods between the layers. The body sizes of ground invertebrates were generally larger than those of soil invertebrates. Body size can restrict movement between two layers with differing detrital particle sizes (Setälä and Aarnio 2002). Thus, the faunal and body size difference implied that the invertebrate food web is compartmentalized between litter and humus layers. However, the interpretation of the differences found in stable-isotopic values between layers is not straightforward (Table 1).
We examined the effects of site, season, and microhabitat (detrital layer) for nine metrics with stable-isotopic values (Table 1). Site exhibited no effect, whereas season had a significant effect only on NR and TA, probably due to the seasonal change in the number of trophic species. Microhabitat exhibited significant effects on five metrics: NR, Nmean, Nmax, Cmean and TA. In general, the δ13C metrics are indicators of the differences in the basal resources of a community food web. However, the difference in mean δ13C (Cmean) was only 0.1–0.7‰ between microhabitats, and there was no difference in the range of δ13C (CR, Cmin, and Cmax). Therefore, contrary to our expectation, no differences in basal resources that were used by consumers could be detected between litter and humus layers based on δ13C value. This lack of clear difference could in part be due to small differences in the δ13C values among types of plant organic matter. In addition, detritivores of the two layers could utilize the same detrital resource, as suggested by the observation that detritivores (Magascolecidae, Diplopoda, and Crustacea) in the litter layer exhibited similar δ13C values to those of small lower-order consumers (Diplopoda, Crustacea, and a portion of Collembola) in the humus layer. Conversely, given that herbivores (mainly lepidopteran larvae) with lower δ13C values than detritivores occurred in the litter layer, the small difference in Cmean between the layers may be a result of the presence of herbivores and their predators in the litter layer.
Interestingly, the δ13C fractionation from basal resources to lower-order consumers on the forest floor, approximately 2–4‰, was larger than the empirical value of δ13C fractionation, approximately 0–1‰ (Vander Zanden and Rasmussen 2001; Post 2002). The large fractionation in δ13C was reported in detrital food webs (Tayasu et al. 1997; Uchida et al. 2004; Hishi et al. 2007). In terrestrial ecosystems, the δ13C fractionation from plant-derived organic matter to herbivores and detritivores might be larger than that from herbivores and detritivores to carnivores due to differences in chemical composition of food and/or assimilation rate.
With respect to δ15N, there was a clear difference in Nmax with associated differences in NR and TA (trophic diversity indices) between the layers. Nmax and Nmean were larger in the humus layer than in the litter layer, and this was due to a difference in food resources of higher-order consumers (Figs. 2 and 3). In contrast, Nmin did not differ between microhabitats, providing further evidence for the sharing of basal resource by ground and soil lower-order consumers. Therefore, the wide range of δ15N in the humus layer suggested that there were a larger number of trophic transfers (trophic levels) than in the litter layer. It is possible that the involvement of microorganisms in the detrital chain contributed to an increase in the δ15N values in the humus layer.
Some non-predatory species in the humus layer showed very high δ15N values like the higher-order consumers (Figs. 2 and 3). For example, the highest δ15N value in Collembola was 3.7–6.7‰ higher than that of humus. Similarly, Symphyla exhibited 4.3–5.9‰ higher δ15N values than humus. These enrichment factor values are much higher than the ordinary isotopic enrichment factor for δ15N, which is approximately 3‰ per trophic level (Minagawa and Wada 1984; Vander Zanden and Rasmussen 2001; Post 2002; McCutchan et al. 2003; Vanderklift and Ponsard 2003). Therefore, the above consumers might be secondary decomposers (see Scheu and Falca 2000) that feed selectively on microdecomposers such as fungi, which would have higher δ15N values than substrate feeders (Kohzu et al. 1999; Dijkstra et al. 2006; Hart et al. 2006). By consuming these secondary decomposers, the δ15N values of soil higher-order consumers (i.e., Formicidae and Gamasida) would become higher than those of ground higher-order consumers such as Carabidae and Formicidae, which would not consume secondary decomposers derived from the humus layer. A part of ground carnivores of Araneae showed very high δ15N values and might partly consume secondary decomposers inhabiting the humus layer. Although microdecomposers also inhabit the litter layer (Scheu and Schaefer 1998), and hence secondary decomposers as well, our results with the δ15N profile in the ground invertebrates suggest that the trophic transfer through secondary decomposers in the litter layer was less important than that in the humus layer. However, further study is needed to examine the importance of secondary decomposers in the litter layer, because we did not use Tullgren apparatuses for the ground invertebrates and our sampling might not be sufficient.
The difference in δ15N of higher-order consumers between the litter and humus layers also suggests that the ground and soil invertebrate food web was segregated at higher trophic levels, because if higher-order consumers (e.g., predators) could consume prey in opposite layers, isotopic differences would disappear. Given that ground higher-order consumers are generally large, they may not be able to capture small prey in the humus layer. Thus, one factor contributing to vertical heterogeneity of the forest floor food web might be the restriction of vertical movements by large body sizes (Setälä and Aarnio 2002). In addition, small soil higher-order consumers likely cannot capture large prey animals in the litter layer. If this segregation is true, the detrital food-web structure of invertebrate community is more complex than previously appreciated, since it is commonly assumed that top predators couple different energy channels starting from detritus (De Ruiter et al. 1995; Scheu 2002; Rooney et al. 2006). In the layered habitat, each layer was thought to have a distinct top predator group, and the coupling of energy channels may be effective within layers but not between layers. For example, different channels are coupled by carabid beetles feeding on invertebrates from both grazing and detrital chains in the litter layer (Sota 1985) and by soil higher-order consumers feeding on organisms of various functional groups in the humus layer (De Ruiter et al. 1998). However, our study did not involve large animals (e.g., mammals and birds), which feed on the ground at the study sites. These animals might feed relatively large soil invertebrates (e.g., insect larvae and Chilopoda) and couple distinct energy channels derived from the litter and humus layers.
In conclusion, our study using stable-isotope analysis did not clearly show that the two layers in the temperate forest floors harbor distinct invertebrate community food webs based on the organic matter of different decomposition states. Nevertheless, we did find heterogeneity in trophic structure associated with differences in species composition and δ15N values of invertebrates between the layers. This finding may be useful for future analyses of the dynamic properties of invertebrate communities in detrital food webs. In detrital food webs, trophic positions of organisms and their functions in decomposition process may be evaluated by δ15N more properly than by δ13C. The use of community-wide metrics with stable isotope data in comparing food web structure is still in its infancy, and further accumulation of data will facilitate more substantial analyses of community food web variations.
References
Balesdent J, Girardin C, Mariotti A (1993) Site-related 13C of tree leaves and soil organic matter in a temperate forest. Ecology 74:1713–1721. doi:10.2307/1939930
Billing SA, Richter DD (2006) Changes in stable isotope signatures of soil nitrogen and carbon during 40 years of forest development. Oecologia 148:325–333. doi:10.1007/s00442-006-0366-7
Briand F, Cohen JE (1984) Community food webs have scale-invariant structure. Nature 307:264–267. doi:10.1038/307264a0
Caner L, Zeller B, Dambrine E, Ponge JF, Chauvat M, Lianque C (2004) Origin of the nitrogen assimilated by soil fauna living in decomposing beech litter. Soil Biol Biochem 36:1861–1872. doi:10.1016/j.soilbio.2004.05.007
Chahartaghi M, Langel R, Scheu S, Ruess L (2005) Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol Biochem 37:1718–1725. doi:10.1016/j.soilbio.2005.02.006
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
De Ruiter PC, Neutel AM, Moore JC (1995) Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269:1257–1260. doi:10.1126/science.269.5228.1257
De Ruiter PC, Neutel AM, Moore JC (1998) Biodiversity in soil ecosystems: the role of energy flow and community stability. Appl Soil Ecol 10:217–228. doi:10.1016/S0929-1393(98)00121-8
Dicks LV, Corbet SA, Pywell RF (2002) Compartmentalization in plant-insect flower visitor webs. J Anim Ecol 71:32–43. doi:10.1046/j.0021-8790.2001.00572.x
Dijkstra P, Ishizu A, Doucett R, Hart SC, Schwartz E, Menyailo OV, Hungate BA (2006) 13C and 15N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–3266. doi:10.1016/j.soilbio.2006.04.005
Ehleringer JR, Buchmann N, Flanagan LB (2000) Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10:412–422. doi:10.1890/1051-0761(2000)010[0412:CIRIBC]2.0.CO;2
Fonseca CR, Ganade G (1996) Asymmetries, compartments and null interactions in an Amazonian ant-plant community. J Anim Ecol 65:339–347. doi:10.2307/5880
Halaj J, Peck RW, Niwa CG (2005) Trophic structure of a macroarthropod litter food web in managed coniferous forest stands: a stable isotope analysis with δ15N and δ13C. Pedobiologia (Jena) 49:109–118. doi:10.1016/j.pedobi.2004.09.002
Hart SC, Gehring CA, Selmants PC, Deckert RJ (2006) Carbon and nitrogen elemental and isotopic patterns in macrofungal sporocarps and trees in semiarid forests of the south-western USA. Funct Ecol 20:42–51. doi:10.1111/j.1365-2435.2005.01058.x
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218. doi:10.1146/annurev.ecolsys.36.112904.151932
Hishi T, Hyodo F, Saitoh S, Takeda H (2007) The feeding habits of collembolan along decomposition gradients using stable carbon and nitrogen isotope analyses. Soil Biol Biochem 39:1820–1823. doi:10.1016/j.soilbio.2007.01.028
Högberg P (1997) Tansley review No. 95 15N natural abundance in soil-plant systems. New Phytol 137:179–203. doi:10.1046/j.1469-8137.1997.00808.x
Hyodo F, Tayasu I, Konaté S, Tondoh JE, Lavelle P, Wada E (2008) Gradual enrichment of 15N with humification of diets in a below-ground food web: relationship between 15Nand diet age determined using 14C. Funct Ecol 22:516–522. doi:10.1111/j.1365-2435.2008.01386.x
Ikeda H, Kubota K, Kagaya T, Abe T (2007) Flight capabilities and feeding habits of silphine beetles: are flightless species really “carrion beetles”? Ecol Res 22:237–241. doi:10.1007/s11284-006-0012-1
Ingham ER, Coleman DC, Moore JC (1989) An analysis of food-web structure and function in a shortgrass prairie, a mountain meadow, and a lodgepole pine forest. Biol Fertil Soils 8:29–37. doi:10.1007/BF00260513
Kohzu A, Yoshioka T, Ando T, Takahashi M, Koba K, Wada E (1999) Natural 13C and 15N abundance of field-collected fungi and their ecological implications. New Phytol 144:323–330. doi:10.1046/j.1469-8137.1999.00508.x
Krause AE, Frank KA, Mason DM, Ulanowicz RE, Taylor WW (2003) Compartments revealed in food-web structure. Nature 426:282–285. doi:10.1038/nature02115
Layman CA, Arrington DA, Montaña CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48. doi:10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2
May RM (1972) Will a large complex system be stable? Nature 238:413–414. doi:10.1038/238413a0
May RM (1973) Stability and complexity in model ecosystems. Princeton University Press, Princeton
McCann K, Hastings A, Huxel GR (1998) Weak trophic interactions and the balance of nature. Nature 395:794–798. doi:10.1038/27427
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102:378–390. doi:10.1034/j.1600-0706.2003.12098.x
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140. doi:10.1016/0016-7037(84)90204-7
Moore JC, Walter DE, Hunt HW (1988) Arthropod regulation of micro- and mesobiota in below-ground detrital food webs. Annu Rev Entomol 33:419–439
Paine RT (1980) Food webs: linkage, interaction strength and community infrastructure. J Anim Ecol 49:667–685. doi:10.2307/4220
Petersen H, Luxton M (1982) A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 39:288–388. doi:10.2307/3544689
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320. doi:10.1146/annurev.es.18.110187.001453
Pimm SL (1979) The structure of food webs. Theor Popul Biol 16:144–158. doi:10.1016/0040-5809(79)90010-8
Pimm SL, Lawton JH (1980) Are food webs divided into compartments? J Anim Ecol 49:879–898. doi:10.2307/4233
Ponge JF (2003) Humus forms in terrestrial ecosystems: a framework to biodiversity. Soil Biol Biochem 35:935–945. doi:10.1016/S0038-0717(03)00149-4
Ponsard S, Arditi R (2000) What can stable isotopes (delta N-15 and delta C-13) tell about the food web of soil macro-invertebrates? Ecology 81:852–864
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM, Conners ME, Goldberg DS (2000) Prey preference by a top predator and the stability of linked food chains. Ecology 81:8–14
Raffaelli D, Hall SJ (1992) Compartments and predation in an estuarine food web. J Anim Ecol 61:551–560. doi:10.2307/5610
Rooney N, McCann K, Gellner G, Moore JC (2006) Structural asymmetry and the stability of diverse food webs. Nature 442:265–269. doi:10.1038/nature04887
Scheu S (2002) The soil food web: structure and perspectives. Eur J Soil Biol 38:11–20. doi:10.1016/S1164-5563(01)01117-7
Scheu S, Falca M (2000) The soil food web of two beech forests (Fagus sylvatica) of contrasting humus type: stable isotope analysis of a macro- and mesofauna-dominated community. Oecologia 123:285–296. doi:10.1007/s004420051015
Scheu S, Schaefer M (1998) Bottom-up control of the soil macrofauna community in a beechwood on limestone: manipulation of food resources. Ecology 79:1573–1585
Schneider K, Migge S, Norton RA, Scheu S, Langel R, Reineking A, Maraun M (2004) Trophic niche differentiation in soil microarthropods (Oribatida, Acari): evidence from stable isotope ratios (15N/14N). Soil Biol Biochem 36:1769–1774. doi:10.1016/j.soilbio.2004.04.033
Schröter D, Wolters V, De Ruiter PC (2003) C and N mineralisation in the decomposer food webs of a European forest transect. Oikos 102:294–308. doi:10.1034/j.1600-0579.2003.12064.x
Setälä H, Aarnio T (2002) Vertical stratification and trophic interactions among organisms of a soil decomposer food web—a field experiment using 15N as a tool. Eur J Soil Biol 38:29–34. doi:10.1016/S1164-5563(01)01119-0
Sota T (1985) Activity patterns, diets and interspecific interactions of coexisting spring and autumn breeding carabids: Carabus yaconinus and Leptocarabus kumagaii (Coleoptera, Carabidae). Ecol Entomol 10:315–324. doi:10.1111/j.1365-2311.1985.tb00728.x
Sticht C, Schrader S, Giesemann A (2006) Influence of chemical agents commonly used for soil fauna investigations on the stable C-isotopic signature of soil animals. Eur J Soil Biol 42:S326–S330. doi:10.1016/j.ejsobi.2006.07.009
Tayasu I, Abe T, Eggleton P, Bignell DE (1997) Nitrogen and carbon isotope ratios in termites: an indicator of trophic habit along the gradient from wood-feeding to soil-feeding. Ecol Entomol 22:343–351. doi:10.1046/j.1365-2311.1997.00070.x
Tiunov AV (2007) Stable isotopes of carbon and nitrogen in soil ecological studies. Biol Bull 34:395–407. doi:10.1134/S1062359007040127
Uchida T, Kaneko N, Ito MT, Futagami K, Sasaki T, Sugimoto A (2004) Analysis of the feeding ecology of earthworms (Megascolecidae) in Japanese forests using gut content fractionation and δ15N and δ13C stable isotope natural abundances. Appl Soil Ecol 27:153–163. doi:10.1016/j.apsoil.2004.04.003
Van Veen FJF, Müller CB, Pell JK, Godfray HCJ (2008) Food web structure of three guilds of natural enemies: predators, parasitoids and pathogens of aphids. J Anim Ecol 77:191–200. doi:10.1111/j.1365-2656.2007.01325.x
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnol Oceanogr 46:2061–2066
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136:169–182. doi:10.1007/s00442-003-1270-z
Acknowledgments
We thank Y. Takami, Y. Takeuchi and H. Nakagawa for statistics, M. Yoshida, M. Ito, H. Takeda, S. Saito, T. Tanigaki, H. Nishi, S. Yamamoto, S. Gotou for taxonomy, C. Hori, T. Takeyama and N. Nagata for technical assistance, and D. Gustafson for English text assistance. We also thank Prof. M. Hori and the members of the Laboratory of Animal Ecology, Kyoto University, for advice and discussion. This research was supported in part by grants-in-aid for Biodiversity Research of 21st Century COE (A14) and Global COE Program “Formation of a Strategic Base for Biodiversity and Evolutionary Research: from Genome to Ecosystem” from the Ministry of Education, Culture, Sports and Technology, Japan, and a grant-in-aid from the Japan Society for the Promotion of Science (No. 2037011 to TS and No. 19681002 to IT).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Okuzaki, Y., Tayasu, I., Okuda, N. et al. Vertical heterogeneity of a forest floor invertebrate food web as indicated by stable-isotope analysis. Ecol Res 24, 1351–1359 (2009). https://doi.org/10.1007/s11284-009-0619-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-009-0619-0