Abstract
Large differences in leaf physiology and morphology between ontogenetic stages of a single woody species have often been observed. Far less attention, however, has been devoted to studying the ontogenetic changes observed in leaf phenology patterns, despite the relevance of leaf phenology in determining the leaf carbon balance and leaf and plant mortality. Leaf emergence patterns and leaf longevity were studied in the saplings and mature trees of the evergreen Quercus ilex and the deciduous Quercus faginea. Our aim here was to analyze and interpret the possible tree-age related differences in these leaf traits. Unlike the adults, in which only one flush of leaf growth was observed, several leaf cohorts were produced within each year in the saplings. Sapling leaves showed a lower mean duration than those of the adults. However, Q. faginea saplings exhibited large plasticity in leaf longevity, which was not seen in the case of Q. ilex. The differences in leaf emergence patterns and in leaf longevity between growth stages seemed to be related to differences in resource availability for leaf production and in leaf mass per unit area, respectively. We propose that the sequential leaf development in saplings may be an important mechanism enabling tree species to cope with resource limitation in the early stages of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large differences between ontogenetic stages of a single woody species have often been observed. Accordingly, characterizing a species and understanding its strategies and long-term responses to environmental factors requires careful study of such tree-age related changes. Differences among growth stages have been reported in different woody species as regards hydraulic conductance (Mencuccini and Grace 1996; Ryan and Yoder 1997; Hubbard et al. 1999) and water use patterns (Donovan and Ehleringer 1992; Cavender-Bares and Bazzaz 2000; Mediavilla and Escudero 2004), in leaf gas-exchange rates (Bond 2000; Day et al. 2001), and in different leaf traits related to differences in leaf physiology, such as leaf mass per unit area (LMA), leaf lamina thickness, and nitrogen concentrations (Ishida et al. 2005; England and Attiwill 2006; Juárez-López et al. 2008). Far less attention, however, has been devoted to studying the ontogenetic changes observed in other characteristics, such as leaf phenology patterns, despite the relevance of leaf phenology in determining the leaf carbon balance (Kikuzawa 1995; Gill et al. 1998) and leaf and plant mortality (Jones et al. 1994, 1997).
The few studies comparing leaf phenology patterns in different growth stages have reported differences in certain traits, such as the timing and duration of leaf emergence, with an earlier emergence in initial stages than in mature specimens of deciduous tree species (Gill et al. 1998; Seiwa 1999a, 1999b; Augspurger and Bartlett 2003). The earlier phenology of seedlings of deciduous species has been interpreted as a mechanism that allows them to avoid shade stress (Gill et al. 1998; Seiwa 1999a, 1999b; Augspurger and Bartlett 2003), and that provides advantage of ephemerally available resources—not only light but also water and nutrients (Jones et al. 1989), thus increasing carbon gain and the survival of the plants during their initial stages. However, many other aspects of leaf phenology may also be important in understanding the strategies used by a given species in different parts of its life cycle, such as the time needed to deploy leaf biomass and the mean leaf life span, which may be especially relevant in environments subjected to both cold and drought stress during the life of the leaf, as is usual in Mediterranean climates (Mitrakos 1980; Terradas and Savé 1992).
In the present work, leaf emergence patterns and leaf longevity were studied in the saplings and mature trees of two oak species with different leaf habits in a cold Mediterranean climate. The low winter temperatures and the summer drought typical of cold Mediterranean climates often constrain photosynthetic activity to a short period during late spring and early summer (Mediavilla and Escudero 2003a). Under these circumstances, a rapid deployment of photosynthetic material in spring can be crucial for plant fitness (Fenner 1998). We hypothesize that although the Mediterranean species should develop their leaf biomass as fast as possible at the start of the growing season in order to take advantage of most of this short favorable period, low resource availability for leaf production during the seedling stage could induce differences in leaf phenology between saplings and adult trees, which would in turn affect the final leaf carbon balance at the different stages. In previous studies (Juárez-López et al. 2008), we have reported lower N concentrations in leaves of saplings in comparison with their counterparts from the adult stage, because of the smaller root systems and the patterns of root distribution of the saplings. The differences in the availability of nitrogen between saplings and adults determined differences in the biochemical capacity and photosynthetic rates (Juárez-López et al. 2008). Our aim here was to analyze if the differences in resource availability also lead to tree-age related differences in leaf traits and leaf phenology and interpret these differences in terms of their implications for the competitive ability of our two oak species during the most critical part of the life cycle of woody species: i.e., the initial growth stages (Kozlowski et al. 1991).
Materials and methods
Study species and site
We studied Quercus ilex L. subsp ballota (Desf.) Samp. and Quercus faginea Lam., two species widely distributed throughout the interior of the Iberian Peninsula, where they are used extensively for reforestation programs. Both oak species are typical components of Mediterranean plant communities (Barbero et al. 1992), and their leaf phenology has been addressed by a few authors, although only at the adult tree stage (Escudero and Del Arco 1987; Castro-Díez and Montserrat-Martí 1998; Mediavilla and Escudero 2003b), being described Q. ilex as evergreen and Q. faginea as deciduous.
The two species were studied over 3 years (1997–1999) at two plots located close to the city of Salamanca, in central-western Spain, between latitudes 40°54′N and 40°58′N and longitudes between 6°07′W and 6°35′W. Both species were present at each of the selected plots.
One plot consisted of sparse populations (about 50 trees ha−1) of isolated mature trees over 100 years old with open pasture areas between the individuals. These savannah-like formations (“dehesas”) are very frequent in the western part of the Iberian Peninsula. Trunk diameter at 1.3 m height ranged from 20 to 60 cm and mean heights were 6–10 m. At the other plot, saplings, planted at a density of 1,000 ha−1, were studied. Planting was carried out during November 1994 with seedlings obtained from acorns collected in areas close to the stands studied. Before planting, the seedlings had been grown in a nursery during their first growth season, and hence our study was conducted during their fourth, fifth, and sixth year of life. During the last growth season, sapling height was around 50–70 cm, and maximum trunk diameter varied between 9 and 12 mm for the deciduous species and 6 and 8 mm for Q. ilex.
The climate (precipitation and temperature) and soil characteristics are fairly homogeneous over the whole study area (Table 1). The climate is cold Mediterranean, with cold wet winters and a period of summer drought that occurs each year. The soils, in all cases dystric Cambisols, are poor in organic matter and in nutrient contents, having a medium/low water holding capacity (Dorronsoro 1992) (Table 1). The soil N contents were analyzed according to the methods described in Bremner (1960) in two samples taken at each plot in 1997: one at the surface (excluding the forest floor) and the second at around 50 cm depth. Each sample was a composite of 12 subsamples collected at random.
Sampling methods and data analyses
In order to analyze the leaf emergence patterns and to calculate leaf life span in the adult specimens, sampling of terminal branches with leaves from different crown positions of each canopy was performed on ten specimens of each species selected at random over 3 years of study. The samples were immediately taken to the laboratory and the branches were separated into annual segments (shoots) of different age classes. Only one flush of leaf growth per year was observed in both species. Accordingly, all the leaves born in one particular year were considered to belong to the same age class. Sub-samples of 40–50 shoots per tree were used to calculate the average number of leaves per shoot on each date and for each age class. In the saplings, on each census date over the 3 years of study, we estimated the total number of leaves of each age class present in the crown in ten specimens selected for each species. Unlike the adults, several leaf cohorts were produced within each year, which were distinguished by marking them with colored wires, and leaf number analysis was performed separately for each flush. The censuses, both in saplings and in mature trees, were conducted at monthly intervals during most of the year, and at 10-day intervals during the leaf emergence period (from May to July). The mean number of leaves of a given age on each census date were used to elaborate life tables, which enabled us to calculate the mean life span for the leaves of each species according to standard methods (Begon and Mortimer 1986).
Leaf mass per unit area (LMA) of each leaf type was also estimated in order to look for possible differences in LMA associated with differences in leaf life span. In the adult trees, the LMA was estimated for each of the ten specimens used to estimate leaf longevity on each year as a mean of the values obtained for 25 mature and fully expanded leaves of each age class randomly sampled in the crowns. In the seedlings we estimated leaf longevity by non-destructive measurements, and, accordingly, the leaves used to estimate LMA were collected on individuals next to the specimens selected for leaf longevity measurements. The area (with a Delta-T Image Analysis System, delta-T Devices Ltd., Cambridge, UK) and dry mass (after desiccation at 70°C to constant mass) of the leaves collected were measured in the laboratory, and dry mass per unit area was derived from these data. One single LMA value was obtained for each leaf type and growth stage (and for each of the different intra-annual flushes in the saplings) as an average of the data obtained during the 3 years of study, after ascertaining that there were no significant differences among years (data not shown).
One-way analysis of variance and Fisher’s protected LSD test were used to establish significant differences at a level of P ≤ 0.05 between means after applying the Levene test to check for homogeneity of variances. For data analysis we used the SPSS Statistical Package (SPSS Inc., Chicago, IL, USA).
Results
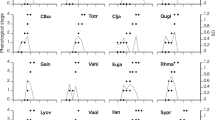
In mature trees, there was only one flush of leaf emergence at the beginning of the growth season, such that the maximum leaf biomass was attained rapidly (Fig. 1). In the saplings, leaf emergence occurred almost 1 month earlier than in the adults of the same species (Fig. 1). In the initial growth stages, there was an average of three flushes of leaf emergence in both species and hence the total leaf number per tree gradually increased during spring and the first part of the summer, the maximum number having been reached towards mid-July or even after (Fig. 2). Within each species, no relationship was observed between the number of flushes and the total number of leaves produced per tree in any of the study years (n = 10 specimens per species and year; average R 2 = 0.025, P = 0.6829 for Q. faginea; R 2 = 0.003, P = 0.8908 for Q. ilex). In both species, the first leaf flush was the most abundant, usually producing approximately 50% of the total number of leaves finally generated by each individual (Fig. 2).
In Q. ilex saplings, the number of leaves retained in the canopy in all the flushes was very high until the spring (March–April) of the following year, when a gradual decrease of the number of leaves was observed, which was especially accelerated during summer (between June and September) (Figs. 1, 2). Thus, mean leaf life span was about 15 months for the saplings of this species (Table 2). In the adults, however, the number of leaves per shoot remained close to the initial maximum values until the end of summer (September) of the following year, when leaf loss started to increase. The mean leaf life span in the adults was therefore longer than 2 years (Table 2). In Q. faginea saplings, most of the leaves produced at the start of the growth season were retained until July–August of the same year, when an intense leaf abscission occurred (Figs. 1, 2). Consequently, in this case, the mean leaf life span was about 7 months. This is only slightly lower than the mean leaf duration in mature trees of the same species (Table 2), in which leaf abscission was delayed to late autumn–early winter (November–December) (Fig. 1). However, unlike the Q. ilex saplings, which did not exhibit differences in leaf longevity among the different flushes produced within each growth season, in Q. faginea the different flushes exhibited different phenological patterns, especially in respect of leaf abscission. Most of the leaf loss observed in the Q. faginea saplings during late summer and early autumn (August–September) corresponded to the leaves that had emerged during the first flush produced in the spring (April) of the same year, which almost completely disappeared before the end of the year (Fig. 2). By contrast, most of the leaves that had emerged in the following flushes of the same growth season (second and third order) were retained in the canopy throughout the first autumn-winter period after their emergence and were shed when new leaf cohorts emerged at the start of the following growth season (Fig. 2). The same trends were observed for the different flushes produced both in 1997 and 1998. As a consequence, whereas the first leaf flush that appeared each year attained a mean longevity of about 161 days, the mean duration of the leaves produced in the subsequent flushes increased to about 285 days (Table 2). However, no significant differences in LMA were observed among the different flushes in the saplings of both species (Table 2).
Discussion
Leaf emergence
The low winter temperatures and the summer drought typical of cold Mediterranean climates often constrain photosynthetic activity to a short period during late spring and early summer (Mitrakos 1980; Terradas and Savé 1992). Mediterranean oak species exhibit a strong stomatal sensitivity to increases in evaporative demands and decreases in leaf water potential along summer (Mediavilla and Escudero 2003c). On the other side, very little CO2 assimilation was measured at temperatures around 10°C during sunny days in winter in the species used in the present study (Mediavilla and Escudero 2003a). Under these circumstances, it would be expected that plants would develop their leaf biomass as fast as possible at the start of the growing season in order to take advantage of most of this short favorable period. This was in fact the type of behavior observed for the mature specimens of the two species studied here, which tended to concentrate leaf emergence in a single flush at the beginning of the growth season, as has been observed for these same species in many other sites and under different climatic conditions (Mediavilla and Escudero 2003b, 2003c). As has been reported for other species (Gill et al. 1998; Seiwa 1999a, 1999b; Augspurger and Bartlett 2003), leaf emergence in the saplings occurred earlier than in the adults. However, in the saplings, leaf production was only completed after several flushes, and hence occurred relatively late in the growth season, in agreement with the findings of other authors studying other oak species (Hanson et al. 1988; Norby and O’Neill 1989; Welandera and Ottosson 1998).
The differences in leaf emergence patterns between adults and saplings of the same species are probably a consequence of the differences in resource availability for leaf production during both stages. With increasing age (and size) the plant produces larger resource-acquiring organs (e.g., roots and foliage area), and its storage capacity increases, whereas growth rates decrease (Bond 2000; Boege and Marquis 2005). As well as providing a greater reserve of nutrients and carbohydrates that can be translocated from the different reserve organs, a more developed root system (Cavender-Bares and Bazzaz 2000; Juárez-López et al. 2008) allows adult specimens to fulfill the heavy requirements derived from concentrating the whole annual leaf production in a single flush. In saplings, however, reduced nutrient uptake, and especially low carbohydrate reserves, probably limits the number of leaves that the saplings are able to deploy when environmental conditions become favorable at the start of the growth season. New leaves may only be deployed when the previous leaf flushes become productive and supply sufficient resources for the production of new leaf biomass.
Taking into account the greater resource limitations in saplings with respect to adults, it may be expected that losses of leaf biomass through herbivory will have a greater negative impact on plant performance in the initial growth stage than in later stages (Haukioja and Koricheva 2000; Warner and Cushman 2002). Non-synchronous leaf production could also be important for minimizing the impacts of herbivory, decreasing the degree of synchrony between plant phenology and that of herbivores and helping to guarantee the survival of at least a fraction of the total number of leaves produced by a plant. In any case, it is possible that an early development of the photosynthetic apparatus during the sapling stage is not as important as it is for the adult stage, because saplings are capable of maintaining positive assimilation rates during relatively dry periods of the growth season, whereas the adults maintain a much more conservative gas-exchange strategy (Mediavilla and Escudero 2004).
Leaf longevity
The mean duration of the leaves of Q. ilex saplings was shorter than that of their counterparts from mature trees. This may be explained in terms of the lower LMA of the former, which renders their leaves more vulnerable to physical hazards such as frosts and droughts (Wright and Cannon 2001; Shipley et al. 2006). However, the saplings behaved as typical evergreens, their leaves lasting about two growing seasons. In Q. faginea saplings, the leaves attained a mean duration that was only slightly shorter than that of mature trees. Similarly to Q. ilex, the LMA of saplings of Q. faginea was lower than that of the adults, but the differences in leaf mass per area between both stages were considerably smaller in Q. faginea than in Q. ilex. These smaller differences in the LMA could help to explain why the differences in leaf longevity between growth stages were also relatively reduced in the deciduous species.
However, in Q. faginea saplings, important differences were seen between the leaves produced in the different flushes. In some cases, the leaves were truly deciduous, being shed during their first growing season, whereas in other cases they were retained up to the following growth season, thus exhibiting evergreen behavior. Surprisingly, there were no significant differences in LMA between deciduous and evergreen leaf cohorts of Q. faginea. This plasticity in leaf longevity observed within individuals of Q. faginea saplings suggests that factors other than genetics and climate would contribute to determining the patterns of leaf demography in the earlier stages of Q. faginea.
The first leaf flush of Q. faginea produced in 1997 disappeared after approximately 5 months, whereas leaf longevity in the other two or three flushes was almost double, most of the leaves being shed when a new leaf cohort emerged at the start of the following growth season. Leaf production in Q. faginea entails greater nitrogen investments per unit leaf mass than in Q. ilex (Mediavilla and Escudero 2003a; Juárez-López et al. 2008). In addition, previous studies have shown that during the seedling or sapling stage, the root distribution pattern involves a more severe N limitation in Q. faginea than in Q. ilex (Juárez-López et al. 2008), which is exacerbated by the shorter residence time of N in the leaf biomass in Q. faginea (Silla and Escudero 2004). In Q. faginea, leaf construction during the growth season may thus require amounts of N larger than those supplied by root uptake. Retaining the leaves from the previous growth season in the crown would provide an extra N reserve that could be remobilized and allocated to the new leaves, and minimize the dependence of the plants upon soil nutrients (Jonasson and Chapin 1985). Retranslocation of nutrients from aging leaves could support nutrient input into new, actively growing leaves as a consequence of the sequential leaf development. The retention of leaves until the following growth season is not necessary in later growth stages, when a larger size permits increases in the root uptake ability and storage capacity in different parts of the plant. Accordingly, leaf senescence in the initial growth stages of Q. faginea seems to be largely determined by N limitations. The sequential leaf development could be then an important mechanism enabling Q. faginea to cope with N deficiency in the early stages of life.
References
Augspurger CK, Bartlett EA (2003) Leaf phenology of juvenile vs. adult trees in a temperate deciduous forest. Tree Physiol 23:517–525
Barbero M, Loisel R, Quézel P (1992) Biogeography, ecology and history of Mediterranean Quercus ilex ecosystems. Vegetatio 99–100:19–34. doi:10.1007/BF00118207
Begon M, Mortimer A (1986) Population ecology. Blackwell Scientific Publications, Oxford
Boege K, Marquis RJ (2005) Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends Ecol Evol 20:441–448. doi:10.1016/j.tree.2005.05.001
Bond BJ (2000) Age-related changes in photosynthesis of woody plants. Trends Ecol Evol 20:441–448
Bremner JM (1960) Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci 55:11–31
Castro-Díez P, Montserrat-Martí G (1998) Phenological pattern of fifteen Mediterranean phanaerophytes from Quercus ilex communities of NE-Spain. Plant Ecol 139:103–112. doi:10.1023/A:1009759318927
Cavender-Bares J, Bazzaz FA (2000) Changes in drought response strategies with ontogeny in Quercus rubra: implications for scaling from seedlings to mature trees. Oecologia 124:8–18. doi:10.1007/PL00008865
Day ME, Greenwood MS, White AS (2001) Age-related changes in foliar morphology and physiology in red spruce and their influence on declining photosynthetic rates and productivity with tree age. Tree Physiol 21:1195–1204
Donovan LA, Ehleringer JR (1992) Contrasting water-use patterns among size and life-history classes of a semi-arid shrub. Funct Ecol 6:482–488. doi:10.2307/2389287
Dorronsoro F (1992) El medio físico-químico: suelos. In: Junta de Castilla y León (eds) El Libro de las Dehesas Salmantinas. Salamanca, Spain, pp 71–124
England JR, Attiwill PM (2006) Changes in leaf morphology and anatomy with tree age and height in the broadleaved evergreen species, Eucalyptus regnans F. Muell. Trees (Berl) 20:79–90. doi:10.1007/s00468-005-0015-5
Escudero A, Del Arco JM (1987) Ecological significance of the phenology of leaf abscission. Oikos 49:11–14. doi:10.2307/3565549
Fenner M (1998) Phenology of growth and reproduction in plants. Perspect Plant Ecol Evol Syst 1:78–91. doi:10.1078/1433-8319-00053
Gill DS, Amthor JS, Bormann FH (1998) Leaf phenology, photosynthesis, and the persistence of saplings and shrubs in a mature northern hardwood forest. Tree Physiol 18:281–289
Hanson PJ, Isebrands JG, Dickson RE, Dixon RK (1988) Ontogenetic patterns of CO2 exchange of Quercus rubra L. leaves during three flushes of shoot growth II. Insertion gradients of leaf photosynthesis. For Sci 34:69–76
Haukioja E, Koricheva J (2000) Tolerance to herbivory in woody vs. herbaceous plants. Evol Ecol 14:551–562. doi:10.1023/A:1011091606022
Hubbard RM, Bond BJ, Ryan MG (1999) Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol 19:165–172
Ishida A, Yazaki K, Lai Hoe A (2005) Ontogenetic transition of leaf physiology and anatomy from seedlings to mature trees of a rain forest pioneer tree, Macaranga gigantea. Tree Physiol 25:513–522
Jonasson S, Chapin FS III (1985) Significance of sequential leaf development for nutrient balance of the cottonsedge, Eriophorum vaginatum L. Oecologia 67:511–518. doi:10.1007/BF00790022
Jones RH, Allen BP, Sharitz RR (1989) Potential advantages and disadvantages of germinating early for trees in floodplain forests. Oecologia 81:443–449. doi:10.1007/BF00378950
Jones RH, Sharitz RR, Dixon PM, Segal DS, Schneider RL (1994) Woody plant regeneration in four floodplain forests. Ecol Monogr 64:345–367. doi:10.2307/2937166
Jones RH, Allen B, Sharitz RR (1997) Why do early-emerging tree seedlings have survival advantages?: a test using Acer rubrum (Aceraceae). Am J Bot 84:1714–1718. doi:10.2307/2446470
Juárez-López FJ, Escudero A, Mediavilla S (2008) Ontogenetic changes in stomatal and biochemical limitations to photosynthesis of two co-occurring Mediterranean oaks differing in leaf life span. Tree Physiol 28:367–374
Kikuzawa K (1995) Leaf phenology as an optimal strategy for carbon gain in plants. Can J Bot 73:158–163. doi:10.1139/b95-019
Kozlowski TT, Kramer PJ, Pallardy SG (1991) The physiological ecology of woody plants. Academic Press, New York, p 657
Mediavilla S, Escudero A (2003a) Photosynthetic capacity, integrated over the lifetime of a leaf, is predicted to be independent of leaf longevity in some tree species. New Phytol 159:203–211. doi:10.1046/j.1469-8137.2003.00798.x
Mediavilla S, Escudero A (2003b) Relative growth rate of leaf biomass and leaf nitrogen content in several Mediterranean woody species. Plant Ecol 168:321–332. doi:10.1023/A:1024496717918
Mediavilla S, Escudero A (2003c) Stomatal responses to drought at a Mediterranean site: a comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol 23:987–996
Mediavilla S, Escudero A (2004) Stomatal responses to drought of mature trees and seedlings of two co-occurring Mediterranean oaks. For Ecol Manage 187:281–294
Mencuccini M, Grace J (1996) Developmental patterns of above-ground hydraulic conductance in a Scots pine (Pinus sylvestris L.) age sequence. Plant Cell Environ 19:939–948. doi:10.1111/j.1365-3040.1996.tb00458.x
Mitrakos KA (1980) A theory for Mediterranean plant life. Acta Oecol 1:245–252
Norby RJ, O’Neill EG (1989) Growth dynamics and water use of seedlings of Quercus alba L. in CO2-enriched atmospheres. New Phytol 111:491–500. doi:10.1111/j.1469-8137.1989.tb00712.x
Ryan MG, Yoder BJ (1997) Hydraulic limits to tree height and tree growth. Bioscience 47:235–242. doi:10.2307/1313077
Seiwa K (1999a) Changes in leaf phenology are dependent on tree height in Acer mono, a deciduous broad-leaved tree. Ann Bot (Lond) 83:355–361. doi:10.1006/anbo.1998.0831
Seiwa K (1999b) Ontogenetic changes in leaf phenology of Ulmus davidiana var. Japonica, a deciduous broad-leaved tree. Tree Physiol 19:793–797
Shipley B, Lechowicz MJ, Wright I, Reich PB (2006) Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87:535–541. doi:10.1890/05-1051
Silla F, Escudero A (2004) Nitrogen use efficiency: trade-offs between N productivity and mean residence time at organ, plant and population level. Funct Ecol 18:511–521. doi:10.1111/j.0269-8463.2004.00872.x
Terradas J, Savé R (1992) The influence of summer and winter stress and water relationships on the distribution of Quercus ilex L. Vegetatio 99–100:137–145. doi:10.1007/BF00118219
Warner PJ, Cushman JH (2002) Influence of herbivores on a perennial plant: variation with life history stage and herbivore species. Oecologia 132:77–85. doi:10.1007/s00442-002-0955-z
Welandera NT, Ottosson B (1998) The influence of shading on growth and morphology in seedlings of Quercus robur L. and Fagus sylvatica L. For Ecol Manage 107:117–126
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low nutrient, sclerophyll flora. Funct Ecol 15:351–359. doi:10.1046/j.1365-2435.2001.00522.x
Acknowledgments
This paper has received financial support from the Spanish Ministry of Education (Projects No. FOR89-0845 and AMB95-0800) and from the Junta de Castilla y León (Projects No. SA47/95 and SA72/00B).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mediavilla, S., Escudero, A. Ontogenetic changes in leaf phenology of two co-occurring Mediterranean oaks differing in leaf life span. Ecol Res 24, 1083–1090 (2009). https://doi.org/10.1007/s11284-009-0587-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-009-0587-4