Abstract
Using data from a global positioning system (GPS), seven adult red deer (Cervus elaphus L.) were tracked in the Parc National des Cévennes, southern France, between November 1998 and December 2000 to assess the factors affecting large-range movement patterns and habitat use. The home range varied from a single compact area for females to three distinct seasonal ranges for males, which used alternative migratory strategies (i.e. non-, downward- and upward-migrants). The migrants used mainly southerly and easterly aspects, and wintered in areas having steeper slopes than were used during summer or the rut season. For males, the time of rut migration was mid-September and they finally entered wintering ranges from mid-December to the beginning of January. Exploratory behaviour (i.e. individuals found outside the limits of their familiar area but returning to it a few days later) occurred in both sexes and for all individuals monitored during at least a 6-month period. Velocity and efficiency of exploratory movements were higher than usual movements. During these exploratory movements, hinds may have used different landscape attributes (elevation, slope, canopy cover) while stags did not. These results provide new empirical information that could be used for building and applying broad-scale spatial and landscape use models in ecological research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aim of home-range analysis is to delimit the area used by an individual in the course of its normal activities (Burt 1943), i.e. to make “an assumption of stability” (Worton 1987). Consequently, to minimise the influence of brief extra-territorial forays (Doerr and Doerr 2005), methods of home-range analysis generally exclude both the auto-correlated data (but see De Solla et al. 1999) and the most outlying locations. Hence, Cushman et al. (2005) felt that, for many questions about animal movement and ecology, it is important to understand the details of animal movement patterns, and pointed out that doing so will help to address questions about the relationships between time, season, space, resources, social interactions, and animal movement patterns that are otherwise difficult or intractable.
Johnson et al. (2002a) claimed that most studies of animal movements and habitat use do not empirically recognise that different components of the environment are important at different scales, and that few researchers have attempted to stratify observed movements or use of habitats according to the scales at which those behaviours occur. Thus, there is a serious need for empirical information on the ranging behaviour of wildlife species to properly describe individual variability, and to take search and orientation strategies into account, in order to make reliable predictions on population models in complex, heterogeneous landscapes (Johnson et al. 1992; Conradt et al. 2003; Benhamou 2004).
In a review of literature on ungulate movements, Gates et al. (2005) pointed out that the primary benefits of adopting a movement strategy appear to be the ability to find new resources, to escape predators, to find new mates during rut, or to improve reproductive potential within a changing environment. Often weather-related, environments can change through natural variation in resource accessibility as dictated by snow, or in forage availability as dictated by grazing intensity and season (Johnson et al. 2002b), and the success of a movement strategy depends on the environment in which the population persists. Gates et al. (2005) devoted a section to the awareness of destination: following Baker’s (1978) definitions, they distinguished exploratory migration (i.e. migration beyond the limits of the familiar area, but with the animal retaining the ability to return) from calculated migration, which is usually regular and occurs with a fixed periodicity over the course of a year (i.e. movement to a specific destination known to the animal at the time of migration, either through direct perception, previous acquaintance, or social communication).
Investigations into large- and medium-sized mammal movements have been possible through the use of very high frequency radio-tracking systems since the early 1960s (e.g. Cochran et al. 1965). More recently, the adaptation of global positioning system (GPS) devices to wildlife studies (Rodgers and Anson 1994) has offered new opportunities to monitor fine-scale movements and behaviour at various time periods (e.g. Johnson et al. 2002a, b, or Franke et al. 2004 on caribou, Rangifer tarandus). In the present paper, we used red deer (Cervus elaphus L.) as a model species because movement patterns and spatial behaviour are sex-related (Clutton-Brock et al. 1982). Indeed, except during the rutting period, red deer is a gregarious species. Adult hinds live in matriarchal groups including calves and yearlings, while adult stags spend most of the time alone or in bachelor groups. In the northern hemisphere, the rut begins in mid-September and ends in mid-October, with the adult stags becoming intolerant of each other and moving to rutting areas to meet and defend groups of adult hinds (Ahlén 1965; Clutton-Brock et al. 1982). To cope with environmental (e.g. snow conditions, food availability) and social (need to be alone) constraints, the stags may be migratory by definition in that they may have discrete winter and summer ranges, and may move over large distances (e.g. Knight 1970; Craighead et al. 1972; Blankenhorn et al. 1978; Jarnemo 2007). Consequently, the annual home range of stags may be very large, whereas the hind annual home range is generally more compact and smaller than that of adult males (Lincoln et al. 1970; Gonzalez and Pépin 1996; Szemethy et al. 1998; Adrados 2002).
Using differential global positioning system (dGPS) devices, we carried out a study on the temporal and spatial behaviour of free-ranging adult red deer in a mountainous temperate forest area (Adrados 2002). We defined usual movements as being short-range when individuals travelled regularly within a seasonal range. Among all other movements defined as long-range, we distinguished migratory movements (i.e. travel to join another zone known by the animal at a fixed periodicity over the course of a year and staying there for more than a week) from exploratory movements (i.e. individuals found outside their current familiar area but returning to it a few days later). The main aims of the present study were to use locations obtained every 3 h to: (1) describe the home range structure of both sexes for individuals monitored for at least 1 year (n = 4); (2) detail the migration patterns for deer having distinct seasonal home ranges, and the exploratory behaviour of individuals monitored over a period of at least 6 months (n = 7), and (3) examine the landscape attributes during the short time period framing exploratory movements. In the latter case, assuming that the regular movements carried out during the days prior to and after exploratory movements correspond to normal behaviour, we compared their characteristics with those of exploratory movements.
Methods
Study area
The study was carried out in the Parc National des Cévennes (44°19′N, 03°45′E) located in the eastern part of the Massif Central (France). Within the study area, elevation ranges between 800 and 1,700 m above sea level. In this mountainous area the landscape is a mosaic of mixed forest (Pinus sylvestris, Abies alba, Fagus sylvatica, Quercus sp., Castanea sativa) and heath (Erica sp., Calluna vulgaris, Sarothammnus purgans, Vaccinium sp.). Rainfall ranges from 900 to 1,500 mm/year.
Capture and radio-tracking
We trapped red deer with the authorisation of the Parc National des Cévennes and the French Ministry of Agriculture. We used ten cage traps baited with apple and salt, and organised trapping sessions from September to April in 1997–2000. We fitted the cage traps with VHF beacons to verify their status (open or closed); in this way, captured deer would not be held in the trap for more than 6 h during daytime or 12 h at night before handling in the best way possible to prevent panic or injury. We caught five adult males and four adult females at least 5 years old, which were immobilised in the trap using a dart gun containing medetomidine (Orion Pharma, Espoo, Finland) and ketamine hydrochloride (Merial, Lyon, France). Maximum handling time never exceeded 40 min. The GPS collar fitted to the animals weighed less than 2 kg (i.e. never exceeding 2.5% of their body mass). We injected atipamezole (Orion Pharma, Espoo, Finland) as an antidote to medetomidine before their release at the capture site. Deer were active within 2–5 min of release and left the capture site without showing signs of stress (such as protracted or very fast movement; Pépin et al. 2004).
Depending on the objectives of our research (fine-scale site use, e.g. Adrados et al. 2003; analytical study of daily distance travelled, e.g. Pépin et al. 2004), we planned various time intervals between location records (from 5 min to 3 h, which correspond to a range from 288 to 8 attempted locations per day, respectively). Due to the logistics of capture campaigns, the reliability of GPS devices and hunting harvest, the start and end dates, and thus the duration of monitoring, varied among animals (Table 1). The distance between the centre of the study area and our 12-channel GPS Pathfinder base station (Trimble Navigation, Sunnyvale, CA) was about 240 km. We used this base station to differentially correct GPS locations using N3WIN software (version 2.40) before January 2000, and N4WIN software version 1.1895 thereafter (Lotek Wireless, Newmarket, ON, Canada). Among the locations sampled at scheduled 3-h intervals, we selected the 3-dimensional (3-D) and 2-dimensional (2-D) values with dilution of precision values <10 (Rempel and Rodgers 1997; Adrados et al. 2003). More than 99% of relevant fixes had an accuracy of <30 m (Adrados et al. 2002).
Study analyses
Data consisted of sequential locations of individuals monitored for at least 6 months (Table 1). We used a geographic information system (ArcView GIS 3.2) with habitat information (three classes of vegetation) extracted from a SPOT satellite image with a 20 m resolution from the Centre National d’Etudes Spatiales (CNES, Toulouse, France), and topographic characteristics (elevation, slope, aspect) extracted from an Institut Géographique National (IGN) elevation map. We inspected the locations in sequential order since a moving animal describes a continuous trajectory. The spatial and temporal distribution of locations allowed us to better detect clusters or congregations of locations within the home range area (D’Eon and Serrouya 2005). Doing so will help delimit well separated geographical ranges, which are linked by corridors used by animals only during short time periods each year (Georgii and Schröder 1983). For each deer monitored for at least 1 year, we checked for potential seasonal differences in the topographic characteristics of locations within the individual’s home range by using cumulative data distribution.
Connecting the consecutive locations with straight lines results in an approximation of the total movement path of an individual (Doerr and Doerr 2005), which relates the velocity and tortuosity of an animal’s path to the efficiency of the behavioural mechanism involved, e.g. oriented path to reach a goal along a straight line versus random search path adjusting search effort to local profitability of the environment (Benhamou 2004). For a deer migrating from one seasonal range to another, an efficient path should be close to the straight line linking the start point to the goal. Consequently, the efficiency of an oriented path can be reliably estimated by a straightness index computed as the ratio between the distance from the start point to the goal (D), and the path length travelled to reach the goal (L), ranging between 0 and 1 (Benhamou 2004). The path travelled, measured as the sum of step lengths (i.e. L), tends to decrease with a decrease in recording frequency, causing the straightness index to tend towards 1 if the recording frequency is low. Consequently, as recommended by Pépin et al. (2004), for locations recorded with time intervals of 3 h for red deer, we applied a correction factor (1.45) to the perceived straight line distance to estimate real distance travelled. We focussed on the analysis of exploratory movements by first comparing the speed and efficiency of paths travelled by animals on the days before, during and after those exploratory movements. The time spans used before and after the exploratory movement were time adjusted to obtain comparable samples. We used mixed effects linear models with the behavioural indices obtained within the three time periods as fixed effect factors, and the exploratory movements and individuals as nested random factors (several exploratory movements sometimes being recorded for a single animal). Among landscape indices, we also used elevation, slope, and canopy cover (coniferous, broad-leaved trees, and heath) as fixed effect factors.
Results
Home range characteristics
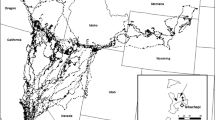
Four individuals (F1, M1, F2, and M4) were monitored for at least a whole year (Table 1). The home range of females was continuous while the home range of males included three distinct seasonal areas, i.e. winter, summer and rutting ranges (see Fig. 1 for M4). The distances between the centres of winter and summer ranges were 3.0 km for M1 and 4.9 km for M4, and the distances from the centre of the rutting area to the centre of summer and winter ranges were 2.7 and 3.9 km for M1, and 10.3 and 7.7 km for M4, respectively. F1 inhabited a restricted south–east border portion of her home range from 20 January to 4 April, and then from 13 to 30 April 1999 (Fig. 2), while F2 wintered most often in the central core area of her home range (Fig. 3).
Distribution of locations of three neighbouring adult stags (Cervus elaphus), in the Parc National des Cévennes, France. Map coordinates are in metres, using the French Lambert III Congruent Conic Projection. Two contour lines (1,000 and 1,500 m) are indicated. The sampling periods are listed in Table 1. The males occasionally make more or less direct forays to rejoin various parts of their home range (e.g. as in the case of M4, and on the 23–24 October and 3–4 November 1999 for M3), or make ellipsoidal loops away from and back to their start points (e.g. on the 2–4 October 1999 for M2, and on the 14–16 February 2000 for M3)
Distribution of locations of two neighbouring adult hinds (Cervus elaphus) in the Parc National des Cévennes, France. Map coordinates are in metres using the French Lambert III Congruent Conic Projection. Four contour lines (800, 1,000, 1,200 and 1,400 m) are indicated. The sampling periods are listed in Table 1. The south–eastern part of the home range of F1 used from 20 January to 4 April 1999, and 13–30 April 1999, corresponded to her wintering area. During summer (i.e. 15–20 June and 27–30 July 2000), F3 made two very long loops away from and back to start points, both in the same direction and with the same end point
Distribution of locations of an adult hind Cervus elaphus recorded from 29 February to 1 April 2000 (small circles and black broken lines) within her home range (minimum convex polygon, from data recorded between mid-October 1999 and mid-December 2000), in the Parc National des Cévennes, France. Map co-ordinates are in metres, using the French Lambert III Congruent Conic Projection. Three contour lines (800, 1,000 and 1,200 m) are indicated. During this period, the hind preferentially used a central core area, but also made regular loops up to the boundary of her annual home range (e.g. 29 February–1 March, 19–21 March, and 30 March–1 April)
From the cumulative data distribution, for the two males we found greater elevation and slope values for winter ranges compared to rutting ranges (see Fig. 1 for M4 and Fig. 4 for both males). For F1, elevation values were smaller and slope values were larger in her winter range than in her contiguous summer range (Figs. 2, 4). Both F1 and M4 used higher ranges in summer than in other seasons, while the reverse occurred for M1 (Fig. 4). Whatever the season, 58.7% of locations were facing south and south-west for M4, 64.5% of locations were facing east and south-east for F1, and 48.4% of locations were facing south-east and south for F4. The home range of M1 changed from south and south-west aspects in summer (65.3% of locations) to east and south-east aspects in winter (65.5% of locations).
Cumulative distribution of the elevation and slope values of locations of two adult stags (M1 and M4) and one adult hind (F1) monitored at least a whole year within their seasonal home range (black circles for summer, white squares for winter, white triangles for rut) in the Parc National des Cévennes, France. The corresponding sampling periods for stags are indicated in Table 2. For the hind, the summer and winter periods correspond to her ranging behaviour, the rut values being data recorded from 15 September to 14 October
Migratory behaviour of stags
All migrations between seasonal ranges occurred within 1 day (Table 2). Presumably due to the general geographical distribution of the home ranges, no migratory movement was found between rut and winter ranges for M1, or between rut and summer ranges for M4. Stags entered their rutting area in mid-September and moved to their wintering area between mid-December and the beginning of January. More or less direct migratory movements to rejoin the summer ranges occurred earlier for M1 than for M4 (i.e. end of April–beginning of May and June, respectively). Direct migratory movements from rut to winter ranges were also found for M3, between the end of October and the beginning of November (Fig. 1).
Exploratory behaviour
As reported in Table 2, M1 exhibited an exceptional exploratory movement on 28–30 June 1999. During these days, he left his summer range for his rutting range and then returned. F2 made at least three exploratory movements to the periphery of her winter home range between the end of February and the beginning of April (Fig. 3). Some exploratory movements also occurred during rut for M2 (2–4 October 1999) and during winter for M3 (14–16 February 2000) (Fig. 1), and twice in June–July 2000 for F3 (Fig. 2).
Only 32% of accurate fixes were obtained for F3 (Table 1). Consequently, data concerning this hind were excluded from our analysis of behavioural characteristics associated with exploratory movements. Considering within-individual variation as well as between-individual variation, the use of mixed effects linear models revealed that, on average, both the velocity (m/h) and efficiency (D/L) of the 11 remaining movements were higher during exploratory forays than for regular movements travelled on the days before and after such forays (F = 10.26 and 4.80, df = 2, P = 0.0038 and 0.0345, respectively). More precisely, the speed of movements was on average 202.5 m/h higher during the exploratory movements than on the days before (t = 4.24, P = 0.0017), followed by a 167.5 m/h decrease during subsequent days (t = 3.50, P = 0.0057). Similarly, the efficiency of exploratory movements was higher than movements during the days flanking the foray (+ 0.20 and + 0.24, t = 2.39 and 2.90, P = 0.0379 and 0.0157, respectively).
The use of mixed effects linear models did not enable us to draw such generalisations from data analysis of habitat characteristics (for instance F = 1.52 and 0.56, df = 2, P = 0.253 and 0.584 for elevation and slope, respectively). However, by considering individual exploratory movements as experimental units, and thus by looking for within-individual variation in habitat use, we found that F3 used higher elevation (Fig. 2) and more closed canopy cover during her exploratory movements than on the days before and after these forays in both June and July. This hind also used smaller slopes during her exploratory movements than on the flanking days in July. Similarly, F2 used higher elevation during her second exploratory movement than on the flanking days, but lower elevation during her first exploratory movement than on subsequent days (Fig. 3). In contrast, we were unable to detect any change in the habitat used by males on the days before, during, and after their exploratory movements.
Discussion
Our description of the spatial temporal dynamics of adult red deer movements complements previous studies of ranging behaviour, habitat use and activity patterns in the Parc National des Cévennes (Adrados et al. 1999, 2003; Pépin et al. 2004) and elsewhere in Europe (Georgii 1980; Georgii and Schröder 1983; Jeppesen 1987; Schaal 1995; Hamann et al. 1997; Klein and Hamann 1999). Because of individual differences in home range use (from one compact annual home range to distinct seasonal areas), we found that three alternative migratory strategies co-exist (i.e. non-, downward- and upward-migrants).
Red deer, Rocky Mountain elk (Cervus elaphus), and mule deer (Odocoileus hemionus) living in mountainous areas are generally known as downward migrants, i.e. moving from high elevation summer ranges to low elevation winter ranges (Georgii and Schröder 1983; Gates et al. 2005; Ager et al. 2003; D’Eon and Serrouya 2005). This vertical movement is a typical pattern of migration assumed to be a strategy to cope with energetic needs (Hudson and White 1985). The proximal cause of this strategy is usually attributed to deep snow accumulations at high elevations during winter and ultimately to seasonal changes in the quality and quantity of available forage within the annual home range of the animal (e.g. Langvtan and Albon 1986; Garrott et al. 1987). Some red deer remain in the lowlands throughout the year near artificial feeding places in the main valleys of the Bavarian Alps (Georgii 1980). Both to retain red deer in hunting grounds and to substitute for forested areas lost to human encroachment, alpine pastures in some regions of Austria are covered with a network of winter feeding stations (Schmidt 1992). However, during winters with heavy snowfall, both fed and non-fed herds are confined for the whole winter period to windblown alpine areas above the treeline where sinking depth and forage accessibility are highly reduced due to patchy sown distribution (Schmidt 1993). In mild winters, Schmidt and Gossow (1991) noted that non-fed red deer remained in lower elevated timber stands adjacent to cultivated meadows, while a fed herd used alpine meadows consistently by making returns of 800 m in elevation every 2 days.
Studying the migration patterns of female sika deer (Cervus nippon) in eastern Hokkaido, Japan, Igota et al. (2004) found that 12 hinds used the intermediate-altitude home ranges of deer all year round (residents), 29 were downward migrants (below 300 m elevation), and 10 were upward migrants (above 300 m elevation). This reverse altitudinal migration (upward migration), which had never been reported before in other cervid populations, was also found in our red deer population, and may be attributed to the use of a wintering area having steeper slopes and, consequently, less grounded snow than in the summer range. This is in agreement with the findings of Igota et al. (2004), who reported that upward migrants wintered in areas with less snow, and more coniferous, cover than their summer home ranges. Sakuragi et al. (2003) found that the dietary quality of upward migrants that migrated over a long distance was similar to that of residents.
In agreement with data recorded in the Bavarian Alps by Georgii (1980) and Georgii and Schröder (1983), we found that all migrations between seasonal home ranges of red deer occurred within 1 day. Georgii and Schröder (1983) concluded that, as for most cervid species, the seasonal home ranges of both male and female red deer vary only slightly from year to year in spatial position and dimension. This suggests that spatial memory provides long-term, large-scale navigational information about where to migrate (Bennett and Tang 2006). Most of the stags exhibited seasonal migrations—which either connect lowland wintering and mountainous summering grounds or normally occupied home ranges with special rutting areas—and they leave the valley for their upland summer range migrations from the end of March to mid-April, moving to their rutting areas in mid-September (Georgii and Schröder 1983). Most of the adult hinds enter their summer home range at the end of May–beginning of June, and they leave this range from the end of July to late September, the length of stay on the summer range being quite variable (Georgii 1980).
Georgii (1980) cited the case of a female that made two short-time excursions (1-day trips) out of her home range in 3 years of observation, i.e. she went from spring to summer home range a week before seasonal range change to test the nutritive values of forage (cited as ‘test trips’ Bertram and Rempel 1977). In our study, longer “test trips” were found in the case of stags, with a first trip between 23 April and 6 May 1999 for M1, 5 days before his seasonal use of his summer range, and a first trip between 8 and 15 June 2000 for M4, 6 days before his seasonal use of his summer range (see Table 2). Mysterud et al. (2001) suggested that, among northern temperate ungulates, migration to high elevation during summer is not necessary for increased energy intake, but is rather a strategy to have prolonged access to newly emerged forage as they migrate along a gradient in altitude during early summer (i.e. due to the link between plant phenology and topography). Consequently, we can imagine that migrating stags search for plants at a relatively early stage of growth, which doubtless having greater palatability and nutritive value (Hudson and White 1985).
Conceptually, decisions regarding when to migrate can balance the possibility of finding better forage at a distance against the energy required to travel to that location by means of a learned behaviour (e.g. when the ratio between the snow depth and the brisket height of the animal exceeds a threshold value) (Bennett and Tang 2006). In a study coupling local climate–topography interactions (i.e. the climatological “downscaling process” that determines the prevalent weather conditions at the plant and animal scale) with large-scale vegetation dynamics and red deer (> 1 year old) performances along the west coast of Norway, Pettorelli et al. (2005) found that an early spring vegetation start had positive effects on both the subsequent autumn body masses of harvested animals and on the starting date of the spring migration of females.
From our detailed analysis of movement patterns of adult red deer, we also showed that excursions occasionally occurred within the home range for both sexes in other circumstances, e.g. to the periphery of home range and back to the central core area during the winter season, or from the summer range to the rutting range in the middle of summer. If snow-free areas become available by thaw in the Bavarian Alps, Georgii and Schröder (1983) noted that stags occasionally exhibit short excursions out of their normally small winter ranges.
To understand large-scale ranging behaviour, it is important to consider not only the dispersal capabilities of the animal but also the complex interaction between animal behaviour and landscape pattern (e.g. Lima and Zollner 1996; Vuilleumier and Metzger 2006). The use of GPS with attempted locations planned every 3 h allowed us to iron out some of the difficulties in gathering and interpreting experimental results on animal dispersal processes, and to both detect and characterise some forays of adult red deer. From our results, we can state that animals involved in such exploratory trips showed apparent similarities in their ranging behaviour, i.e. higher velocity and greater straightness index values to reach their end destination. Moreover, we found that females travelled under particular individual–landscape attributes (elevation, slope, canopy cover) while males did not. Human-related disturbance risks (e.g. hunting, wandering dogs, gathering of mushrooms, predator reintroduction, conversion of working ranches to amenity homes) (Hamann et al. 1997; Bennett and Tang 2006) may explain such forays. Until now, relatively little attention has been paid to documenting such large-scale movement of deer, which could be useful in building models adapted to landscape characteristics, topographical features, and resource-management strategies (Kie et al. 2005; Bennett and Tang 2006; Vuilleumier and Metzer 2006).
References
Adrados C (2002) Occupation de l’espace et utilisation de l’habitat par le Cerf (Cervus elaphus L.) en forêt tempérée de moyenne montagne: approche au moyen du GPS. Doctorat Université Paul Sabatier, Toulouse
Adrados C, Janeau G, Joachim J, Pépin D (1999) Preliminary results on spatio-temporal behaviour in red deer (Cervus elaphus) obtained with differential GPS. Entretiens de Chizé, Juillet 1999, Session I:15–21
Adrados C, Girard I, Gendner JP, Janeau G (2002) Global Positioning System (GPS) location accuracy improvement due to selective availability removal. C R Biol 325:165–170
Adrados C, Verheyden H, Cargnelutti B, Pépin D, Janeau G (2003) GPS approach to study fine-scale site use by wild red deer during active and inactive behaviors. Wildl Soc Bull 31:544–552
Ager AA, Johnson BK, Kern JW, Kie JG (2003) Daily and seasonal movements and habitat use by female rocky mountain elk and mule deer. J Mammal 84:1076–1088
Ahlén I (1965) Studies on the red deer, Cervus elaphus L., in Scandinavia. III. Ecological investigations. Viltrery 3:177–376
Baker RR (1978) The evolutionary ecology of animal migration. Holmes and Meier, New York
Benhamou S (2004) How to reliably estimate the tortuosity of an animal’s path: straightness, sinuosity, or fractal dimension? J Theor Biol 229:209–220
Bennett DA, Tang W (2006) Modelling adaptive, spatially aware, and mobile agents: Elk migration in Yellowstone. Int J Geogr Inf Sci 20:1039–1066
Bertram RC, Rempel RD (1977) Migration of the north Kings deer herd. California Fish Game 63:157–179
Blankenhorn HJ, Buchli CH, Voser P (1978) Wanderungen und jahreszeitliches Verteilungsmuster der Rothirspopulation (Cervus elaphus L.) in Engalin, Münstertal und Schweizerischen Nationalpark. Rev Suisse Zool 85:779–789
Burt WH (1943) Territoriality and home range concepts as applied to mammals. J Mammal 24:346–352
Clutton-Brock TH, Albon SD, Gibson RM, Guinness FE (1982) Red deer: behaviour and ecology of two sexes. University of Chicago Press, Chicago
Cochran WWD, Warner R, Tester JR, Kuechle VB (1965) Automatic radio-tracking system for monitoring animal movements. BioScience 15:98–100
Conradt L, Zollner PA, Roper TJ, Frank K, Thomas CD (2003) Foray search: an effective systematic dispersal strategy in fragmented landscapes. Am Nat 161:905–915
Craighead JJ, Craighead FC, Ruff RL, O’Gara BW (1972) Elk migrations in and near Yellowstone National Park. Wildl Monogr no. 29
Cushman SA, Chase M, Griffin C (2005) Elephants in space and time. Oikos 109:331–341
D’Eon RG, Serrouya R (2005) Mule deer seasonal movements and multiscale resource selection using global positioning system radiotelemetry. J Mammal 86:736–744
De Solla SR, Bonduriansky R, Brooks RJ (1999) Eliminating autocorrelation reduces biological relevance of home range estimates. J Anim Ecol 68:221–234
Doerr ED, Doerr VAJ (2005) Dispersal range analysis: quantifying individual variation in dispersal behaviour. Oecologia 142:1–10
Franke A, Caeli T, Hudson RJ (2004) Analysis of movements and behavior of caribou (Rangifer tarandus) using hidden Markov models. Ecol Model 173:259–270
Garrott RAG, White GC, Bartman RM, Carpenter LH, Allredge AW (1987) Movements of female mule deer in northwest Colorado. J Wildl Manag 51:634–643
Gates CC, Stelfox B, Muhly T, Chowns T, Hudson RJ (2005) Review of literature on ungulate movements. In: The ecology of bison movements and distribution in and beyond Yellowstone National Park. A critical review with implications for winter use and transboundary population management. http://www.nps.gov/yell/parkmgmt/upload/2.pdf
Georgii B (1980) Home range patterns of female red deer in the Alps. Oecologia 47:278–285
Georgii B, Schröder W (1983) Home range and activity patterns of male red deer (Cervus elaphus L.) in the Alps. Oecologia 58:238–248
Gonzalez G, Pépin D (1996) Le cerf (Cervus elaphus) en milieu montagnard et nordique. I. Paléontologie, occupation de l’espace, utilisation du temps et des ressources. Revue bibliographique. Gibier Faune Sauvage 13:27–52
Hamann JL, Klein F, Saint-Andrieux C (1997) Domaine vital diurne et déplacements de biches (Cervus elaphus) sur le secteur de la Petite Pierre (Bas Rhin). Gibier Faune Sauvage, Game Wildl 14:1–17
Hudson RJ, White RG (1985) Bioenergetics of wild herbivores. CRC, Boca Raton
Igota H, Sakuragi M, Uno H, Kaji K, Kaneko M, Akamatsu R, Maekawa K (2004) Seasonal migration patterns of female sika deer in eastern Hokkaido, Japan. Ecol Res 19:169–178
Jarnemo A (2007) Seasonal migration of male red deer (Cervus elaphus) in southern Sweden and consequences for management. Eur J Wildl Res (in press) doi:10.1007/s10344-007-0154-7
Jeppesen JL (1987) Impact of human disturbance on home range, movements and activity of red deer (Cervus elaphus) in a Danish environment. Dan Rev Game Biol 13:1–37
Johnson AR, Wiens JA, Milne BT, Crist TO (1992) Animal movements and population dynamics in heterogeneous landscapes. Landsc Ecol 7:63–75
Johnson CJ, Parker KL, Heard DC, Gillingham MP (2002a) Movement parameters of ungulates and scale-specific responses to the environment. J Anim Ecol 71:225–235
Johnson CJ, Parker KL, Heard DC, Gillingham MP (2002b) A multiscale behavioral approach to understanding the movements of woodland caribou. Ecol Appl 12:1840–1860
Kie JG, Ager AA, Bowyer RT (2005) Landscape-level movements of North American elk (Cervus elaphus): effects of habitat patch structure and topography. Landsc Ecol 20:289–300
Klein F, Hamann JL (1999) Domaines vitaux diurnes et déplacements de cerfs mâles (Cervus elaphus) sur le secteur de La Petite Pierre (Bas-Rhin). Gibier Faune Sauvage, Game Wildl 16:251–271
Knight RR (1970) The Sun River Elk herd. Wildl Monogr no. 23
Langvtan R, Albon SD (1986) Geographic clines in body weight of Norwegian red deer, a novel explanation of Bergmann’s rule. Holarct Ecol 9:285–293
Lima SL, Zollner PA (1996) Towards a behavioral ecology of ecological landscapes. Trends Ecol Evol 11:131–135
Lincoln A, Yougson RW, Short RV (1970) The social and sexual behaviour of the red deer stag. J Reprod Fertil Suppl 11:71–103
Mysterud A, Langvatn R, Yoccoz NG, Stenseth NC (2001) Plant phenology, migration and geographic variation in body weight of a large herbivore: the effect of a variable topography. J Anim Ecol 70:915–923
Pépin D, Adrados C, Mann C, Janeau G (2004) Assessing real daily distance traveled by ungulates using differential GPS locations. J Mammal 85:774–780
Pettorelli N, Mysterud A, Yoccoz NG, Langvatn R, Stenseth NC (2005) Importance of climatological downscaling and plant phenology for red deer in heterogeneous landscapes. Proc R Soc B 272:2357–2364
Rempel RS, Rodgers AR (1997) Effects of differential correction on accuracy of a GPS animal location system. J Wildl Manage 61:525–530
Rodgers AR, Anson P (1994) Animal-borne GPS: tracking the habitat. GPS World 5:20–32
Sakuragi M, Igota H, Uno H, Kaji K, Kaneko M, Akamatsu R, Maekawa K (2003) Benefit of migration in a female sika deer population in eastern Hokkaido, Japan. Ecol Res 18:347–354
Schaal A (1995) Observations sur les déplacements et l’utilisation de l’espace par le cerf en région d’Arc-en-Barrois (Haute-Marne). In: Dronneau C, Klein F (eds) Le cerf à Arc-en-Barrois 1982–1986. Office national de la chasse, Bar-le-Duc, 52:32–72
Schmidt K (1992) High alpine pastures as alternative wintering habitat for alpine red deer. In: Spitz F, Janeau G, Gonzalez G, Aulagnier S (eds) ‘Ongulés/Ungulates 91’. Société Française pour l’Etude et la Protection des Mammifères–Institut de Recherche sur les Grands Mammifères, Toulouse, pp 255–261
Schmidt K (1993) Winter ecology of nonmigratory Alpine red deer. Oecologia 95:226–233
Schmidt K, Gossow H (1991) Winter ecology of alpine red deer with and without supplemental feeding: management implications. In: Csanyi S, Ernhaft J (eds) Transactions of the XXth international congress of game biologists. University of Agricultural Science, Gödöllö, pp 180–185
Szemethy L, Heltai M, Matrai K, Peto Z (1998) Home ranges and habitat selection of red deer (Cervus elaphus) on a lowland area. Gibier Faune Sauvage, Game Wildl 15:607–615 Special issue part 2
Vuilleumier S, Metzger R (2006) Animal dispersal modelling: handling landscape features and related animal choices. Ecol Model 190:159–170
Worton BJ (1987) A review of models of home range for animal movement. Ecol Model 38:277–298
Acknowledgements
We thank J.-M. Angibault, B. Cargnelutti, N. Cebe, D. Picot and Hélène Verheyden for helping capturing and collaring the red deer, and for assisting with the collection of data. Capture methods were approved by the French Ministry of Environment, and two authors of this paper (D.P. and G.J.) were entitled by the French Ministry of Agriculture to experiment on free-living mammals (certificates no. 7060 and 7382). Funds were provided by the ‘Institut National de la Recherche Agronomique’ (INRA) and by the ‘Conseil régional de Languedoc Roussillon’ (arrêté no. 994187). C. Adrados was supported by a grant from both the ‘Office National des Forêts’ (ONF) and INRA. We would like to acknowledge the staff of the ‘Parc National des Cévennes’ and ONF for allowing the work be done and for their assistance with field-work. Very helpful comments from J.-F. Gerard and two anonymous reviewers improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pépin, D., Adrados, C., Janeau, G. et al. Individual variation in migratory and exploratory movements and habitat use by adult red deer (Cervus elaphus L.) in a mountainous temperate forest. Ecol Res 23, 1005–1013 (2008). https://doi.org/10.1007/s11284-008-0468-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-008-0468-2