Abstract
Fungi play a crucial role in the decomposition of lignin in fallen leaves but few studies have examined the functional roles of ligninolytic fungi associated with the decomposition of fallen leaves on tropical forest soils. This study examined fungal populations responsible for lignin decomposition in Castanopsis sieboldii leaves in a subtropical evergreen broad-leaved forest in southern Japan. Fallen leaves of C. sieboldii are characterized by the occurrence of bleached portions attributable to fungal colonization of leaf tissues and decomposition of lignin. The bleached area accounted for 29.7%, on average, of the total area of C. sieboldii fallen leaves in the study site. Leaf mass per unit area (LMA) and lignin content were lower in the bleached area than in the surrounding nonbleached area of the same leaves, indicating that removal of lignin enhanced mass loss from leaf tissues and created small-scale heterogeneity of decomposition within single leaves. An unidentified species of Lachnocladiaceae (Basidiomycetes) was isolated frequently from the bleached area and caused selective decomposition of lignin in leaves under pure culture conditions, indicating that this fungus was responsible for the bleaching. The greater hyphal length of basidiomycetes in the bleached area than in the nonbleached area supported the finding that this Lachnocladiaceae sp. was associated with the bleaching. The relatively rapid decomposition of C. sieboldii leaves on the subtropical forest soil is partly attributable to colonization of the litter by this Lachnocladiaceae sp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungi play a crucial role in leaf litter decomposition and contribute to soil respiration, nutrient recycling, and build-up of soil organic matter (Swift et al. 1979). This is because they decompose the lignocellulose matrix in litter that other organisms are rarely able to decompose (Cooke and Rayner 1984). Tropical forests have been investigated for the diversity and species composition of litter-inhabiting macro and microfungi (Bills and Polishook 1994; Lodge and Cantrell 1995; Lodg 1996, 1997; Sharma et al. 1995; Paulus et al. 2003, 2006b; Rambelli et al. 2004; Santana et al. 2005) and the succession of fungal assemblages during leaf litter decomposition (Promputtha et al. 2002; Tokumasu and Aoki 2002; Tang et al. 2005; Paulus et al. 2006a). Nevertheless, we still know little about the functional roles of fungi in the decomposition of lignin in tropical leaf litter (Osono 2007). This is despite the fact that the decomposition process of leaf litter in tropical soils is characterized by more rapid removal of lignin (Musvoto et al. 2000; Hirobe et al. 2004b), which leads to faster decomposition of leaf litter and lower accumulation of soil organic carbon than in temperate soils (Takeda 1998).
Osono (2006a) recently developed an approach to examine the abundance and activity of ligninolytic fungi in tropical and temperate forests with reference to the occurrence of bleached portions on leaves of broad-leaved woody species. The presence of bleached portions is associated with fungal colonization of leaf tissues and decomposition of lignin (Osono and Takeda 2001; Koide et al. 2005a, b). Osono (2006a) showed in a preliminary survey that the bleached area on the leaf surfaces was generally greater in tropical forests than in temperate forests. The bleaching agents in temperate forests included some ascomycetous genera and their anamorphs and basidiomycetes, but the agents in tropical forests were yet to be identified. More studies are necessary to explain the occurrence of the bleached area and fungal populations responsible for lignin decomposition in leaf litter on tropical forest soils.
The purpose of this study was to examine fungal populations responsible for lignin decomposition in fallen leaves in a subtropical evergreen broad-leaved forest in southern Japan. Leaves of Castanopsis sieboldii (Makino) Hatusima, a dominant fagaceous tree in the forest, were studied, because this litter is characterized by the occurrence of bleached portions (Fig. 1). Fallen leaves of C. sieboldii collected from the forest floor was examined for bleached area and chemical composition of the bleached and surrounding nonbleached portions of the same leaves to evaluate lignin decomposition by fungi. Fungi were isolated and hyphal abundance was determined in the bleached and nonbleached portions to describe fungal assemblages associated with the decomposition and bleaching of C. sieboldii leaves. Fungi were also isolated from green leaves to test the possibility that fungi from live leaves can persist after litter fall and take part in the decomposition of the litter. Finally, major fungal species were examined their ability to decompose lignin in sterilized leaves under pure culture conditions to identify fungal species responsible for the bleaching of the litter.
Methods
Study site and sample collection
Sampling was conducted in an evergreen broad-leaved subtropical forest in the University Forest, University of the Ryukyus, in the northern part of Okinawa Island in the Ryukyu Islands, south-western Japan (26˚9′N, 128˚5′E, ca 250–330 m a.s.l.). The mean annual temperature was 22°C and the annual precipitation was 2456 mm during the years between 1992 and 1999 (Yona Experimental Forest, University of the Ryukyus). Minimum temperature was 5.4°C in January and minimum mean monthly precipitation was 130 mm in December. Typhoons frequently strike the island between July and October. Foliage litterfall peaks in March and August (Xu et al. 1998). The topography is hilly and dissected. The bedrock is composed of sandstone and slate, and yellow soil has developed. The forest stand was dominated by C. sieboldii, Schima wallichii ssp. liuliuensis (Nak.) Bloemb. with a maximum height of 20 m (Enoki 2003). Logging and other forest practices have not occurred for at least 50 years. Decomposition of leaf litter of the dominant tree species, including C. sieboldii, has been studied previously at the study site (Alhamd et al. 2004; Xu et al. 2004; Xu and Hirata 2005).
C. sieboldii leaves were collected in December 2004 and December 2006 in a forest stand adjacent to the study plot (200 m × 200 m) established by Enoki (2003). In December 2004, six quadrats (15 cm × 15 cm) were set on the forest floor along a 11-m transect at two-meter intervals, and C. sieboldii fallen leaves were collected from the forest floor within the quadrats and used for measurement of leaf area and leaf mass per unit area (LMA), and for chemical analysis. A total of 20 fallen leaves on which bleaching was evident were collected around the quadrats and used for isolation of fungus. A total of 20 healthy-looking green leaves attached to five C. sieboldii trees 2–4 m in height were also harvested around the transect and used for isolation of fungus. In December 2006, ten sets of bleached leaves of C. sieboldii, each including 30–40 leaves, were collected from the forest floor along the transect at one-meter intervals and used for hyphal length estimation. The leaves were placed in paper bags, preserved at room temperature (ca 15–20°C), and taken back to the laboratory.
Measurement of leaf area and leaf mass per unit area
Leaves for leaf-area measurement were pressed between board papers and oven-dried at 40°C for 1 week. The leaves were then photocopied and scanned with a photoscanner (Epson GT-8000). The area of bleached portions and total leaf area were measured (Koide et al. 2005a) by image analysis performed on a Macintosh computer using public domain NIH image software (written by Wayne Rasband, US NIH). The bleached area as a proportion of total leaf area was expressed as a percentage and mean values of the proportions were calculated. Leaf disks (6 mm in diameter) were then punched from the bleached area and from the surrounding nonbleached area of the same leaves, by use of a cork borer. Ten to 14 disks were obtained for each quadrat. The disks were oven-dried again at 40°C for 1 week to calculate LMA. The disks were then combined to make one sample each of bleached and nonbleached parts from each quadrat, ground in a laboratory mill to pass through a 0.5-mm screen, and used for chemical analysis.
Chemical analysis
The lignin content in the samples was estimated by gravimetry, according to a standardized method using hot sulfuric acid digestion (King and Heath 1967). Samples were extracted with alcohol–benzene at room temperature (15–20°C), and the residue was treated with 72% (v/v) sulfuric acid for 2 h at room temperature with occasional stirring. The mixture was diluted with distilled water to furnish a 2.5% sulfuric acid solution and autoclaved at 120°C for 60 min. After cooling, the residue was filtered and washed with water through a porous crucible (G4), dried at 105°C, and weighed as acid-insoluble residue. The filtrate (autoclaved sulfuric acid solution) was used for total carbohydrate analysis. The amount of carbohydrates in the filtrate was estimated by means of the phenol–sulfuric acid method (Dubois et al. 1956). One milliliter 5% (v/v) phenol and 5 mL 98% (v/v) sulfuric acid were added to the filtrate. The optical density of the solution was measured by use of a spectrophotometer at 490 nm, using known concentrations of D-glucose as standards. Total N content was measured by automatic gas chromatography (Sumigraph NC-900 NC analyzer; Sumitomo Chemical, Osaka, Japan). The methods are described in detail elsewhere (Osono 2004). The term “lignin” is commonly used for the acid-insoluble material as determined by the sulfuric acid digestion method. Although the lignin fraction contains not only true lignin but also lignin-like materials such as melanins and humic substances produced during decomposition, in this study, for the sake of simplicity, the term “lignin” is used for the suite of lignin and lignin-like materials.
Isolation of fungus
A surface disinfection method (Kinkel and Andrews 1988) and a modified washing method (Tokumasu 1996) were used for isolation of fungi from green and fallen leaves of C. sieboldii (Osono 2005). Fungus was isolated 4 days after harvesting. Two leaf disks were punched from a single green leaf with a sterile cork borer (6 mm in diameter), making a total of 40 disks from the 20 green leaves; 20 of the 40 disks were used for the surface disinfection method and the other 20 for the washing method. Using a sterile cork borer, two leaf disks were punched from the bleached portion of a single fallen leaf and another two disks from a nonbleached portion of the same leaf, making a total of 80 disks from the 20 fallen leaves; 40 of the 80 disks were used for the surface disinfection method and the other 40 for the washing method.
For surface disinfection, leaf disks were submerged for 30 s in 70% (v/v) ethanol, to wet the surface, then surface-disinfected for 15 s in a solution of 15% (v/v) hydrogen peroxide and submerged for 30 s in 70% ethanol. The disks were rinsed with sterile, deionized water, transferred to sterile filter paper in Petri dishes (9 cm in diameter), and dried for 24 h to suppress vigorous bacterial growth after plating (Widden and Parkinson 1973). The disks were placed on 9-cm Petri dishes containing lignocellulose agar (LCA) modified in accordance with Miura and Kudo (1970), two disks per plate. LCA contains glucose 0.1%, KH2PO4 0.1%, MgSO4.7H2O 0.02%, KCl 0.02%, NaNO3 0.2%, yeast extract 0.02%, and agar 1.3% (w/v).
For modified washing, leaf disks were washed in a sterile test tube and agitated in a vertical shaker for 1.5 min to isolate fungi growing actively on the surface. The disks were washed serially in five changes of 0.005% (w/v) Aerosol-OT (di-2-ethylhexyl sodium sulfosuccinate) solution and rinsed five times with sterile distilled water. The washed disks were treated in the same manner as that used in the plating-out procedure for the surface disinfected disks.
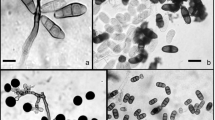
Plates were incubated at 20°C in the dark and observed 3 days and 2, 4, and 8 weeks after surface disinfection. Any hyphae or spores on the plates were subcultured on fresh LCA plates, incubated, and identified. Identification was based on micromorphological observations, with reference to Domsch et al. (1980) and Ellis (1971, 1976). Two fungal morphotaxa which were isolated frequently from live or fallen leaves were identified by DNA analysis according to the method described by Hirose and Osono (2006). The taxon of Lachnocladiaceae sp. and Valsaceae sp. were determined on the basis of the DNA sequences of 28S rRNA gene D1/D2 region of the isolates using primers D1 (Peterson 2000) and NL4 (O’Donnell 1993) (DDBJ accession numbers AB334107 and AB334108, respectively). The frequency of occurrence of individual species was calculated as a percentage based on the number of leaf disks with the species of the 20 leaf disks tested for each leaf type with each isolation method. A fungal species was arbitrarily regarded as frequent when the frequency of occurrence of the species determined by either of the two methods was more than or equal to 30% on any of green leaves, bleached portions of fallen leaves, or nonbleached portions of fallen leaves. Fungal species other than the frequent species are mentioned only when of special interest.
Hyphal length estimation
Bleached and nonbleached portions of leaves from ten quadrats were cut separately and used for hyphal length estimation by means of the agar-film method of Jones and Mollison (1948) but with several modifications (Koide et al. 2005a). Samples (1.0 g fresh weight) were homogenized in a blender at 10,000 rev/min in 49 ml deionized water for 3 min. The suspension (20 ml) was diluted with 20 ml molten agar solution (final concentration 1.5% w/v) and mixed at low speed on a magnetic stirring plate. Two agar films were prepared for each suspension in a hemocytometer (0.1 mm depth), transferred to glass slides, and dried for 24 h. The films were stained with fluorescent brightener (FB) for 1 h. The FB binds to chitin in the fungal cell wall (West 1988) and enables visualization of all hyaline hyphae.
The stained films were mounted between slides and coverslips with one drop of immersion oil (type DF; Cargille Laboratories, Cedar Grove, NJ, USA) and examined with a Nikon Microphot-SA epifluorescent microscope equipped with a high-intensity mercury light source. A Nikon UV-1A filter cube was used for examination of FB-stained hyphae. Darkly pigmented hyphae that were not stained with FB were observed by bright-field microscopy. Microscope fields were selected randomly and 25 fields were observed for each slide at 1,000× magnification. Hyphal lengths were estimated using an eyepiece grid and the grid-intersection method (Olson 1950). Total hyphal length was calculated as the sum of FB-stained hyphal length and darkly pigmented hyphal length. In this study, hyphae with clamp connections were classified as Basidiomycota. It was difficult, however, to estimated the hyphal length of individual basidiomycete species in decomposing leaves. The length of clamp-bearing hyphae in leaves was, therefore, estimated as total biomass of basidiomycetes, even though this may have resulted in underestimation of basidiomycete biomass because the frequency of clamp formation varies between species and because every hyphal segment observed does not necessarily bear clamp connections. Separate litter samples were dried at 40°C for 1 week to convert fresh weight to dry weight.
Pure culture decomposition test
Nineteen isolates of 19 species isolated from C. sieboldii leaves and obtained from culture collections (Table 1) were used for pure culture decomposition testing. Thirteen isolates were obtained from green leaves or the bleached or nonbleached portions of fallen leaves collected in December 2004 at the study site. In addition, Trametes versicolor, Collybia dryophila, and Mycena polygramma were obtained from a culture collection (IFO/NBRC, Chiba, Japan) and used in the previous decomposition tests (Osono and Takeda 2002, 2006; Osono et al. 2003, 2006). Microporus affinis, Xylaria sp.2, and Fistulina hepatica were isolated from sporocarps on decaying wood in Castanopsis forests. These isolates from culture collection and from wood were used for comparisons. Freshly fallen leaves of C. sieboldii were collected in December 2004 from the forest floor of the study site and used for the tests. The leaves were cut into pieces 1 cm in width, oven-dried at 40°C for 1 week, and preserved in a PVC bag until the experiment started.
The leaves (300 mg) were sterilized by exposure to ethylene oxide gas at 60°C for 6 h. The sterilized litter was placed on the surface of Petri dishes (9 cm in diameter) containing 20 ml 2% agar. Inocula for each assessment were cut from the margin of the previously inoculated Petri dishes on 2% malt-extracted agar (malt extract 2% and agar 2% (w/v)) with a sterile cork borer (6 mm in diameter) and placed on the agar adjacent to the litter, one plug per plate. The plates were incubated for 12 weeks at 20°C in the dark. The plates were sealed firmly with laboratory film during incubation so that moisture did not limit decomposition on the agar. After incubation the leaves were retrieved, oven-dried at 40°C for 1 week, and weighed. The undecomposed initial litter was also sterilized, oven-dried at 40°C for 1 week, and weighed to determine original mass. Four plates were prepared for each isolate, and four uninoculated plates served as a control. Mass loss of litter was determined as a percentage of the original mass, taking the mass loss of control litter into consideration. The duplicated leaves were then combined and used for chemical analyses as described above. Chemical analyses were performed for those fungi that caused mass loss of litter more than or equal to 5% of original litter mass.
Lignin/carbohydrate loss ratio (L/C) is a useful index of substrate-use pattern of each fungal species (Osono et al. 2006). L/C of each fungal species was calculated according to the equation:
L/C = mass loss of lignin (% original lignin mass)/mass loss of carbohydrates (% original carbohydrate mass).
Results
Bleached area, leaf mass per unit area, and chemical composition
Bleached area accounted for 29.7 ± 3.2% (SE, n = 6) of the total area of C. sieboldii fallen leaves at the study site. LMA was significantly (P < 0.001) lower in the bleached area (8.1 ± 0.5 mg/cm2, SE, n = 6) than in the nonbleached area of the same leaves (10.0 ± 0.5 mg/cm2). Lignin content was lower and amounts of total carbohydrates and nitrogen were higher in the bleached area than in the nonbleached area (Table 2).
Hyphal length and species composition of fungi
Total hyphal length was significantly greater in the bleached area than in the nonbleached area (Table 2). Darkly-pigmented hyphal length and darkly-pigmented hyphal length as a percentage of total hyphal length were significantly greater in the nonbleached area than in the bleached area (Table 2). In contrast, clamp-bearing hyphal length and clamp-bearing hyphal length as a percentage of total hyphal length were significantly greater in the bleached area than in the nonbleached area (Table 2).
A total of 47 fungal taxa were isolated from C. sieboldii leaves, of which 11 taxa were isolated frequently (Table 3). Geniculosporium sp.1, Valsaceae sp., Pestalotiopsis distincta, and P. neglecta were frequently isolated from green leaves. Lachnocladiaceae sp. was isolated frequently from the bleached area of fallen leaves. Pestalotiopsis neglecta and Cylindrocladium spp. were frequent on the nonbleached area of fallen leaves. Penicillium lividum, Trichoderma viride, T. harzianum, T. hamatum, and Pochonia suchlasporia were frequent on both the bleached and nonbleached area of fallen leaves.
Decomposing ability of fungi
Mass loss of C. sieboldii leaves caused by 19 isolates ranged from 0.6 to 55.2% (Table 1). Bleaching of the litter was noticeable on leaves inoculated with Lachnocladiaceae sp. and Xylaria sp.1 obtained from C. siebodii fallen leaves and with T. versicolor, M. affinis, and C. dryophila obtained from culture collection and from wood. Of the 13 isolates from C. sieboldii leaves, the greatest mass loss was caused by Lachnocladiaceae sp., followed by Geniculosporium sp.1, P. distincta, Xylaria sp.1, and Beltrania rhombica. In contrast, mass losses caused by P. suchlasporia, P. lividum, and P. neglecta were lower than 5%. Valsaceae sp., three Trichoderma species, and Cylindrocladium sp. caused intermediate mass loss between 5 and 10%. Trametes versicolor, M. affinis, C. dryophila, and Xylaria sp.2 obtained from culture collection and from wood caused mass loss greater than or at a similar level to those caused by fungi isolated from C. sieboldii leaves, whereas M. polygramma and F. hepatica caused negligible mass loss.
Substantial mass loss of lignin was caused by Lachnocladiaceae sp., T. versicolor, M. affinis, and C. dryophila (Table 1). The lower mass loss of lignin was caused by P. distincta, Xylaria sp.1, T. harzianum, and T. hamatum. Mass loss of lignin was negative for B. rhombica, Valsaceae sp., and Cylindrocladium sp., indicating the net mass increase of acid-insoluble substances during the incubation. Mass loss of total carbohydrates was observed for all fungal isolates that caused mass loss of litter more than or equal to 5% (Table 1). Lachnocladiaceae sp. caused selective delignification with L/C of 2.2 that was greater than L/C of 0.7–1.0 for the other ligninolytic T. versicolor, M. affinis, and C. dryophila (Table 1).
Discussion
The results for frequency of fungal species in the bleached area and pure culture decomposition tests demonstrated that Lachnocladiaceae sp. was primarily responsible for selective delignification and bleaching of C. sieboldii leaves. This is supported by the greater hyphal length of basidiomycetes in the bleached area, suggesting that the bleaching was associated with the activity of basidiomycetes in the Lachnocladiaceae. L/C of 2.2 for Lachnocladiaceae sp. is at the highest of the range previously reported for litter-decomposing fungi from temperate forests (Osono and Takeda 2002, 2006; Osono et al. 2003, 2006). Previous studies also gave results consistent with those in this study that the bleaching of fallen leaves was caused by fungi capable of selective delignification—e.g., Xylaria sp. in Fagus crenata Bl. (Osono and Takeda 2001), Coccomyces sp. in Camellia japonica (Koide et al. 2005a, b), and Coccomyces sp. and Marasmius sp. in Gaultheria shallon (Osono et al. unpublished data). As far as we are aware, this study is the first to demonstrate fungal bleaching of leaves in the tropics.
Geniculosporium sp.1, Xylaria sp.1, Pestalotiopsis distincta, and three Trichoderma species had some ligninolytic abilities. Xylariaceous fungi are known to have ligninolytic ability (Whalley 1996). Pestalotiopsis species have been found to be cellulolytic (Osono et al. 2003; Koide et al. 2005a; Osono and Takeda 2006), but a species in Pestalotiopsis was recently shown to produce a ligninolytic enzyme (Hao et al. 2007). Thus, those fungi detected on the litter can partly participate in lignin decomposition in the bleached area and lignin can be decomposed to some extent in nonbleached portions simultaneously with carbohydrate decomposition. Beltrania rhombica and Cylindrocladium sp. were regarded as cellulolytic species and can utilize nonlignified cellulose in the nonbleached area and delignified cellulose in the bleached area. Penicillium lividum and Pochonia suchlasporia causing negligible mass loss of litter can be regarded as sugar fungi associated with the decomposition products and (or) intermediates produced by other ligninolytic and cellulolytic decomposers, for example Lachnocladiaceae sp. Saito (1960) demonstrated that the growth of sugar fungi can be supported by reducing sugars produced during the selective delignification of leaves by ligninolytic fungi.
The biodiversity and species richness of microfungi in tropical Asian forests have been addressed recently on fallen leaves of Castanopsis fissa (Tang et al. 2005) and other tropical trees (Promputtha et al. 2002; Paulus et al. 2003, 2006a, b). Tang et al. (2005) examined fungal succession on senescent untreated and autoclaved leaves of C. fissa over a four-month period in Hong Kong Island and identified a total of 38 fungal taxa. Paulus et al. (2006b) recorded 31–81 species per tree species on fallen leaves of six trees in an Australian tropical forest. The number of fungal taxa recorded on C. sieboldii is at the low end of this range, i.e. 47 taxa for both green and fallen leaves or 37 taxa for fallen leaves only. This may be partly because of the different method of fungal isolation and the location of study site in previous studies. Paulus et al. (2006b) reported that species accumulation curves against the number of leaves examined did not reach asymptotes when a total of 60 fallen leaves was examined for each of six tree species, suggesting that more species remain to be detected. Thus, the number of microfungal taxa recorded on C. sieboldii could increase when more leaves are examined.
Endophytic phyllosphere fungi of forest trees often take part in the decomposition of lignin in fallen leaves (Osono 2006b). Koide et al. (2005b) reported that endophytic fungi of live leaves of a temperate tree Camellia japonica L. were responsible for lignin decomposition in litter after leaf death. In the current study, Geniculosporium sp.1, Valsaceae sp., P. distincta, and P. neglecta were isolated from green leaves but occurred only infrequently on fallen leaves and (or) had limited ability to decompose lignin in fallen leaves. Thus, it is not probable that these fungi participate in lignin decomposition in fallen C. sieboldii leaves. The current result does not necessarily indicate the insignificant roles of tropical fungal endophytes in the decomposition. Biodiversity of tropical fungal endophytes has been addressed in recent studies (Arnold et al. 2001; Gamboa and Bayman 2001; Suryanarayanan et al. 2002; Arnold 2005; Promputtha et al. 2005; Duong et al. 2006), and extracellular enzymatic activity was detected for tropical endophytes (Lumyong et al. 2002). Promputtha et al. (2007) recently demonstrated that fungal endophytes of the tropical tree Magnolia liliifera existed as saprobes. Further studies are necessary regarding the roles of endophytic fungi in the decomposition in tropical forests.
Lignin content and LMA were lower in the bleached area of fallen C. sieboldii leaves than in the nonbleached area, indicating the removal of lignin by Lachnocladiaceae sp. enhanced the mass loss of leaf tissues and created a small-scale heterogeneity of decomposition within the single leaves. This difference between bleached and nonbleached area has been found on fallen leaves of other tree species on which the occurrence of a bleached area was evident (Osono 2006a). Lignin has been known as a major factor limiting decomposition of leaf litter not only in temperate regions (Berg and McClaugherty 2003; Osono and Takeda 2005) but also in tropical regions (Laishram and Yadava 1988; Arunachalam et al. 1998; Loranger et al. 2002; Hirobe et al. 2004a; Pandey et al. 2007). The level of colonization of fallen leaves by Lachnocladiaceae sp. (i.e. approximately 30% of leaf area available for fungal colonization) was at the highest of the range previously reported from tropical regions (Osono 2006a). Therefore, the relatively rapid decomposition of C. sieboldii leaves on the subtropical forest soil (Alhamd et al. 2004; Xu et al. 2004; Xu and Hirata 2005) is partly attributable to colonization of the litter by Lachnocladiaceae sp. This is in good agreement with the suggestion by Osono (2006a) that the colonization of fallen leaves by ligninolytic fungi plays a crucial role in decomposition processes in tropical forests.
Bleached areas can frequently be observed on fallen leaves of tree species other than C. sieboldii both at the study site and in several other tropical forests (T. Osono personal observation). More studies are necessary to examine whether the result of this study can be applied to fallen leaves of other tree species and to other tropical forest soils. Species richness and functional biodiversity of ligninolytic fungi and the succession of ligninolytic fungi during the decomposition processes also should be studied further in tropical forests.
References
Alhamd L, Arakaki S, Hagihara A (2004) Decomposition of leaf litter of four tree species in a subtropical evergreen broad-leaved forest, Okinawa Island, Japan. For Ecol Manag 202:1–11
Arnold AE (2005) Diversity and ecology of fungal endophytes in tropical forests. In: Deshmukh SK, Rai MK (eds) Biodiversity of fungi, their role in human life. Science Publishers, Plymouth, pp 49–68
Arnold AE, Maynard Z, Gilbert GS (2001) Fungal endophytes in dicotyledonous neotropical trees: patterns of abundance and diversity. Mycol Res 105:1502–1507
Arunachalam A, Maithani K, Pandey NH, Tripathi RS (1998) Leaf litter decomposition and nutrient mineralization patterns in regrowing stands of a humid subtropical forest after tree cutting. For Ecol Manag 109:151–161
Berg B, McClaugherty C (2003) Plant litter, decomposition, humus formation, carbon sequestration. Springer, Berlin
Bills GF, Polishook JD (1994) Abundance and diversity of microfungi in leaf litter of a lowland rain forest in Costa Rica. Mycologia 86:187–198
Cooke RC, Rayner ADM (1984) Ecology of Saprotrophic Fungi. Longman, London
Domsch KH, Gams W, Anderson TH (1980) Compendium of soil fungi. Vol. 1 and 2. Academic, London
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Duong LM, Jeewon R, Lumyong S, Hyde KD (2006) DGGE coupled with ribosomal DNA gene phylogenies reveal uncharacterized fungal phylotypes. Fungal Divers 23:121–138
Ellis MB (1971) Dematiaceous hyphomycetes. CAB International, Oxon
Ellis MB (1976) More dematiaceous hyphomycetes. CAB International, Oxon
Enoki T (2003) Microtopography and distribution of canopy trees in a subtropical evergreen broad-leaved forest in the northern part of Okinawa Island, Japan. Ecol Res 18:103–113
Gamboa MA, Bayman P (2001) Communities of endophytic fungi in leaves of a tropical timber tree (Guarea guidonia: Meliaceae). Biotropica 33:352–360
Hao J, Song F, Huang F, Yang C, Zhang Z, Zheng Y, Tian X (2007) Production of laccase by a newly isolated deuteromycete fungus Pestalotiopsis sp. and its decolorization of azo dye. J Ind Microbiol Biotechnol 34:233–240
Hirobe M, Sabang J, Bhatta BK, Takeda H (2004a) Leaf-litter decomposition of 15 tree species in a lowland tropical rain forest in Sarawak: decomposition rates and initial litter chemistry. J For Res 9:341–346
Hirobe M, Sabang J, Bhatta BK, Takeda H (2004b) Leaf-litter decomposition of 15 tree species in a lowland tropical rain forest in Sarawak: dynamics of carbon, nutrients, and organic constituents. J For Res 9:347–354
Hirose D, Osono T (2006) Development and seasonal variations of Lophodermium populations on Pinus thunbergii needle litter. Mycoscience 47:242–247
Jones PCT, Mollison JE (1948) A technique for the quantitative estimation of soil microorganisms. J Gen Microbiol 2:54–69
King HGC, Heath GW (1967) The chemical analysis of small samples of leaf material and the relationship between the disappearance and composition of leaves. Pedobiologia 7:192–197
Kinkel LL, Andrews JH (1988) Disinfestation of living leaves by hydrogen peroxide. Trans Br Mycol Soc 91:523–528
Koide K, Osono T, Takeda H (2005a) Fungal succession and decomposition of Camellia japonica leaf litter. Ecol Res 20:599–609
Koide K, Osono T, Takeda H (2005b) Colonization and lignin decomposition of Camellia japonica leaf litter by endophytic fungi. Mycoscience 46:280–286
Laishram ID, Yadava PS (1988) Lignin and nitrogen in the decomposition of leaf litter in a sub-tropical forest ecosystem at Shiroy hills in north-eastern India. Plant Soil 106:59–64
Lodg DJ (1996) Microorganisms. In: Reagan DP, Waide RB (eds) The food web of a tropical forest. The University of Chicago Press, Chicago, pp 53–108
Lodge DJ (1997) Factors related to diversity of decomposer fungi in tropical forests. Biodivers Conserv 6:681–688
Lodge DJ, Cantrell S (1995) Fungal communities in wet tropical forests: variation in time and space. Can J Bot 73(suppl. 1):S1391–S1398
Loranger G, Ponge JF, Imbert D, Lavelle P (2002) Leaf decomposition in two semi-evergreen tropical forests: influence of litter quality. Biol Fertil Soils 35:247–252
Lumyong S, Lumyong P, McKenzie EHC, Hyde KD (2002) Enzymatic activity of endophytic fungi of six native seedling species from Doi Suthep-Pui National Park, Thailand. Can J Microbiol 48:1109–1112
Miura K, Kudo M (1970) An agar-medium for aquatic hyphomycetes. Trans Mycol Soc Japan 11:116–118 (in Japanese)
Musvoto C, Campbell BM, Kirchmann H (2000) Decomposition and nutrient release from mango and miombo woodland litter in Zimbabwe. Soil Biol Biochem 32:1111–1119
O’Donnell K (1993) Fusarium and its near relatives. In: Reynolds DR, Taylor JW (eds) The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics. CAB International, Wallingford, pp 225–233
Olson FCW (1950) Quantitative estimates of filamentous algae. Trans Am Microsc Soc 69:272–279
Osono T (2005) Colonization and succession of fungi during decomposition of Swida controversa leaf litter. Mycologia 97:589–597
Osono T (2006a) Fungal decomposition of lignin in leaf litter: comparison between tropical and temperate forests. In: Meyer W, Pearce C (eds) Proceeding for the 8th International Mycological Congress, Cairns, Australia. Medimond, Italy, pp 111–117
Osono T (2006b) Role of phyllosphere fungi of forest trees in the development of decomposer fungal communities and decomposition processes of leaf litter. Can J Microbiol 52:701–716
Osono T (2007) Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 22:955–974
Osono T, Takeda H (2001) Effects of organic chemical quality and mineral nitrogen addition on lignin and holocellulose decomposition of beech leaf litter by Xylaria sp. Eur J Soil Biol 37:17–23
Osono T, Takeda H (2002) Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 94:421–427
Osono T, Takeda H (2005) Decomposition of lignin, holocellulose, polyphenol and soluble carbohydrate in leaf litter of 14 tree species in a cool temperate forest. Ecol Res 20:41–49
Osono T, Takeda H (2006) Fungal decomposition of Abies needle and Betula leaf litter. Mycologia 98:172–179
Osono T, Fukasawa Y, Takeda H (2003) Roles of diverse fungi in larch needle-litter decomposition. Mycologia 95:820–826
Osono T, Bhatta BK, Takeda H (2004) Phyllosphere fungi on living and decomposing leaves of giant dogwood. Mycoscience 45:35–41
Osono T, Hobara S, Koba K, Kameda K (2006) Reduction of fungal growth and lignin decomposition in needle litter by avian excreta. Soil Biol Biochem 38:1623–1630
Pandey RR, Sharma G, Tripathi SK, Singh AK (2007) Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. For Ecol Manag 240:96–104
Paulus B, Gadek P, Hyde K (2003) Estimation of microfungal diversity in tropical rainforest leaf litter using particle filtration: the effects of leaf storage and surface treatment. Mycol Res 107:748–756
Paulus B, Gadek P, Hyde K (2006a) Successional patterns of microfungi in fallen leaves of Ficus pleurocarpa (Moraceae) in an Australian tropical rain forest. Biotropica 38:42–51
Paulus B, Kanowski J, Gadek P, Hyde KD (2006b) Diversity and distribution of saprobic microfungi in leaf litter of an Australian tropical rainforest. Mycol Res 110:1441–1454
Peterson SW (2000) Phylogenetic analysis of Penicillium species based on ITS and lsu-rDNA nucleotide sequences. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood, Amsterdam, pp 163–178
Promputtha I, Lumyong S, Lumyong P, McKenzie EHC, Hyde KD (2002) Fungal succession on senescent leaves of Manglietia garrettii in Doi Suthep-Pui National Park, northern Thailand. Fungal Divers 10:89–100
Promputtha I, Jeewon R, Lumyong S, McKenzie EHC, Hyde KD (2005) Ribosomal DNA fingerprinting in the identification of non-sporulating endophytes from Magnolia liliifera (Magnoliaceae). Fungal Divers 20:167–186
Promputtha I, Lumyong S, Dhanasekaran V, McKenzie EHC, Hyde KD, Jeewon R (2007) A phylogenetic evaluation of whether endophytes become saprotrophs at host senescence. Microb Ecol 53:579–590
Rambelli A, Mulas B, Pasqualetti M (2004) Comparative studies on microfungi in tropical ecosystems in Ivory Coast forest litter: behaviour on different substrata. Mycol Res 108:325–336
Saito T (1960) An approach to the mechanism of microbial decomposition of beech litter. Sci Rep Tohoku Univ Ser IV (Biol) 26:125–131
Santana ME, Lodge DJ, Lebow P (2005) Relationship of host recurrence in fungi to rates of tropical leaf decomposition. Pedobiologia 49:549–564
Sharma GD, Mishra RR, Kshattriya S (1995) Fungi and litter decomposition in the tropics. In: Reddy MV (ed) Soil organisms and litter decomposition in the tropics. Westview Press, Boulder, pp 39–57
Suryanarayanan TS, Murali TS, Venkatesan G (2002) Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can J Bot 80:818–826
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Studies in Ecology Vol. 7. Blackwell, Oxford
Takeda H (1998) Decomposition processes of litter along a latitudinal gradient. In: Sassa K (ed) Environmental forest science. Kluwer, Dordrecht, pp 197–206
Tang AMC, Jeewon R, Hyde KD (2005) Succession of microfungal communities on decaying leaves of Castanopsis fissa. Can J Microbiol 51:967–974
Tokumasu S (1996) Mycofloral succession on Pinus densiflora needles on a moder site. Mycoscience 37:313–321
Tokumasu S, Aoki T (2002) A new approach to studying microfungal succession on decaying pine needles in an oceanic subtropical region in Japan. Fungal Divers 10:167–183
West AW (1988) Specimen preparation, stain type, and extraction and observation procedures as factors in the estimation of soil mycelial length and volumes by light microscopy. Biol Fertil Soils 7:88–94
Whalley AJS (1996) The xylariaceous way of life. Mycol Res 100:897–922
Widden P, Parkinson D (1973) Fungi from Canadian coniferous forest soils. Can J Bot 51:2275–2290
Xu X, Enoki T, Tokashiki Y, Hirata E (1998) Litterfall and the nutrient returns in evergreen broadleaved forests in northern Okinawa Island. Sci Bull Fac Agr Univ Ryukyus 45:195–208
Xu X, Hirata E, Enoki T, Tokashiki Y (2004) Leaf litter decomposition and nutrient dynamics in a subtropical forest after typhoon disturbance. Plant Ecol 173:161–170
Xu X, Hirata E (2005) Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 273:279–289
Acknowledgments
We thank Dr T. Enoki, Mr A. Takashima, and the staff of Yona Experimental Forest, University of the Ryukyus, for help with field work, Professor Dr H. Takeda for useful discussion, and Dr I Okane and Mr Y. Fukasawa for providing fungal isolates for the experiments. This study received partial financial support from the Sumitomo Foundation to T.O.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Osono, T., Ishii, Y. & Hirose, D. Fungal colonization and decomposition of Castanopsis sieboldii leaves in a subtropical forest. Ecol Res 23, 909–917 (2008). https://doi.org/10.1007/s11284-007-0455-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0455-z