Abstract
Seeds of the Japanese walnut, Juglans ailanthifolia, are usually scatter-hoarded by two rodent species, the Japanese squirrel Sciurus lis and the field mouse Apodemus speciosus, but only by the latter in several areas where S. lis is absent. We examined seed-size-mediated interactions of these three species across a wide geographic range. Field tracking of walnuts with miniature radio-transmitters revealed that squirrels hoarded larger seeds more frequently and at greater distances than smaller seeds. In contrast, mice hoarded smaller seeds more frequently and transported them farther than larger seeds. These seed dispersers could affect the evolution of seed size because seeds hoarded at sites farther from source trees are known to survive better until germination and as seedlings. We expect that larger seeds may be advantageous in regeneration if the main seed dispersers are squirrels, whereas smaller seeds may be advantageous if mice are the dominant dispersers. These predictions were supported by the fact that seed size was smaller on islands inhabited only by mice and at the edge of the squirrel distribution, compared to areas where mice and squirrels are both common.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species interactions are thought to be a major force driving evolutionary change and promoting biodiversity (Thompson 2005). Thompson (2005) formalised the geographic perspective for the study of species interactions within the framework of the geographic mosaic theory of coevolution. Species do not coevolve as a unit across their geographic ranges; that is, interacting species coevolve in different ways in different populations, often creating a geographic mosaic of traits. Seed and seed predator interactions provide a good example because plant species often interact with an assemblage of predators that varies in species composition across the plant’s distribution area, rather than with a single predator species. Plant seeds are vulnerable to predation by animals because they are concentrated sources of nutrients and energy. Plants have generally evolved defensive characteristics such as hard shells, chemical compounds, and spines to protect seeds from predation (e.g. Elliott 1974; Steele et al. 1993; Coffey et al. 1999; Kirkpatrick and Pekins 2002). Local adaptations in interspecific interactions and resultant local differences in traits occur because the main seed predators of a plant species often change with species composition among different localities (Benkman 1995, 1999; Benkman et al. 2001; Mezquida and Benkman 2005).
On the other hand, some plant species use animals as mechanisms of seed dispersal through endozoochory or scatter-hoarding (Vander Wall 1990). Seed traits may be affected not only by protection against predation but also by the preferences of seed dispersers. Geographic mosaic perspectives could also be applied to seed and seed disperser interactions if a single species of seed disperser is not distributed throughout the range of a target plant species. Plant species that depend on scatter-hoarding animals for seed dispersal generally produce smaller numbers of larger, more nutritious seeds than most plants with other dispersal modes (Vander Wall 1990). Vander Wall (2001) reviewed fossil records and suggested that the seed sizes of tree genera dispersed by scatter-hoarding animals have increased since the Palaeocene. In feeding experiments in which rodents were provided with different species of seeds, larger seeds tended to be hoarded in preference to smaller ones (Stapanian and Smith 1984; Hurley and Robertson 1987; Vander Wall 1995). Within the same plant species, rodents transport larger seeds farther and hoard them at more distant locations than they do smaller seeds (Hurley and Robertson 1987; Jansen et al. 2002). Therefore, strict directional selection by seed hoarders might be included in the process of seed dispersal.
Seed sizes often vary among individuals within plant species (e.g. Janzen 1982; Venable 1992; Moegenburg 1996; Seiwa 2000; Brewer 2001; Seiwa et al. 2002). It was thought that several conflicting selective forces maintain a range of seed sizes, for example, the ability to escape pathogens and predators and to germinate and become successfully established (e.g. Howe and Richter 1982; Morse and Schmitt 1985; Silvertown 1989; Moegenburg 1996; Seiwa 2000; Seiwa et al. 2002; Gómez 2004). The Japanese walnut, Juglans ailanthifolia Carriere, is widely distributed throughout riparian forests in Japan (Horikawa 1976). Seiwa (2000) reported the seed-size variation of this species as ranging from 4.00 to 13.99 g in mass; however, differences in seed sizes did not reflect the growth and survival rates in seedlings. In the present study, we examined dispersal distances by hoarding as one of the factors affecting geographic variation in walnut seed size. In central to southern Japan, two rodent species—the Japanese squirrel Sciurus lis Temminck and the Japanese field mouse Apodemus speciosus (Temminck)—are known to be important predators, and hoarding by these animals contributes to walnut seed dispersal (Tamura and Shibasaki 1996; Tamura et al. 1999, 2005; Tamura 2001). Bears and crows also occasionally consume the seeds, but do not play important roles in seed dispersal (Nihei 1995; Koike et al. 2003). Sciurus lis stores seeds separately in the litter layer on the ground (terrestrial scatter-hoarding) although seeds are sometimes stored among twigs on trees at 0.5–12.0 m above the ground (arboreal hoarding) (Tamura and Shibasaki 1996). Apodemus speciosus also scatter-hoards seeds in the litter layer but occasionally stores two or more seeds in the same burrows (larder hoarding) (Tamura 2001). Tracking of seeds using miniature radio transmitters has revealed that some of the scatter-hoarded seeds on the ground and some fallen seeds from arboreal sites on the ground are not retrieved until spring, and can germinate (Tamura and Shibasaki 1996; Tamura 2001). Seeds larder-hoarded by mice also have a chance to germinate, although germination rates decrease with seeded depth (80% at 50 mm to 13% at 300 mm depth; Saito and Miyaki 1983).
Because these two rodent species differ greatly in body size, i.e. 250–310 g for S. lis and 20–60 g for A. speciosus (Abe 2002), we expect interspecific differences in transport and hoarding behaviour to be mediated by seed size. Furthermore, A. speciosus is distributed widely throughout central to southern Japan, including on small islands, whereas S. lis is restricted to more northern areas and is absent on small islands (Fig. 1). We expect that spatial variation in the presence or absence of S. lis may affect geographic differences in walnut size. In this study, we first observed the hoarding behaviour of small and large walnuts by the two rodent species. Second, we compared seed-size across localities with and without squirrels, and finally we tested the idea that the variation may illustrate the geographic mosaic caused by differences in local interactions among walnuts, squirrels, and mice.
Distribution of the Japanese squirrel, Sciurus lis (hatched area) and the 11 locations at which seeds of the Japanese walnut, Juglans ailanthifolia, were collected for comparisons of seed size. The squirrel is distributed in only a part of Honshu and Shikoku. In contrast, the Japanese field mouse, Apodemus speciosus, is commonly distributed at all collection locations, including on two small islands. 1 Aomori, 2 Akita, 3 Sado Island, 4 Niigata, 5 Nagano, 6 Tokyo, 7 Fukui, 8 Oki Island, 9 Tottori, 10 Tokushima on Shikoku, 11 Kumamoto on Kyushu. Numerals indicate latitude (°N) and longitude (°E)
Materials and methods
Seed-size mediated hoarding behaviour
We made observations in a forest in the Tama Forest Science Garden (TFSG), Hachioji, Tokyo (35°38′N, 139°17′E). The study sites were natural forests surrounded by various types of forests including conifer plantation, secondary deciduous forest, and arboretum (Tamura 2004). The tall tree layer is dominated by Abies firma and Quercus glauca with a few trees of Aucuba japonica and Q. glauca in a lower tree layer. A single feeding box (100 × 150 × 200 mm) was placed on the ground at each of three study sites (sites A, B, and C). The three sites were at least 200 m apart. The entrances of the feeding boxes were covered with wire net that allowed mice to enter, but prevented squirrels from doing so. Groups of large and small walnut seeds, collected from ten individual trees at Chiyoda, Ibaraki Prefecture, were used in the experiments after each seed was weighed. Three large and three small seeds were placed in each feeding box together. Each walnut was fitted with miniature radio-transmitters on the surface of the shell (each specific frequency around 50 MHz; R1650, Advanced Telemetry Systems, Isanti, MN) by adhesives including epoxy resins (CA-152, Cemedine, Tokyo, Japan). The weight of a transmitter was 4 g, including adhesive material. According to Tamura (1996), S. lis took away the provided walnuts with sham transmitters (ranging from 0.8 to 5.0 g in weight) regardless of their weight at the feeding stand, and the transport distance for hoarding did not differ between walnuts with two types of transmitters (2.5 and 4.0 g in weight). Sone and Kohno (1999) reported that transported distances by A. speciosus were not significantly different between acorns numbered with ink and those with transmitters. Therefore, we assumed that the application of transmitters would not have much effect on transporting and hoarding behaviour of S. lis and A. speciosus. The feeding boxes were checked every day; when the radio-tagged seeds were removed, their locations were traced using a receiver (FT-690, Yaesu, Tokyo, Japan). The distances from feeding boxes to hoarding sites were measured using a tape measure to the nearest 0.1 m. This experiment, using the three large and three small radio-tagged seeds, was conducted ten times per feeding box during the period from November 2003 to January 2004.
For squirrels, feeding stands were placed at three study sites (sites D, E, and F) located at least 500 m apart. The stands were 1.5 m in height and the legs were covered with vinyl sheeting to prevent mice from climbing them. Five large and five small seeds with radio-transmitters were placed on each stand and were monitored in the same way as for mice. This procedure was performed six times per feeding stand during the period from November 2004 to February 2005.
We examined the structure of 50 seeds collected at Chiyoda, which was helpful in interpreting the seed preferences of the two rodent species. We measured shell length and shell thickness to the nearest 0.01 mm using slide callipers, and total seed weight and edible material weight to the nearest 0.01 g in fresh weight. Because the basal half of the shell was thicker than the apical half, we used the average thickness of the basal and apical halves of the shell. Allometric relationships were quantified from log-transformed data regressions (least square regressions; Crawley 2002).
Seed-size variation among populations
We compared walnut seed size at 11 localities (Fig. 1). Six populations (No. 1, 2, 4, 5, 6, and 7) were selected in the area where squirrels were common, two (No. 9 and 10) were selected from marginal areas of the squirrel distribution range where squirrels are uncommon, and three (No. 3, 8, and 11) were selected from islands where squirrels are absent. Sado and Oki islands (No. 3 and 8) were isolated from Honshu, a main habitat of S. lis, during the Pleistocene (Minato 1977). Although several historical records of S. lis exist from Kyushu (No. 11), they are doubtful because no reliable specimens have been preserved, and no fossils have been found so far (Yasuda 2007). Mice are commonly observed at all localities. About 20 seeds were sampled from a single tree and the diameter at breast height (DBH) of the tree was recorded. Data were obtained from 11 to 33 trees per locality (ca. 100 km2 in area) in the fruiting seasons from August to October in 2004 and 2005. After the pulp was removed, the seeds were washed with water and dried at room temperature. The maximum shell length, width, and depth were measured, and the seed size was calculated as a spherical shape, [(length/2 × width/2 × depth/2) × 4π/3] mm3.
Seed-size variation within a single tree and among trees was compared using the coefficient of variation (CV%). Intra-specific or inter-specific variation in seed size has often been argued in relation to latitude, elevation, and/or temperature (Ellison 2001; Moles and Westoby 2003). Several studies have investigated whether seed mass increases with tree size or age (Hardin 1984; Venable 1992). In the present study, therefore, annual mean temperature, tree size and seed disperser were considered as factors affecting geographic variation of walnut seed size. A generalised linear model (GLM) in R 2.2.1 software (R Development Core Team 2005) was used to identify the factors that determine the seed size of individual trees (n = 246) according to Akaike Information Criterion (AIC) as a measure of the fit of a model (the smaller the AIC, the better the fit). Collecting sites of each population were dispersed in a 100 km2 area often including complex topography, and elevation differences of collecting sites in a population ranged from 92 m (Oki island) to 1,147 m (Shikoku). Therefore, we estimated the temperature for each collecting site. The annual mean temperature was calculated by a multiple regression equation of temperature (°C) = 38.17 − 0.882 latitude (°N) + 0.0573 longitude (°E) − 0.00585 altitude (m), based on meteorological data recorded at 158 observation sites in Japan (r = 0.986, P < 0.0001; Sota et al. 2000). We obtained the latitude, longitude and altitude of each tree using a Global Positioning System (GPS: Geko201 Garmin International, Olathe, KS). The DBH was used as tree size. The factor “seed dispersers” was categorised to reflect whether the tree was located within, outside, or at the boundary of the current squirrel distribution range.
Results
Seed structure
The thickness of seed shells (y mm) ranged from 3.53 mm to 6.22 mm with a mean of 4.83 mm, and increased with shell length (x mm) with a slightly negative allometric relationship (log y = 0.786 log x − 0.527, r = 0.875, n = 50, P < 0.001; 95% confidence range of the slope 0.660–0.912). The edible material weight (z g) ranged from 1.07 g to 3.66 g with a mean of 2.13 g, and increased isometrically with the total seed weight (w g) (log z = 0.900 log w − 0.558, r = 0.930, n = 50, P < 0.001; 95% confidence range of the slope 0.797–1.003). The proportion of edible to total seed weight was 22.3% (range 17.1–30.0).
Hoarding behaviour
The number of walnuts hoarded by mice at each study site is summarised in Table 1. The large and small seeds provided in the feeding boxes weighed 13.33 ± 0.94 g (mean ± SD, n = 90) and 5.33 ± 0.62 g (n = 90), respectively. Although statistically significant only at site C, mice tended to hoard more small seeds than large seeds. In total, hoarding of 52.2% of 90 large seeds and 76.7% of 90 small seeds occurred, thus small seeds were more likely to be hoarded. Only 4 of the 47 hoarded large seeds were larder-hoarded underground together with eaten walnuts, intact walnuts without transmitters, and several species of acorns. The other 43 large and all the small seeds were scatter-hoarded. None of the provided walnuts had been eaten by the next day when we retrieved them.
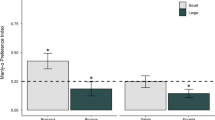
At all study sites, mice transported and hoarded small seeds farther from the feeding box than large ones (Mann–Whitney U-test; site A: U = 57, site B: U = 126, site C: U = 93.5, all P < 0.01; Fig. 2a). The mean transported distance to the cache site was 8.6 m (range 0.1–27.0 m) for large seeds and 16.7 m (1.6–46.5 m) for small seeds.
The number of walnuts hoarded by squirrels at each study site is summarised in Table 2. The large and small seeds used in the experiment weighed 14.02 ± 0.73 g (mean ± SD, n = 90) and 4.94 ± 0.74 g (n = 90), respectively. At all study sites, squirrels tended to hoard larger walnuts and to eat smaller walnuts immediately. In total, squirrels hoarded large seeds more frequently, i.e. 90.0% of 90 large seeds, but only 60.0% of 90 small seeds were hoarded. Of the large seeds, 35 (43.2%) were hoarded arboreally and the remaining 46 (56.8%) were hoarded on the ground. Of the small seeds, 15 (27.8%) were hoarded arboreally and 39 (72.2%) were hoarded on the ground. The ratios of arboreal to terrestrial hoarding were not affected by seed size (Chi-square test: χ2 = 3.31, P > 0.05).
At all study sites, the transported distance of squirrels was significantly greater for large seeds than for small seeds (Mann–Whitney U-test; site D: U = 64, site E: U = 63, site F: U = 81, all P < 0.01; Fig. 2b). The mean transported distance was 16.6 m (range 0.1–108 m) for large seeds and 4.1 m (0.1–13 m) for small seeds.
Seed-size variation among populations
The mean CVs in seed size within single trees ranged from 7.66 to 12.27% among the 11 populations, whereas the CVs among trees ranged from 13.23 to 28.11% (Table 3). This indicates that the variation in seed size within individual trees was much lower than that among individuals. The variation in seed size did not differ between localities with or without squirrels (CV at localities with squirrels: 13.23–21.31, CV at localities without or few squirrels: 15.20–28.11, Table 3).
GLM analysis revealed a negative correlation between annual mean temperature and seed size. Seed dispersers also correlated with seed size significantly, whereas tree size did not. The populations without or with only few squirrels as seed dispersers (crosses and triangles in Fig. 3) tended to have smaller seed sizes than the populations with squirrels (dots in Fig. 3). Based on AIC minimisation, seed dispersers were the most important factor affecting seed size: AIC was never decreased by any model including temperature or tree size with or without their interactions (Table 4).
Relation between seed size [(shell length/2 × width/2 × depth/2) × 4π/3, mm3] of Japanese walnut trees at 11 locations and annual mean temperature. Locations within the distribution range of Japanese squirrels (populations 1, 2, 4, 5, 6, and 7) are indicated by dots. Locations in marginal regions (populations 9 and 10) and those outside of the squirrel’s distribution range (populations 3, 8, and 11) are indicated by triangles and crosses, respectively
Discussion
Japanese squirrels hoarded larger walnuts more frequently and transported them farther than smaller seeds. This tendency may be because of the higher energy gain (larger amount of edible material) from larger seeds for squirrels, making large seeds worthwhile to hoard. Theoretical models of seed hoarding predict that larger seeds should be hoarded at greater distances than smaller ones (Stapanian and Smith 1978; Clarkson et al. 1986). Comparisons of the transport distances and frequencies of hoarding between foods having different size and/or preference for target animals have been widely reported. Larger Carapa seeds (C. procera) are hoarded more frequently than smaller seeds by red acouchis (Myoprocta acouchy; Jansen et al. 2002; Jansen et al. 2004). Fox squirrels (Sciurus niger) transport seeds of black walnut (Juglans nigra) farther than acorns (Quercus macrocarpa and Q. muehlenbergii), probably to protect the relatively valuable walnuts from cache robbers, which are abundant near the source (Stapanian and Smith 1984). American red squirrels (Tamiasciurus hudsonicus) hoard cones of red pine (Pinus resinosa), which contain many seeds, more frequently than cones of Scots pine (P. sylvestris), which contain fewer seeds (Hurley and Robertson 1987). Yellow pine chipmunks (Tamias amoenus) prefer seeds of Jeffrey pine (P. jeffreyi) to those of bitterbrush (Purshia tridentata) and transport the former farther than the latter for hoarding (Vander Wall 1995). Hoarding at farther distances may be advantageous to animals because of a decreased risk of pilfering, although long-distance locomotion incurs higher costs (e.g. Tamura et al. 1999). Hoarders may be willing to incur high costs if they can retrieve sufficient rewards from their caches, which is the case when seeds are large or preferred.
We found the opposite result for the field mouse. This species transported smaller seeds (smaller amount of edible material) more frequently and farther than larger seeds. The different responses by squirrels and mice toward walnut seed sizes may be partly related to their feeding behaviour. Mice shave and make holes in the shells to eat walnuts (Tamura et al. 2005). Smaller nuts have proportionately thinner shells and mice may therefore be able to gnaw holes more easily. This may explain why mice prefer smaller nuts. In contrast, squirrels shave only a part of the raphe of the shells and crack the halves with their teeth (Tamura et al. 2005). Thus, the effect of shell thickness on squirrel feeding behaviour may be weak.
Alternatively, smaller nuts may be easier for mice to carry because of their smaller body size compared to squirrels. The spiny pocket mouse (Heteromys desmarestianus) buries smaller seeds of palm (Astrocaryum mexicanum) farther from the source than larger seeds, although it preferentially removes larger seeds (Brewer 2001). The white-tailed rat (Uromys caudimaculata) hoards medium-sized seeds of Beilschmiedia bancroftii more frequently although the seed size does not affect the hoarding distance (Theimer 2003). Thus, it seems costly for small rodents to eat large seeds and to transport them long distances.
The effect of distance from the mother tree in increasing seedling survival (distance-dependent mortality) has been well examined for various tree species in both tropical and temperate forests (Clark and Clark 1984; Shibata and Nakashizuka 1995; Wada et al. 2000; Wenny 2000). The seed survival pattern of Japanese walnut fits this model; survival rates of artificially hoarded seeds are generally lower near the source tree and increase rapidly with distance from 0 to 40 m (Tamura et al. 1999). Survival rates of seedlings are also higher at sites farther from a mother tree because saplings >2-years old are distributed farther from the mother tree than are current year seedlings (Tamura et al. 2005). Under such conditions, we can expect that larger seeds would be advantageous for regeneration if the main seed dispersers are squirrels, whereas smaller seeds may be advantageous if mice are the dominant dispersers. Presumably, squirrels hoard larger seeds whereas mice hoard smaller seeds, so that all seeds are eventually hoarded and thus there should be more variation in seed size in regions with mice and squirrels than in regions inhabited only by mice.
The observed geographic differences in seed size support the former prediction that seed size is correlated mostly to the disperser species (Table 4). Seed size at localities with squirrels tended to be larger than seed size at sites without or with only a few squirrels (Fig. 3). However, the second prediction was unclear. The CVs of the population seed size were not always greater in regions with mice and squirrels than in those with only mice (Table 3). We think that squirrels are more important seed dispersers than mice even in regions with both species. Squirrels harvest most of the walnuts on the trees before they fall to the ground in late autumn (Tamura et al. 2005). On the other hand, mice use various kinds of seeds that are available on the ground, such as acorns and drupes, rather than walnuts (Tamura et al. 2005). Consequently, the Japanese squirrel may be a major disperser of walnuts at the locality where they are distributed, so that the CVs of the population seed size are not great as expected.
Although the present study indicated the possibility that walnut seed size is affected by the disperser species, seed size is also affected by many other factors. In fact, intraspecific variation in seed size is common in plants (Janzen 1982; Venable 1992; Moegenburg 1996; Seiwa 2000; Brewer 2001; Seiwa et al. 2002; Yamada et al. 2002; Yamada and Miyaura 2005), and several hypotheses have been considered that might account for seed size. One such hypothesis is that the survival rates at the pre-germination stage are considered important in evolution of seed size, i.e. large seeds are known to receive higher predation rates by insects and mammals than small seeds (Moegenburg 1996; Brewer 2001; Gómez 2004). On the other hand, in the post-germination stage, large seeds with more energy reserves are advantageous even in conditions of deep burial, low light availability, nutrient shortage, and soil drought (Salisbury 1974; Foster and Janson 1985; Seiwa 2000; Seiwa et al. 2002; Gómez 2004). In walnuts (J. ailanthifolia), however, an advantage of large seeds in early seedlings has not been shown (Seiwa 2000). Life-history traits of plants, such as the trade-off between seed size and the number of seeds that plants can produce, are another factor affecting seed-size evolution (Salisbury 1974; Smith and Fretwell 1974; Foster and Janson 1985; Jacobsson and Eriksson 2000). These various conflicting factors are thought to help maintain seed size variation between populations or individuals (e.g. Howe and Richter 1982; Morese and Schmitt 1985; Silvertown 1989).
The observed differences in walnut seed size were consistent with the presence/absence of S. lis as a seed disperser; however, we did not indicate a causal relationship between these two factors in the present study. Moreover, the temperature gradient overlaps the distribution of squirrels such that as the temperature decreases so does the squirrel presence. Unfortunately, there is no area lacking squirrels in northern Japan. To further understand the seed size variation observed in the present study, we must consider factors other than seed dispersers.
References
Abe H (2002) A guide to the mammals of Japan. Tokai University Press, Tokyo
Benkman CW (1995) The impact of tree squirrels (Tamiasciurus) on limber pine seed dispersal adaptations. Evolution 49:585–592
Benkman CW (1999) The selection mosaic and diversifying coevolution between crossbills and lodgepole pine. Am Nat 153:s75–s91
Benkman CW, Holimon WC, Smith JW (2001) The influence of a competitor on the geographic mosaic of coevolution between crossbills and lodgepole pine. Evolution 55:282–294
Brewer SW (2001) Predation and dispersal of large and small seeds of a tropical palm. Oikos 92:245–255
Clark DA, Clark DB (1984) Spacing dynamics of a tropical rain forest tree: evaluation of the Janzen-Connell model. Am Nat 124:769–788
Clarkson K, Eden SF, Sutherland WJ, Houston AI (1986) Density dependence and magpie food hoarding. J Anim Ecol 55:111–121
Coffey K, Benkman CW, Milligan B (1999) The adaptive significance of spines on pine cones. Ecology 80:1221–1229
Crawley MJ (2002) Statistical computing: an introduction to data analysis using S-plus. Wiley, West Sussex
Elliott PF (1974) Evolutionary responses of plants to seed eaters: pine squirrel predation on lodgepole pine. Evolution 28:221–231
Ellison AM (2001) Interspecific and intraspecific variation in seed size and germination requirements of Sarracenia (Sarraceniaceae). Am J Bot 88:429–437
Foster S, Janson CH (1985) The relationship between seed size and establishment conditions in tropical woody plants. Ecology 66:773–780
Gómez JM (2004) Bigger is not always better: conflicting selective pressures on seed size in Quercus ilex. Evolution 58:71–80
Hammond DS, Brown VK (1995) Seed size of woody plants in relation to disturbance, dispersal, soil type in wet Neotropical forests. Ecology 76:2544–2561
Hardin ED (1984) Variation in seed weight, number per capsule and germination in Populus deltoides Bartr. trees in southeastern Ohio. Am Midl Nat 112:29–34
Horikawa Y (1976) Atlas of the Japanese flora 2 (in Japanese). Gakken, Tokyo
Howe HF, Richter WM (1982) Effects of seed size on seedling size in Virola surinamensis: a within and between tree analysis. Oecologia 53:347–351
Hurley TA, Robertson RJ (1987) Scatterhoarding by terrestrial red squirrels: a test of the optimal density model. Can J Zool 65:1247–1252
Jacobsson A, Eriksson O (2000) A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos 88:494–502
Jansen PA, Bartholomeus M, Bongers F, Elzinga JA, Ouden JD, Van Wieren SE (2002) The role of seed size in dispersal by a scatter-hoarding rodent. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory. CAB International, Wallingford, pp 209–225
Jansen PA, Bongers F, Hemerik L (2004) Seed mass and mast seeding enhance dispersal by a neotropical scatter-hoarding rodent. Ecol Monogr 74:569–589
Janzen DH (1982) Variation in average seed size and fruit seediness in a fruit crop of guanacaste tree (Enterolobium cyclocarpum). Am J Bot 69:1169–1178
Kirkpatrick RL, Pekins PJ (2002) Nutritional value of acorns for wildlife. In: McShea WJ, Healy WM (eds) Oak forest ecosystems. The Johns Hopkins University Press, Baltimore, pp 173–181
Koike S, Hazumi T, Furubayashi K (2003) Seed dispersal by the Japanese black bear (Ursus thibetanus japonicus) (in Japanese). Wildl Conserv Japan 8:19–30
Mezquida ET, Benkman CW (2005) The geographic selection mosaic for squirrels, crossbills and Aleppo pine. J Evol Biol 18:348–357
Minato M (1977) Japan and its nature (in Japanese). Heibonsha, Tokyo
Moegenburg SM (1996) Sabal palmetto seed size: causes of variation, choice of predators, and consequences for seedlings. Oecologia 106:539–543
Moles AT, Westoby M (2003) Latitude, seed predation and seed mass. J Biogeogr 30:105–128
Morse DH, Schmitt J (1985) Propagule size, dispersal ability, and seedling performance in Asclepias syriaca. Oecologia 67:372–379
Nihei Y (1995) Variations of behaviour of carrion crows Corvus corone using automobiles as nutcrackers (in Japanese). Jpn J Ornithol 44:21–35
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Saito S, Miyaki M (1983) Growth of seedlings of Juglans ailanthifolia germinated from nuts seeded at different depths (in Japanese). Trans Meet Hokkaido Branch Jpn For Soc 32:219–222
Salisbury E (1974) Seed size and mass in relation to environment. Proc R Soc Lond B 186:83–88
Seiwa K (2000) Effects of seed size and emergence time on tree seedling establishment: importance of developmental constraints. Oecologia 123:208–215
Seiwa K, Watanabe A, Saito T, Kanno H, Akasaka S (2002) Effects of burying depth and seed size on seedling establishment of Japanese chestnuts, Castanea crenata. For Ecol Manage 164:149–156
Shibata M, Nakashizuka T (1995) Seed and seedling demography of co-occurring Carpinus species in a temperate deciduous forest. Ecology 76:1099–1108
Silvertown J (1989) The paradox of seed size and adaptation. Trends Ecol Evol 4:24–26
Smith CC, Fretwell SD (1974) The optimal balance between size and number of offspring. Am Nat 108:499–506
Sone K, Kohno A (1999) Acorn hoarding by the field mouse, Apodemus speciosus Temminck (Rodentia: Muridae). J For Res 4:167–175
Sota T, Takami Y, Kubota K, Ishikawa R (2000) Geographic variation in the body size of some Japanese Leptocarabus species (Coleoptera, Carabidae): the “toppled-domino pattern” in species along a geographic cline. Entomol Sci 3:309–320
Stapanian MA, Smith CC (1978) A model for seed scatterhoarding: coevolution of fox squirrels and black walnuts. Ecology 59:884–896
Stapanian MA, Smith CC (1984) Density-dependent survival of scatterhoarded nuts: an experimental approach. Ecology 65:1387–1396
Steele M, Knowles T, Bridle K, Simms E (1993) Tannins and partial consumption of acorns: implications for dispersal of oaks by seed predators. Am Midl Nat 130:229–238
Tamura N (1996) Application of a radio-transmitter for studying seed dispersion by animals. J Jpn For Soc 76:607–610
Tamura N (2001) Walnut hoarding by the Japanese wood mouse, Apodemus speciosus Temminck. J For Res 6:187–190
Tamura N (2004) Effects of habitat mosaic on home range size of the Japanese squirrel, Sciurus lis. Mammal Study 29:9–14
Tamura N, Shibasaki E (1996) Fate of walnut seeds, Juglans ailanthifolia, hoarded by Japanese squirrels, Sciurus lis. J For Res 1:219–222
Tamura N, Hashimoto Y, Hayashi F (1999) Optimal distances for squirrels to transport and hoard walnuts. Anim Behav 58:635–642
Tamura N, Katsuki T, Hayashi F (2005) Walnut seed dispersal: mixed effects of tree squirrels and field mice with different hoarding ability. In: Foreget PM, Lambert JE, Hulme PE, Vander Wall SB (eds) Seed fate. CAB International, Wallingford, pp 241–252
Theimer TC (2003) Intraspecific variation in seed size affects scatterhoarding behaviour of an Australian tropical rain-forest rodent. J Trop Ecol 19:95–98
Thompson JN (2005) The geographic mosaic of coevolution. The University of Chicago Press, Chicago
Vander Wall SB (1990) Food hoarding in animals. University of Chicago Press, Chicago
Vander Wall SB (1995) The effects of seed value on the caching behavior of yellow pine chipmunks. Oikos 74:533–537
Vander Wall SB (2001) The evolutionary ecology of nut dispersal. Bot Rev 67:74–117
Venable DL (1992) Size-number trade-offs and the variation of seed size with plant resource status. Am Nat 140:287–304
Wada N, Murakami M, Yoshida K (2000) Effects of herbivore-bearing adult trees of the oak Quercus crispula on the survival of their seedlings. Ecol Res 15:219–227
Wenny DG (2000) Seed dispersal, seed predation, and seedling recruitment of a neotropical montane tree. Ecol Monogr 70:331–351
Yamada H, Miyaura T (2005) Geographic variation in nut size of Castanopsis species in Japan. Ecol Res 20:3–9
Yamada H, Yamaguchi K, Miyaura T (2002) Effects of Japan sea climate on geographic distribution of Castanopsis sieboldii and C. cuspidata. J For Res 7:67–71
Yasuda M (2007) Notes on the Japanese squirrel Sciurus lis in Kyushu, southern Japan (in Japanese). Sciurid Inf 18:1–3
Acknowledgements
We thank K. Yoko-o, S. Seki, T. Ueyama, T. Kataoka, and C. Nishi for assistance in collecting walnut seeds. We also thank S. Suzuki and K. Tsuchiya for help with statistical analysis. Professor C.W. Benkman provided valuable comments to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tamura, N., Hayashi, F. Geographic variation in walnut seed size correlates with hoarding behaviour of two rodent species. Ecol Res 23, 607–614 (2008). https://doi.org/10.1007/s11284-007-0414-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0414-8