Abstract
Rates of nest predation have frequently been shown to differ between fragmented and unfragmented habitats, but have rarely been compared among natural habitats in the same geographic region. In this study, artificial nests of two types (open cup and domed) were placed in four habitats (mangroves, monsoon rainforests, eucalypt woodlands and paperbark swamps) over 12 months in three localities near Darwin in the Australian monsoon tropics to determine the effects of habitat, season and nest type on the rate of nest predation. A quail egg and a similarly coloured plasticine egg were placed in each nest. Habitat had a strong effect on nest predation rates, with nests in mangroves experiencing predation rates more than four times higher than those in eucalypt woodlands and paperbark swamps. Despite the strong rainfall seasonality of the region, there was no consistent seasonal variation in nest predation rates. Nest type also had little influence on predation rates, except in paperbark swamps where open cup nests suffered a higher predation rate than domed nests. The study indicates that generalised nest predation rates for tropical regions, even for small areas (e.g. <17 km radius), might overlook substantial variation between habitats. Such variation confounds purported differences in nest predation rates between tropical and temperate regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predation of eggs and nestlings is the primary cause of reproductive failure among birds, and a major factor in the evolution of avian life histories (Ricklefs 1969; Slagsvold 1982; Bosque and Bosque 1995; Martin 2002). Nest predation rates are typically higher in the tropical lowlands than in the north temperate region (Skutch 1966; Robinson et al. 2000; Stutchbury and Morton 2001; but see Gibbs 1991). Whilst much attention has been paid to the effects of habitat fragmentation, and in particular anthropogenic forest edges, on nest predation rates in both regions (Söderström 1999; Zanette and Jenkins 2000; Berry 2002), variation in such rates between natural habitats within geographic regions has rarely been assessed. However Bayne et al. (1997) found that artificial nests in coniferous forest experienced much higher predation rates than those in deciduous or mixed wood forest. In the tropics, variation in nest predation rates may be expected to occur between lowland rainforest, montane rainforests, and savannas, due to dramatic differences in vegetation structure, nest conspicuousness, and the diversity and density of nest predators (Janzen 1978). Such variation may limit the validity or applicability of comparisons between tropical and temperate nest predation rates. It may also have management implications, as the nest predators of one habitat may be more or less sensitive to disturbance than those of other, adjacent habitats.
Australian passerines generally show higher nest predation rates than their north temperate ecological counterparts (Robinson 1990; Taylor and Ford 1998), but the large number of endemic families in Australia constrains phylogenetically controlled comparisons with other continents, even neighbouring Indonesia. Moreover, studies of avian reproduction in Australia are strongly biased towards eucalypt forests and woodlands in the temperate south, whereas very few studies have been conducted in the tropical north. The major nest predators in southern Australia are birds, particularly currawongs and butcherbirds (Gardner 1998; Major et al. 1999; Fulton and Ford 2001, 2003; Berry and Lill 2003). In upland wet-tropical rainforests and adjacent farmland of North Queensland, however, native rats were responsible for over 90% of predation at artificial nests (Laurance et al. 1993; Laurance and Grant 1994). Snakes and rats appeared to be the major nest predators of rainbow pittas (Pitta iris) in the Darwin region of the Australian monsoon tropics (Zimmermann and Noske 2003). However, there was a significant difference between monsoon rainforest and adjacent eucalypt-dominated forests in nest predation rates of this species (Zimmermann 1996), suggesting that nest predator assemblages or behaviour could vary between habitats in the region.
We used artificial nests to examine variation in nest predation rates among four coastal and subcoastal habitats in the Darwin region (12°28′S, 130°50′E) of the Northern Territory (NT) of Australia. This monsoon-tropical region is characterised by moderately high temperatures year-round and a strongly seasonal rainfall, in which 90% of the annual rain (average MAR, 1,600 mm) falls during a single wet season (November through April; Taylor and Tulloch 1985). As in the neotropics (Stutchbury and Morton 2001), birds of the Australian monsoon tropics exhibit diverse breeding seasons, some species breeding primarily during the dry season, others during the wet season and several breeding biannually, at the end of both seasons (Noske and Franklin 1999; Noske 2001). Although this diversity in breeding seasons partly relates to food resources, Noske and Franklin (1999) suggested that some small passerines breed during the dry season to avoid nest predation by reptiles that are most active during the warmer wet season. In the present study, we examined seasonal variation in artificial nest predation rates to determine whether such rates were higher in the wet season than at other times of the year.
Domed nests, which possess a roof and side entrance, are more frequent among tropical birds than among North Temperate birds (Ricklefs 1969; Collias and Collias 1984). Various functions have been suggested for the roof of domed nests, including protection from rain, solar and ultraviolet radiation and nest predators. Whilst many studies have compared the breeding success of birds that build open cup nests with those that use tree holes for nesting (Skutch 1966; Oniki 1979; Martin and Li 1992), few studies have compared predation rates of domed and open cup nests (Loiselle and Hoppes 1983; Gardner 1998; Hausmann et al. 2004). We compared predation rates of open cup-shaped artificial nests with those of domed artificial nests in the Darwin region, where approximately one half and one quarter of the small- to medium-sized land bird species build cup- and dome-shaped nests, respectively (R. A. Noske, unpublished data). Although it is now widely recognised that predation rates of artificial nests do not necessarily reflect those of real nests (Major and Kendal 1996; Zanette 2002), studies of this type are the only practical means of standardising nest parameters, such as nest size, shape, and height above the ground, to enable comparisons among habitats and seasons.
Methods
Study sites
The study was undertaken at several sites within 30 km of Darwin, NT, Australia (Fig. 1). Four habitat types were considered in this study and their approximate areal representation in the region (estimated from Wilson et al. 1990) were eucalypt woodland (95%), paperbark swamps (2%), coastal mangroves (henceforth “mangals”; 2%), and monsoon rainforests (1%). Despite the minor proportions they occupy in the local landscape, the last two habitats show high avian diversity and density (Woinarski et al. 1988). The predominant vegetation type in the region is eucalypt woodland and open forest (often called savanna), dominated by evergreen Eucalyptus tetrodonta and E. miniata up to 20 m in height, with a variable mid-storey of tall shrubs, including wattles Acacia species, and usually a dense ground cover of grasses, including annual Sarga species, which form a tall understorey in the late wet season (Wilson and Bowman 1987). These woodlands are extremely fire-prone and are mostly burnt annually or every 2 years (Edwards et al. 2001). Monsoon rainforests in the region are generally small (<5 ha), discrete patches of evergreen forest, often fed by permanent groundwater, and with a diverse range of canopy species rarely exceeding 25 m in height, little understorey, many vines and a ground cover of leaf litter (Wilson et al. 1990).
The other two habitats largely lack an understorey. Mangals occur almost continuously along the coasts and around Darwin Harbour, where the maximum tidal range is 7.8 m above sea level; the mean spring tide range is 5.5 m and mean neap range is 1.9 m (Semenuik 1985). The bare muddy substrate supports forests of mangroves (up to 15 m) such as Rhizophora stylosa along tidal rivers and creeks, and dense thickets (1.5–2.5 m high) of Ceriops australis towards the landward edge, except where hypersaline conditions have led to barren saltflats (Wightman 1989). Paperbark swamps occur patchily in low-lying seasonally inundated areas and are virtually monocultures of Melaleuca viridiflora and/or M. leucadendra (paperbarks), with a sparse ground cover of sedges and grasses (Wilson and Bowman 1987). Structurally, mangals and rainforests may be considered closed forests, whereas the other two habitats considered here are open forests or woodlands.
The effect of landscape scale factors on nest predation rates was minimised by selecting sites that were spatially well separated from others of the same or different habitats (Fig. 1). To minimise edge effects, only large examples of the patchily distributed habitats (monsoon rainforests and paperbark swamps) were chosen, and sampling conducted as far from the edge as practicably possible. Monsoon rainforest and eucalypt woodland sites were located at the Territory Wildlife Park (c. 30 km south of Darwin; 12°42′S, 130°59′E), Howard Springs Nature Park (c. 25 km east of Darwin; 12°27′S, 131°02′E) and Holmes Jungle Nature Park (Darwin fringe). None of these sites were grazed by livestock. Paperbark swamp sites were located at the Territory Wildlife Park, Howard Swamp (ca. 1 km from Howard Springs Nature Park) and Girraween (12°31′S, 131°04′E). Mangal sites were located at East Point (7 km NW of Darwin), Berrimah settlement ponds and Hudson Creek (9–10 km east of Darwin).
Artificial nest and egg construction
We followed the protocols established by Major et al. (1996) for the construction of artificial nests, though some modifications were necessary for the tropical environment. Open cup nests were constructed from halved tennis balls covered with coconut fibre and wool. However, for the paperbark swamp sites, bark from paperbark trees was substituted for coconut fibre as a nest covering, since it is the primary building material of species nesting in this habitat. Prior to plant material being attached using construction adhesive, the tennis balls were dipped in brown paint to eliminate the conspicuous bright yellow covering and then left to air for at least 3 days before, and a week after, the addition of plant material, to reduce any odour. Domed nests were constructed by cutting a 2–3 cm diameter hole (mimicking the nest entrance) in one side of whole tennis balls, and a slightly smaller hole on the other, and then covered and lined with coconut fibre or paperbark. The latter hole mimicked those made by local predators of domed nests and provided access by such predators. All nests also had drainage holes of 0.5 cm diameter bored into the bottom of the nest to prevent saturation. Modelling clay, in two shades of brown and white, was used to make artificial eggs. The eggs were ca. 2.5 × 1.5 cm in size and were moulded the week prior to the experimental field trials to allow for airing. Eggs of captive Japanese Quail (Coturnix coturnix), measuring ca. 3.5 × 2.5 cm, were used to attract predators.
Experimental design
The experimental trials were conducted every 2nd month over the course of a year so as to encompass both the wet and dry seasons, beginning in September 1999 and concluding in July 2000. Three widely separated sites (see above) were used for each habitat, and at each site, three non-parallel transects were established, each being 100 m long and at least 100 m apart. Transects were marked with flagging tape at 20-m intervals, and a nest was placed 3–6 m on either side of each marker, giving a total of 6 nests transect−1 or 18 nests site−1 habitat−1. To compare predation rates between the nest types, open cups were alternated with domed nests. Each nest was attached by wire to one or more branches of suitable shrubs or small trees at heights of 1–2 m, before two eggs (one quail egg and one artificial egg) were introduced. Nests were not placed in exactly the same position in consecutive trials. For each trial, nests were checked at 3-day intervals over a period of 9 days, which is slightly less than the incubation period (10–11 days) of a common mangrove-dwelling species, the Yellow White-Eye (Zosterops luteus) (Noske 1999). Predation was deemed to have occurred if one or both eggs had been removed or marked in some way (e.g. obvious claw, beak or tooth marks on the artificial egg).
Statistical analysis
Ecological systems are often best understood by developing a set of credible hypotheses that then compete with one another for support, using the data as the arbitrator (see Hillborn and Mangel 1997). This multiple working hypothesis approach provides the most robust foundation for strong inference. For this study, an a priori candidate set of generalised linear models with mixed effects (GLMM; Pinheiro and Bates 2000) was evaluated using a “strength of evidence” approach, as opposed to classic dichotomous null hypothesis tests and associated P-values. Model-selection and subsequent inference (using relative weights of evidence) used information-theoretic methods based on the Akaike information criterion (AIC; see Burnham and Anderson 2002), whereby a measure of Kullback–Leibler information (a fundamental conceptual measure of the relative distance of a given model from full reality) is derived and used as an objective basis for ranking the parsimony of models in an a priori candidate set. An a priori model-building strategy tends to avoid data dredging, which leads to over-fitted models and the “discovery” of spurious effects (a common fault with stepwise or best subsets model selection). The AIC highest ranked models are those which explain the most substantial proportion of variance in the data, yet exclude unnecessary parameters that cannot be justified for inference on the basis of the data (Burnham and Anderson 2002).
In this study, we were interested in the separate (and some interactive) effects of habitat type, season and nest type (our “fixed effects”) on nest predation rates. An interaction between habitat type and nest type was hypothesised, on the basis that open cup nests may be less conspicuous, and therefore less heavily depredated, in habitats with a closed canopy (monsoon rainforests and mangals) than more open habitats (eucalypt woodland and paperbark swamps). Our “random effect” was hierarchical, being transect nested within site, and controlled for temporal (repeated measures) and spatial association by decomposing the model variance into autocorrelative and residual components (see Pinheiro and Bates 2000). Analyses were carried out using the R statistical package v1.8.1 (http://www.r-project.org). The mode of analysis took two forms, determined by the nature of the dependent variable being examined:
-
1.
Probability of nest predation: whether a given nest was depredated or not [1, 0] over the entire 9-day period of monitoring (n = 1,290). This was a binary variable and thus presumed to follow a binomial distributional family with a logit link function in the mixed-effects GLM. Fixed effects were habitat type, season and nest type and a habitat × nest type interaction.
-
2.
Timing of nest predation: for those nests that were depredated, whether the timing of the predation event occurred within 3, 6 or 9 days after placement of the nest. This was a trinary ordinal variable, analysed on the subset of the data which were depredated (n = 419), and presumed to follow a multinomial distribution with a logit link function in a mixed effects GLM with gamma frailty dependence (Lindsey 1999). The model set was otherwise identical to the nest predation analysis.
Results
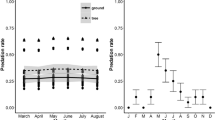
Which of the above effects exert a statistically meaningful influence on the proportion and timing of nest predation? The most parsimonious model for predicting the probability of nest predation included habitat, nest type and their interaction, with the next best model dropping out the interaction term (Table 1A). The highest levels of nest predation occurred in the mangals, where 70% of nests were depredated (Fig. 2, top row). Monsoon rainforests showed the next highest level of nest predation (32% of nests), whilst the eucalypt woodlands and paperbark swamps showed substantially lower levels (with overlapping 95% confidence intervals). Examination of the coefficients of the generalised linear model (Table 2A) supported the trends illustrated in Fig. 2, and indicated a particularly strong effect of nest type in paperbark swamps (lower predation in domed nests), but no effect of nest type in the mangals. The concordance of the AIC best model (a measure of the association between observed responses and predicted probabilities) was 87.5%.
Season effects were only weakly supported, being present in the models ranked 3 and 4 of the full a priori set of ten candidates. The AIC best model received 102 times more support from the data than the best single-factor model (habitat only), based on the information-theoretic evidence ratio (an index of the likelihood of one model over another, calculated for this comparison as W [H+N+H:N]/W [H]), whilst the null model of no fixed effects was 5.92 × 107 times less likely. Of the single factor models, habitat was 9,302 times better supported than nest type, and 9.48 × 105 times superior to season as an explanatory factor.
A similar ranking of predictors was found for the timing of nest predation, with the AIC best model again being habitat, nest type and their interaction (Table 1B), with a concordance of 70.5% (for GLM coefficients, see Table 2B). Nest predation usually occurred within the first 6 days following the placement of the artificial nests (Fig. 2, bottom row), but occurred particularly early in the mangals, with 51% of predation events for this habitat during the first 3 days. As expected, cup-shaped nests were depredated slightly earlier than were domed nests. The next ranked model also included a seasonal component, receiving just over half the support of the best model, and together constituting 99.96% of the total likelihood of the model set (AIC weights). The other eight candidate models received essentially no support from the data. Nests tended to be found by predators earlier during the wet season than during the dry season (Fig. 2).
Of a total of 1,290 nests, 419 (32%) were depredated. Of the 419 depredated nests, 149 (35.6%) lost both eggs without trace, while another 87 nests (20.8%) lost one egg or the other, giving a total of 395 eggs that disappeared. Of the 328 nests in which quail eggs were depredated, only 65 (19.8%) retained egg fragments after predation. Of 107 artificial eggs that showed signs of attack, 50 (46.7%) showed tooth impressions and 31 (29.0%) had beak marks, while the remainder showed markings of indeterminate origin. There was no significant variation between habitats in the proportion of tooth and beak impressions (χ 2 = 0.42, df = 3 and P > 0.05). The common tree snake (Dendrelaphis punctulatus) was observed raiding nests at two sites (monsoon rainforest at Holmes Jungle and eucalypt woodland at Territory Wildlife Park).
Discussion
Effects of habitat
In the present study, the two habitats with a closed canopy (mangals and monsoon rainforests) exhibited higher and faster rates of nest predation than the two open canopy habitats (eucalypt woodlands and paperbark swamps). Several studies in the region suggest that natural nest predation rates in mangals and rainforests are indeed relatively high, but comparative data for other habitats are scarce. Using the Mayfield method of calculation (Mayfield 1975), which adjusts for the bias towards finding successful nests, nest predation rates of large-billed gerygones (Gerygone magnirostris) and mangrove gerygones (G. levigaster) in mangals were 73 and 68%, respectively, with no significant difference in predation rates between eggs and nestlings in either species (Mulyani 2004). Nests of rainbow pittas in the Darwin region suffered higher egg predation rates in monsoon rainforests than in adjacent eucalypt-Pandanus forest (81 and 52%, respectively) (Zimmermann 1996). Moreover, both gerygones and pittas build dome-shaped nests, which should offer better protection from predators than open cup nests. In the suburbs of Darwin, open cup-nesting rufous-banded honeyeaters (Conopophila albogularis) enjoyed a low nest predation rate (30%), presumably due to the absence of many nest predators from the urban environment (Noske 1998). Nevertheless, nest predation rates of 60–70% are typical in temperate Australia (Robinson 1990; Taylor and Ford 1998; Ford and Tremont 2000), suggesting that those of tropical gerygones and pittas are not exceptionally high.
In the neotropics, Skutch (1966) found higher rates of nest predation in forests than in disturbed open habitats (pastures, plantations and second growth), while Oniki (1979) found the reverse. Oniki (1979) argued that the extirpation of large carnivores from anthropogenic habitats resulted in elevated densities of smaller predators, which in turn caused higher nest losses. However, this “meso-predator” effect has limited applicability in tropical Australia, where the only natural predators of nest-robbing rodents are owls and snakes.
Effects of nest type and season
Studies in the Northern Hemisphere have consistently shown that open cup nests suffer higher levels of predation than cavity or enclosed nests (e.g. Ricklefs 1969; Martin 1993). It has also been suggested that domed and pensile nests are less vulnerable to predation than open cup nests (e.g. Ricklefs 1969; Collias and Collias 1984; Frith et al. 1997), but evidence is sparse. Oniki (1979) compared open cup and “enclosed” nests in Brazil, but for the latter, combined oven-shaped and cavity nests, while Skutch (1966) contrasted hole-nesting species with those that build open or roofed nests. Loiselle and Hoppes (1983) found no difference in predation rate of open and “closed” wicker nests placed in Panamanian lowland rainforest. In Panama, two species of flycatchers that built enclosed, pyriform-shaped nests enjoyed higher nesting success than eight other open cup-nesting passerines (Robinson et al. 2000). In southeastern Australia, plasticine eggs placed in disused domed nests of the superb fairy-wren (Malurus cyaneus) suffered much lower rates of predation than those in artificial open cup nests, possibly due to a failure of the major nest predators (birds) to locate cryptic nests in the absence of cues provided by parental activity (Gardner 1998).
In the present study, except for paperbark swamps, there was no supportable difference between the levels of nest predation in open cup and domed nests. In paperbark swamps, however, open cup nests suffered a higher predation rate than domed nests, and since the eggs in the former nests were visible from above, it is possible that birds and other visually orienting, arboreal nest predators were more important in this habitat than in the other three habitats. A preponderance of arboreal predators might be expected in a habitat that is flooded (1–2 m) for several months each wet season, but there was no evidence that visually oriented nest predators were more important in this habitat than in other habitats. If open cup nests suffer more predation than domed nests in paperbark woodland, it may explain why, among honeyeaters (Meliphagidae), domed nests have evolved in the one genus (Ramsayornis), in which the species typically nest over water in tropical paperbark swamps.
Unlike their relatives in temperate Australia, which mostly breed in the warm months of spring and early summer, many passerine birds in the monsoon tropics breed during the dry season, corresponding with the austral winter (Noske and Franklin 1999). Dry season breeding may be a strategy to avoid nest predation by snakes and monitor lizards (Noske 2001), many of which are most active during the wet season (Shine 1991; Christian et al. 1995). In the present study, seasonal variation in nest predation rates was not significant, but the tendency for nests to be depredated more rapidly in the wet season than during the dry season (Fig. 2) may indicate increased reptilian nest predation. Moreover another study in mangals using artificial nests suggested higher nest predation rates during the mid-wet season than during the early dry season (Mulyani 2004), consistent with the reptilian predation hypothesis. However, several snake species are active throughout the year, or even more active during the dry season, including the egg-eating slaty-grey snake (Stegonotus cucullatus) (Brown et al. 2002). Furthermore, seasonal variation in predation rates of natural nests is not necessarily reflected in predation rates of artificial nests (Major et al. 1994; Zanette 2002).
How realistic are artificial nest predation rates?
Although several researchers have warned against the use of artificial nest experiments to infer natural nest predation rates (Major and Kendal 1996; Pärt and Wretenberg 2002; Zanette 2002; Roper 2003), artificial nests permit controlled experiments with larger sample sizes than normally feasible. Predation rates on artificial nests may be higher than on natural nests because they lack parent birds that cover eggs or defend them against nest predators (Angelstam 1986; Yahner et al. 1989; Major et al. 1999; King et al. 1999; Weidinger 2002) or because artificial nests rarely mimic natural nests in construction and placement, and are often more conspicuous (Martin 1987; Bayne et al. 1997; King et al. 1999; Rangen et al. 2000; Berry and Lill 2003). Predators may also be attracted to the greater density of nests found along transect lines, although the effect of nest density on predation rates is unclear (George 1987; Ortega et al. 1998). Conversely, predation rates on artificial nests may be lower than on natural nests due to the lack of parental activity and odour, and nestling calls, which might advertise the location of nests to predators (Willebrand and Marcström 1988; Wilson et al. 1998; Martin et al. 2000).
The widespread use of quail eggs in artificial nest experiments has been criticised due to the potential exclusion of some nest predators, such as small mammals, that may have trouble handling large, thick-shelled quails eggs (Roper 1992; Haskell 1995; Marini and Melo 1998; Bayne et al. 1997; Fulton and Ford 2003). Moreover, Rangen et al. (2000) and Pärt and Wretenberg (2002) found that nests with plasticine eggs were “depredated” more often than nests containing quail and/or finch eggs, apparently due to small mammals being attracted to the unnatural odour of plasticine, although Berry and Lill (2003) found no such effect. Whatever biases exist in the combination of quail and plasticine eggs, however, they did not appear to affect the results of the present study, as the proportions of eggs attacked by birds or mammals were consistent between habitats.
Whilst the proportion of depredated nests showing egg fragments was low, despite high egg removal rates, and the proportion of impressions left by mammals appeared to be higher than those of birds, several other studies have shown that it is not possible to identify nest predators based on the presence and/or condition of remains (e.g. Marini and Melo 1998; Larivière 1999). Nevertheless common tree-snakes were observed raiding nests at two sites, and several lines of evidence suggest that reptiles and mammals were the major nest predators of rainbow pittas in a monsoon rainforest near Darwin (Zimmermann 1996). Further studies of natural nest predation, using remotely triggered cameras (Savidge and Siebert 1988; Whelan et al. 1994; Berry 2002) or other techniques, will be required to determine whether differences in nest predation rates among habitats are due to differences in nest predator assemblages or behaviour.
Conclusions
The present study demonstrates significant variation in predation rates of artificial nests between four, often adjacent, tropical lowland habitats, situated within an area of ca. 17 km radius. Mangals and monsoon rainforests sustained higher and faster nest losses than the more open eucalypt woodlands and paperbark swamps. If either of the first two habitats were fragmented by clearing, it is conceivable that their suitability to nest predators would decline, causing an increase, rather than a decrease, in breeding success. Fortunately there are few threats to these habitats in the Darwin region.
Our experiment does not provide support for the nest predation (by reptiles) explanation for dry season reproduction by many bird species in the region, as there was little evidence of seasonal variation in nest predation rates. However it is quite possible that differences in predator assemblages between habitats contributed to the variation in nest predation rates and that our experimental design created artificial trends or masked real ones, such as seasonal effects. Nevertheless we argue that the magnitude of habitat-related variation reported here is sufficient to cast further doubt on purported differences in general nest predation rates between tropical and temperate regions.
References
Angelstam P (1986) Predation on ground-nesting birds’ nests in relation to predator densities and habitat edge. Oikos 47:365–373
Bayne EM, Hobson KA, Fargey P (1997) Predation on artificial nests in relation to forest type: contrasting the use of quail and plasticine eggs. Ecography 20:233–239
Berry L (2002) Predation rates of artificial nests in the edge and interior of a southern Victorian forest. Wildl Res 29:341–345
Berry L, Lill A (2003) Do predation rates on artificial nests accurately predict predation rates on natural nests. The effects of nest type, egg type and nest site characteristics. Emu 103:207–214
Bosque C, Bosque MT (1995) Nest predation as a selective factor in the evolution of developmental rates in altricial birds. Am Nat 145:234–260
Brown GP, Shine R, Madsen T (2002) Responses of three sympatric snake species to tropical seasonality in northern Australia. J Trop Ecol 18:549–568
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Christian KA, Corbett LK, Green B, Weavers BW (1995) Seasonal activity and energetics of two species of varanid lizards in tropical Australia. Oecologia 103:349–357
Collias NE, Collias EC (1984) Nest building and bird behaviour. Princeton University Press, Princeton, NJ
Edwards A, Hauser P, Anderson M, McCartney J, Armstrong M, Thackway R, Allan G, Hempel C, Russell-Smith J (2001) A tale of two parks: contemporary fire regimes of Litchfield and Nitmiluk National Parks, monsoonal northern Australia. Int J Wild Fire 10:79–89
Ford HA, Tremont S (2000) Life history characteristics of two Australian honeyeaters (Meliphagidae). Aust J Zool 48:21–32
Frith CB, Frith DW, Jansen A (1997) The nesting biology of the Chowchilla Orthonyx spaldingii (Orthonychidae). Emu 97:18–30
Fulton GR, Ford HA (2001) The Pied Currawong’s role in avian nest predation: a predator removal experiment. Pac Conserv Biol 7:154–160
Fulton GR, Ford HA (2003) Quail eggs, modelling clay eggs, imprints and small mammals in an Australian woodland. Emu 103:255–258
Gardner JL (1998) Experimental evidence for edge-related predation in a fragmented agricultural landscape. Aust J Ecol 23:311–321
George TL (1987) Greater land bird densities on island vs mainland: relation to nest predation level. Ecology 68:1393–1400
Gibbs JP (1991) Avian nest predation in tropical wet forest: an experimental study. Oikos 60:155–161
Haskell DG (1995) Forest fragmentation and nest predation: are experiments with Japanese Quail eggs misleading? Auk 112:767–770
Hausmann F, Catterall CP, Piper SD (2004) Effects of edge habitat and nest site characteristics on depredation of artificial nests in fragmented Australian tropical rainforest. Biodivers Conserv 14:2331–2345
Hillborn R, Mangel M (1997) The ecological detective confronting models with data. Princeton University Press, NJ
Janzen DH (1978) Predation intensity on eggs on the ground in two Costa Rican forests. Am Mid Nat 100:467–470
King DI, DeGraaf RM, Griffin CR, Maier TJ (1999) Do predation rates on artificial nests accurately reflect predation rates on natural bird nests? J Field Orn 70:257–262
Laurance WF, Grant JD (1994) Photographic identification of ground-nest predators in Australian tropical rainforests. Wildl Res 21:241–248
Laurance WF, Garesche J, Payne W (1993) Avian nest predation in modified and natural habitats in tropical Queensland: an experimental study. Wildl Res 20:711–723
Larivière S (1999) Reasons why predators cannot be inferred from nest remains. Condor 101:718–721
Lindsey JK (1999) Models for repeated measurements, 2nd edn. Oxford University Press, Oxford
Loiselle BA, Hoppes WG (1983) Nest predation in insular and mainland lowland rain forest in Panama. Condor 85:93–95
Major RE, Christie FJ, Gowing G, Ivison TJ (1999) Elevated rates of predation on artificial nests in linear strips of habitat. J Field Orn 70:351–364
Major RE, Gowing G, Kendal CE (1996) Nest predation in Australian urban environments and the role of the pied currawong, Strepera graculina. Aust J Ecol 21:399–409
Major RE, Kendal CE (1996) The contribution of artificial nest experiments to understanding avian reproductive success: a review of methods and conclusions. Ibis 138:298–307
Major RE, Pyke GH, Christy MT, Gowling G, Hill RS (1994) Can nest predation explain the timing of the breeding season and the pattern of nest dispersion of New Holland Honeyeaters? Oikos 69:364–372
Marini MA, Melo C (1998) Predators of quail eggs, and the evidence of the remains: implications for nest predation studies. Condor 100:395–399
Martin TE (1987) Artificial nest experiments: effects of nest appearance and type of predator. Condor 89:925–928
Martin TE (1993) Nest predation and nest sites: new perspectives on old patterns. Bioscience 43:523–532
Martin TE (2002) A new view of avian life-history evolution tested on an incubation paradox. Proc R Soc Lond B 269:309–316
Martin TE, Li P (1992) Life history traits of open- vs cavity-nesting birds. Ecology 73:579–592
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc Lond B 267:2287–2293
Mayfield HF (1975) Suggestions for calculating nest success. Wilson Bull 87:456–466
Mulyani Y (2004) Reproductive ecology of tropical mangrove-dwelling warblers: the roles of nest predation, brood parasitism and food limitation. Ph.D. thesis, Charles Darwin University, Darwin
Noske RA (1998) Breeding biology, demography and success of the Rufous-banded Honeyeater Conopophila albogularis in Darwin, a monsoonal tropical city. Wildl Res 25:339–356
Noske RA (1999) Notes on the breeding biology of the tropical mangrove-dwelling Yellow White-eye Zosterops luteus. Aust Bird Watcher 18:3–7
Noske RA (2001) The breeding biology of the Mangrove Gerygone Gerygone laevigaster in the Darwin region, with notes on brood parasitism by the Little Bronze-cuckoo Chrysococcyx minutillis. Emu 101:129–135
Noske RA, Franklin DC (1999) Breeding seasons of land birds in the Australian monsoon tropics: diverse responses to a highly seasonal environment. Aust Biol 12:72–90
Oniki Y (1979) Is nesting success of birds low in the tropics? Biotrop 11:60–69
Ortega CP, Ortega JC, Rapp C, Backensto SA (1998) Validating the use of artificial nests in predation experiments. J Wildl Manage 62:925–932
Pärt T, Wretenberg J (2002) Do artificial nests reveal relative nest predation risk for real nests? J Avian Biol 33:39–46
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Rangen SA, Clark RG, Hobson KA (2000) Visual and olfactory attributes of artificial nests. Auk 117:136–146
Ricklefs RE (1969) The nesting cycle of songbirds in tropical and temperate regions. Living Bird 8:165–175
Robinson D (1990) The nesting ecology of sympatric Scarlet Robin Petroica multicolor and Flame Robin P. phoenicea in open eucalyptus forest. Emu 90:40–52
Robinson WD, Robinson TR, Robinson SK, Brawn JD (2000) Nesting success of understory forest birds in central Panama. J Avian Biol 31:151–164
Roper JJ (1992) Nest predation experiments with quail eggs: too much to swallow? Oikos 65:528–530
Roper JJ (2003) Nest-sites influence nest predation differently at natural and experimental nests. Orn Neotrop 14:1–14
Savidge JA, Seibert TF (1988) An infrared trigger and camera to identify predators at artificial nests. J Wildl Manage 52:291–294
Semenuik V (1985) Mangrove environments of Port Darwin, Northern Territory: the physical framework and habitats. J R Soc West Aust 67:81–97
Shine R (1991) Australian snakes: a natural history. Reed Books, Balgowlah, NSW, Australia
Skutch AF (1966) A breeding bird census and nesting success in Central America. Ibis 108:1–16
Slagsvold T (1982) Clutch size variation in tropical birds: the nest predation hypothesis. Oecologia 54:159–169
Söderström B (1999) Artificial nest predation rates in tropical and temperate forests: a review of the effects of edge and nest site. Ecography 22:455–463
Stutchbury BJM, Morton ES (2001) Behavioural ecology of tropical birds. Academic, San Diego, CA
Taylor JA, Tulloch D (1985) Rainfall in the wet-dry tropics: extreme events at Darwin and similarities between years during period, 1870–1983 inclusive. Aust J Ecol 10:281–295
Taylor LNH, Ford HA (1998) Predation of artificial nests in a fragmented landscape on the New England Tablelands of New South Wales. Wildl Res 25:587–594
Weidinger K (2002) Interactive effects of concealment, parental behaviour and predators on the survival of open passerine nests. J Anim Ecol 71:424–437
Whelan CJ, Dilger ML, Robson D, Hallyn N, Dilger S (1994) Effects of olfactory cues on artificial-nest experiments. Auk 111:945–952
Wightman GM (1989) Mangroves of the Northern Territory. Conservation Commission of the Northern Territory, Darwin, Australia
Willebrand T, Marcström V (1988) On the danger of using dummy nests to study predation. Auk 105:378–379
Wilson BA, Bowman DMJS (1987) Fire, storm, flood and drought: the vegetation ecology of Howard’s Peninsula, Northern Territory, Australia. Aust J Ecol 12:165–174
Wilson BA, Brocklehurst PS, Clark MJ, Dickinson KJM (1990) Vegetation survey of the Northern Territory, Australia. Technical report No. 49. Conservation Commission of the Northern Territory, Darwin, Australia
Wilson GR, Brittingham MC, Goodrich LJ (1998) How well do artificial nests estimate success of real nests? Condor 100:357–364
Woinarski JCZ, Tidemann SC, Kerin S (1988) Birds in a tropical mosaic: the distribution of bird species in relation to vegetation patterns. Aust Wildl Res 15:171–196
Yahner RH, Morrell TE, Rachael JS (1989) Effects of edge contrast on depredation of artificial avian nests. J Wildl Manage 53:1135–1138
Zanette L (2002) What do artificial nests tell us about nest predation? Biol Conserv 103:323–329
Zanette L, Jenkins B (2000) Nesting success and nest predators in forest fragments: a study using real and artificial nests. Auk 117:445–454
Zimmermann UM (1996) Ecology of the monsoon-rainforest endemic Rainbow Pitta Pitta iris. Ph.D. thesis, Northern Territory University, Darwin
Zimmermann UM, Noske RA (2003) Breeding biology of the rainbow pitta Pitta iris, a species endemic to Australian monsoon-tropical rainforests. Emu 103:245–254
Acknowledgements
The fieldwork for this study was conducted by SF as part of her B.Sc. Honours at Charles Darwin University (formerly Northern Territory University). We thank Peter Whitehead, Key Centre of Tropical Wildlife Management, for financial support. SF thanks Robert and Yvonne Fischer, Julie Marriott, Peter Phillpott, Rosalinda Isorena, Mike Bellamy, Philip McMahon, Bineeta Sharma and all Library staff at CDU for their support over the 2 years of the project. The Parks and Wildlife Commission of the Northern Territory gave permission to work in the Territory Wildlife Park, Howard Springs and Holmes Jungle, and we thanks the staff in these Parks, especially Brian Delaney and Jasmine Jan. We extend our sincere thanks to Pacific Dunlop, Australia, for their donation of 300 tennis balls. Quail eggs were supplied by The Game Farm, NSW. Finally we thank Hugh Ford and an anonymous referee for helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Noske, R.A., Fischer, S. & Brook, B.W. Artificial nest predation rates vary among habitats in the Australian monsoon tropics. Ecol Res 23, 519–527 (2008). https://doi.org/10.1007/s11284-007-0403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0403-y