Abstract
Zn uptake by maize plants may be affected by the presence of arbuscular mycorrhizal fungi (AMF). Collembola often play an important controlling role in the inter-relationship between AMF and host plants. The objective of this experiment was to examine whether the presence of Collembola at different densities (0.4 and 1 individuals g−1 dry soil) and their activity have any effect on Zn uptake by maize through the plant–AMF system. The presence of the AMF (Glomus intraradices) and of the Collembola species Folsomia candida was studied in a laboratory microcosm experiment, applying a Zn exposure level of 250 mg kg−1 dry soil. Biomass and water content of the plants were no different when only AMF or when both AMF and Collembola were present. In the presence of AMF the Zn content of the plant shoots and roots was significantly higher than without AMF. This effect was reduced by Collembola at both low and high density. High densities of Collembola reduced the extent of AMF colonization of the plant roots and hyphal length in the soil, but low densities had no effect on either. The results of this experiment reveal that the F. candida–G. intraradices interaction affects Zn uptake by maize, but the mechanisms are still unknown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to some estimates, 80–90% of all continental plants live in a symbiotic relationship with arbuscular mycorrhizal fungi (AMF) (Malloch et al. 1980; Jakucs 1999). AMF can facilitate water and P uptake by plants, especially in stress situations (Bethlenfalvay et al. 1988; Posta and Füleky 1997, 2000). The effect of the AMF on heavy metal uptake by plants from polluted soils is not clear. AMF may protect plants from heavy metal toxicity, because plants take up less heavy metal from the soil in the presence of AMF (Zhu et al. 2001). In these circumstances the heavy metals remain in the root zone without translocation to the shoot. Heavy metals can, however, accumulate in larger quantities in the roots and shoots of plants with AMF (Jansa et al. 2003; Oudeh et al. 2002), although results cannot be generalized, because the circumstances of the experiments described above (host plant and AM fungi species, heavy metal, applied concentration, circumstances of plant growth, etc.) were different. Many species of Collembola graze on AM fungi (Larsen and Jakobsen 1996) and different Collembola species have a preference for different AMF species (Moore et al. 1985). Other authors stress the role of saprophytic fungi because evidence shows that Collembola prefer conidial fungi over AMF (Klironomos and Kendrick 1996).
Several studies report that because of AMF consumption by Collembola, mycorrhizal colonization decreases; this has a negative effect on nutrient uptake, plant growth, and production (Warnock et al. 1982; Finlay 1985; McGonigle 1995; Hodge 2000). In fact, this effect depends on Collembola densities. Collembola at an optimum density may stimulate AMF growth and development by consuming it (Bakonyi et al. 2002; Gange and Ayres 1999). The mineralized N and P found in animal faeces can be taken up again by the AMF and the plants (Gange 2000). It has also been shown that although the AMF spores are too large to pass the Collembola’s gut intact, the presence of Collembola helps the AMF colonize the plant (Klironomos and Moutoglis 1999). The dispersal mechanism of AMF by Collembola is still unknown. It is not clear whether the hyphae, the spores, or another form of the AMF play a role in the dispersal. The presence of Collembola may have different effects on the growth of the AM and on the functioning of the symbiotic relationship. In this study three questions were addressed:
-
1
does Collembola density have any effect on growth and development of the AM-fungus Glomus intraradices;
-
2
is Folsomia candida able to affect Zn uptake of maize through the AMF from a Zn-polluted soil; and
-
3
does Collembola density have any effect on Zn uptake by the plant?
Methods
Raman type brown forest soil was used in the experiment. The main chemical characteristics of the soil were: total C 1.6%, total N 0.15%, P 712 mg kg−1, K 0.29%, Fe 0.78%, Zn 43 mg kg−1, Mn 480 mg kg−1, pH (H2O) 7.5, WHC 33% (w/w). The soil was collected in the Botanical Garden of the Szent István University (Gödöllő, Hungary), and was sterilized by autoclaving to eliminate all indigenous organisms of the soil.
Maize (Zea mays L., variety Pioneer) was used in the experiment. Maize seeds were surface-sterilized by applying 30% H2O2 for 10 min, and washed with sterile distilled water. Before planting, the seeds were germinated for 2 days on filter paper. Subsequently, the seedlings were planted in 10 cm×14 cm×6 cm (L×h×w) PVC boxes filled with 800 g dry sterile soil. Two seeds were planted in each microcosm and were thinned to one plant per microcosm after emergence. Plants were grown in a greenhouse for 8 weeks. Plants were watered with 20 mL deionized water when a decrease in their turgor pressure was observed.
A mycorrhizal inoculum of G. intraradices (BEG 2) was propagated on maize and was grown in a greenhouse for 7 weeks. Roots of the plants with the adhering soil were used as an inoculum in the sterilized soil used in the main experiment. Ten grams of inoculum contained approximately 400 infective propagules and additional rhizosphere microorganisms. Fifteen grams of inoculum was used in each mycorrhizal treatment whereas non-mycorrhizal plants received an equivalent amount of sterilized soil. The inoculum was mixed thoroughly with the soil of the microcosms at time of planting of the maize.
Adult Collembola (F. candida) were introduced into the microcosms together with the maize plants and remained in the microcosms for 8 weeks. F. candida is a widespread species in laboratory tests. F. candida feed on the hyphae of several Glomus species (Moore et al. 1985) including the G. intraradices (personal observation). Two densities were applied—0.4 individual g−1 dry soil (low density) and 1 individual g−1 dry soil (high density). In a previous work (Bakonyi et al. 2002), 0.4 individual g−1 dry soil proved to be most stimulating and 1 individual g−1 dry soil diminishing for AMF under our experimental conditions. The number of Collembola was calculated in the upper 5 cm layer of the soil (300 g soil), because most individuals live here (Larink 1997). Consequently, 300 and 120 F. candida individuals were added for the high-density and low-density treatments, respectively. Curry (1994) reported 5.7×104 Collembola m−2 in different soils. This value is equal to our high density. Microcosms were covered with a close-woven net to prevent the Collembola from escaping.
Soil was spiked with 100 mL ZnSO4.7H2O (Merck, GR for analysis) solution at a nominal concentration of 250 mg Zn kg−1 dry soil which is the pollution threshold limit value in Hungary. Treatments without Zn received an equivalent quantity of distilled water. Zn was added 4 weeks after planting the maize. This concentration was chosen to demonstrate the effects of moderate Zn pollution.
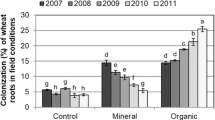
The experiment consisted of eight treatments (Table 1). Five replicates were prepared for each treatment. Microcosms with maize were destructively sampled 8 weeks after planting. Roots were carefully removed from the soil and gently washed in tap water. Shoots were cut at the upper part of the crown. Plant height was measured from the crown to the highest end of the leaves. The weight of the shoots and roots was determined after drying at 70°C for 72 h. The C and N content of thoroughly mixed dry plant roots and shoots were determined by use of a Carlo-Erba NA 1500 elemental analyser. Zn analyses were performed on soil, root, and shoot samples. After digestion with conc. HNO3 the extracts were analysed for Zn with plasma emission spectrometry using a Jobin-Yvon JY24 ICP instrument. Microbial biomass was determined by following the method described by Amato and Ladd (1988). Subsamples of the washed roots were cut into pieces approximately 1 cm in length. Root segments were cleared in 10% KOH for 15 min at 90°C and washed with distilled water. Cleared samples were soaked for 1 h in 25% HCl solution. The formation of mycorrhizae was quantified by measuring the arbuscular formation of the AMF hyphae after staining in 0.1% trypan blue and lactophenol. The percentage colonization was estimated by the grid-line intersect method (Giovanetti and Mosse 1980).
Determination of AM fungal hyphal length in the soil was based on the methods of Bååth and Söderström (1979). A 1-g sample of dry soil was dispersed in 100 mL deionized water in a blender for 1 min. Fungal hyphae were separated by wet-sieving and centrifugation. The separated fungal hyphae were placed in a Petri dish with 5 mL deionized water. Ten millilitres of agar solution (0.75%) containing trypan blue (0.05%) was added to each dish, and the mixture was then dried for 24 h at 70°C. The hyphal length was measured in the dried agar film by the intersection method (Tennant 1975) under a binocular microscope (16× magnification).
The method of Gerdemann and Nicholson (1963) was used for counting the AMF spores. A soil sample of 20 g (dry weight) was washed through a series of sieves, and spores were captured on the 63-μm screen. Four grams from the sievings were added to water and centrifuged for 4 min at 900 rpm. The supernatant contained the dead spores. The pellet was resuspended in a 50% sugar solution and the suspension was centrifuged for 15–30 s at 900 rpm. Spore numbers were counted in the supernatant.
After checking the normality of the data, the variance of the data was analysed with two-way ANOVA. Tukey HSD was used as post-hoc test. The mycorrhizal effect was examined with t-test.
Results
Collembola had significant effect (Table 2) on shoot dry weight and the total dry weight of the plant. The plants were larger when Collembola were present at low density than at high density. There were no significant differences between the root dry weight and water content of the plants. Plants infected by AMF had higher shoot dry weight than plants without AMF, but the difference was not significant. In tests with Zn pollution inoculated plants had significantly higher shoot/root ratio than not inoculated plants (Table 3).
The amount of mycorrhizal colonization was less than 5% in the uninoculated plants (Table 3) which is in the range of experimental error. Mycorrhizal inoculation had a great positive effect on mycorrhizal colonization, spore number, and hyphal length (Table 3). Both Zn and Collembola had a great effect on AMF colonization, spore number, and hyphal length (Table 2). High Collembola densities reduced mycorrhizal colonization compared with treatment without Collembola and with Collembola at low density. The results for hyphal length were similar. High Collembola densities had a negative effect compared with treatment without Collembola and the treatment with few Collembola. Low Collembola densities had no effect on AMF except that it reduced spore numbers in Zn-spiked soil. The presence of Collembola at both density levels reduced AMF spore numbers in the polluted soil. Spore number had a weak interaction with Collembola and Zn. Added Zn increased both hyphal length and the amount of AMF colonization.
Application of Zn considerably enhanced plant Zn concentrations, both in the shoots and in the roots (Table 2) and had a large positive effect on Zn concentration in the soil. Mycorrhizal infection increased shoot and root Zn concentrations (Table 3) but did not affect the Zn content of the soil. Lower root Zn concentrations were found when Collembola were present, irrespective of density. Root Zn concentration had interaction between Collembola and Zn. Soil Zn content and shoot Zn concentration was not affected by Collembola.
C and N concentrations of the plants were similar in all treatments. The microbial biomass values were too low, even zero in some treatments, therefore their further evaluation was not possible.
Discussion
The positive effect of AMF on the growth of plants is well documented (Bethlenfalvay et al. 1988; Biró et al. 2000). In the our experiment mycorrhizal infection did not significantly increase shoot dry weight but the shoot/root ratio was higher in plants inoculated with AMF. This result is consistent with the data of Kaiser and Lussenhop (1991), Posta and Füleky (1997), and Warnock et al. (1982), who found that plants with AMF have a higher shoot/root ratio than plants without AMF.
Effects of the AMF on plant Zn uptake were found to be variable. Usually, AMF is supposed to protecting plants against the toxic effects of Zn. In an experiment conducted with white clover, increasing Zn concentrations (0–400 mg kg−1) enhanced Zn uptake in the shoots and roots but this increase was greater without AMF (Zhu et al. 2001). Similar results were found in another experiment with ryegrass in which metal concentrations were 0, 30, 90, and 270 mg kg−1. The presence of AMF was correlated with higher Zn content in the root, preventing Zn from penetrating the shoot, and thereby reduced the effects of metal toxicity (Takács and Vörös 2003). For maize, concentrations of Zn were lower in mycorrhizal plants than in non-inoculated plants (Weissernhorn et al. 1995).
In other experiments more Zn was taken up by plants inoculated with AMF, both in the roots and shoots, than without AMF. Zn concentrations were higher in the roots than in the shoots (Jansa et al. 2003; Joner and Leyval 2001; Oudeh et al. 2002). Our results support these findings, because mycorrhizal inoculation increased the Zn content of the roots and the shoots. Weissernhorn et al. (1995) suggested that the effect of AMF on metal uptake depends on the conditions of plant growth, on the fungal partner, and on the metal. The Zn concentration applied and the host plant species are also important factors. This is why the results cannot yet be generalized.
Zn contamination may reduce (Bi et al. 2003; Takács and Vörös 2003) or enhance (Zhu et al. 2001) AMF colonization or have no effect (Li and Christie 2001). Chen et al. (2001) demonstrated that the concentration of Zn in AM fungal mycelia is approximately ten times higher than in the host plant tissues. In our experiment, Zn significantly increased the amount of AMF colonization, and Zn had a positive effect on hyphal length in all treatments. In our work the high concentration of Zn in the soil probably enhanced the hyphal production of the AMF. In this way AMF helps to keep the Zn in the root zone, because AMF infection enhanced Zn uptake by the root more than by the shoot. This supports the hypothesis that AMF can protect plants against heavy metal intoxication.
Folsomia candida consumes the hyphae of some Glomus species (Moore et al. 1985). According to our data (unpublished), F. candida fed on the hyphae of G. intraradices but did not consume the spores. The results of this experiment suggest that Collembola also fed on mycorrhiza. High densities of F. candida significantly reduced the amount of AMF colonization and hyphal length but low densities did not have any effect on these. A significant difference was observed between the effect of the two density levels on the extent of colonization. Finlay (1985) obtained similar results from a study of the efficiency of mycorrhizal infection for different densities of Collembola Onychiurus ambulans. Collembola at high density were found to reduce the length of the external hyphae. At optimal density, however, dispersal of the AMF inoculum and stimulation of hyphal growth compensated for the effect of feeding. In our experiment hyphal length correlated with colonization rates—high Collembola densities reduced hyphal length and low densities did not affect it. There was a significant difference between hyphal length in the soil at different Collembola densities. Bakonyi et al. (2002) found that the extent of AMF colonization was highest for a density of 0.2–0.4 Sinella coeca g soil−1. Higher or lower Collembola densities reduced the colonization rate. In this experiment different Collembola densities also affected plant biomass. In treatments applying lower Collembola densities plants grew higher and their dry weight was also greater.
Effects of Collembola on mycorrhizal development and growth also have an indirect effect on Zn uptake by the maize plants. There was an interaction between root Zn concentration and Collembola and Zn. This means that Collembola had a greater effect on root Zn concentration in the presence of Zn. At a high density the Collembola reduced AMF colonization and hyphal length in the soil, which resulted in a lower Zn concentration in the plant roots. Low Collembola densities had a similar negative effect on Zn concentration of the root; this density did not, however, have any effect on AMF colonization and hyphal length. We must therefore emphasize the importance of Zn uptake by maize through the root, not through the AM system. In this experiment the difference between the Zn uptake of non-inoculated and mycorrhizal plants was approximately 25% for unpolluted soil and approximately 65% for Zn-spiked soil. Other authors have obtained similar results for Zn uptake (Kothari et al. 1990). These results mean the plants take up a large amount of Zn through the roots. Collembola may feed on the roots of the plants (Thimm and Larink 1995; Petersen 2002). We suppose Collembola can affect Zn uptake not only through the AMF system but also through the plant roots, although the mechanism is not yet known.
We conclude that F. candida can affect the development of AMF and this effect depends on the density of Collembola. The presence of Collembola reduced concentrations of Zn in the roots of the maize plant in both spiked and unspiked soil. Collembola densities were not, however, important in affecting Zn uptake of plants through the plant–AMF system. Collembola seems to directly affect Zn uptake by maize, not only through the AMF–host plant system.
References
Amato M, Ladd JN (1988) Assay for microbial biomass based on ninhydrin-reactive N in extracts of fumigated soils. Soil Biol Biochem 20:107–114
Bååth E, Söderström B (1979) Fungal biomass and fungal immobilization of plant nutrients in Swedish coniferous forest soils. Rev Ecol Biol Sol 16:477–489
Bakonyi G, Posta K, Kiss I, Fábián M, Nagy P, Nosek JN (2002) Density-dependent regulation of arbuscular mycorrhiza by collembola. Soil Biol Biochem 34:661–664
Bethlenfalvay GJ, Brown MS, Ames RN, Thomas RS (1988) Effects of drought on host and endophyte development in mycorrhizal soybeans in relation to water use and phosphate uptake. Physiol Plant 72:565–571
Bi YL, Li XL, Christie P (2003) Influence of early stages of arbuscular mycorrhiza on uptake of zinc and phosphorus by red clover from a low-phosphorus soil amended with zinc and phosphorus. Chemosphere 50:831–837
Biró B, Köves-Péchy K, Vörös I, Takács T, Eggenberger P, Strasser RJ (2000) Interrelations between Azospirillum and Rhizobium nitrogen-fixers and arbuscular mycorrhizal fungi in the rhizosphere of alfalfa in sterile, AMF-free or normal soil conditions. Appl Soil Ecol 15:159–168
Chen BD, Li XL, Christie P (2001) A modified glass bead compartment cultivation system for studies on nutrient and trace metal uptake by arbuscular mycorrhiza. Chemosphere 42:185–192
Curry JP (1994) Grassland invertebrates. Chapman & Hall, London
Finlay RD (1985) Interactions between soil micro-arthropods and endomycorrhizal associations of higher plants. In: Fitter AH, Attkinson D, Read DJ, Usher MB (eds) Ecological interactions in soil. Blackwell Scientific Publications, Oxford, pp 319–331
Gange AC (2000) Arbuscular mycorrhizal fungi, Collembola and plant growth. Trends Ecol Evol 15:369–372
Gange AC, Ayres RL (1999) On the relation between arbuscular mycorrhizal colonization and plant ‘benefit’. Oikos 87:615–621
Gerdemann JH, Nicholson TH (1963) Spores of mycorrhizal endogene species extracted from soil by wet-sieving and decanting. Trans Br Mycol Soc 46:235–244
Giovanetti M, Mosse B (1980) An evaluation techniques for measuring vesicular-arbuscular infection in roots. New Phytol 84:489–500
Hodge A (2000) Microbial ecology of the arbuscular mycorrhiza. FEMS Microbiol Ecol 32:91–96
Jakucs E (1999) Fungal symbioses. Acta Microbiol Hung 46:193–195
Jansa J, Mozafar A, Frossard E (2003) Long-distance transport of P ad Zn through the hyphae of an arbuscular mycorrhizal fungus in symbiosis with maize. Agronomie 23:481–488
Joner EJ, Leyval C (2001) Time-course of heavy metal uptake in maize and clover as affected by root density and different mycorrhizal inoculation regimes. Biol Fert Soils 33:351–357
Kaiser PA, Lussenhop J (1991) Collembolan effects on establishment of vesicular-arbuscular mycorrhizae in soybean (Glycine max). Soil Biol Biochem 23:307–308
Klironomos JN, Kendrick WB (1996) Palatability of microfungi to soil arthropods in relation to the functioning of arbuscular mycorrhizae. Biol Fertil Soils 21:43–52
Klironomos JN, Moutoglis P (1999) Colonization of nonmycorrhizal plants by neighbours as influenced by the Collembolan, Folsomia candida. Biol Fertil Soils 29:277–281
Kothari SK, Marschner H, Römheld V (1990) Direct and indirect effects of VA mycorrhizal hyphae in acquisition of phosphorus and zinc by maize grown in calcareous soil. Plant Soil 131:177–185
Larink O (1997) Springtailes and mites: important knots in the food web of soils. In: Benckiser G (eds) Fauna in soil ecosystems: recycling processes. Nutrient fluxes and agriculture production. Marcel Dekker, New York, pp 225–264
Larsen J, Jakobsen I (1996) Effects of a mycophagous Collembola on the symbioses between Trifolium subterraneum and three arbuscular mycorrhizal fungi. New Phytol 133:295–302
Li X, Christie P (2001) Changes in soil solution Zn and pH and uptake of Zn by arbuscular mycorrhizal red clover in Zn-contaminated soil. Chemosphere 42:201–207
Malloch DW, Pirozynski KA, Raven PH (1980) Ecological and evolutionary significance of mycorrhizal symbiosis in vascular plants (a review). Proc Natl Acad Sci USA 77:2113–2118
McGonigle TP (1995) The significance of grazing on fungi in nutrient cycling. Can J Bot 73:1370–1376
Moore JC, St John TV, Coleman DC (1985) Ingestion of vesicular–arbuscular mycorrhizal hyphae and spores by soil microarthropods. Ecology 66:1979–1981
Oudeh M, Khan M, Scullion J (2002) Plant accumulation of potentially toxic elements in sewage sludge as affected by soil organic matter level and mycorrhizal fungi. Environ Pollut 116:293–300
Petersen H (2002) General aspects of collembolann ecology at the turn of the millennium. Pedobiologia 46:246–260
Posta K, Füleky Gy (1997) Growth and phosphorus nutrition of mycorrhizal maize plants at different soil volumes and phosphorus supplies. Acta Agron Hung 45:135–145
Posta K, Füleky Gy (2000) Phosphate activity in the rhizosphere and hyphosphere of maize induced by different phosphorus sources. Bull Szent István Univ 55–67
Takács T, Vörös I (2003) Effect of metal non-adapted arbuscular mycorrhizal fungi on Cd, Ni and Zn uptake by ryegrass. Acta Agron Hung 51(3):347–354
Tennant D (1975) A test of modified line intersect method of estimating root length. J Ecol 63:995–1001
Thimm T, Larink O (1995) Grazing preferences of some Collembola for endomycorrhizal fungi. Biol Fertil Soils 19:226–268
Warnock AJ, Fitter AH, Usher MB (1982) The influence of a springtail Folsomia candida (Insecta, Collembola) on the mycorrhizal association of leek (Allium porrum) and the vesicular–arbuscular mycorrhizal endophyte Glomus fasciculatus. New Phytol 90:285–292
Weissernhorn I, Leyval C, Belgy G, Berthelin J (1995) Arbuscular mycorrhizal contribution to heavy metal uptake by maize (Zea mays L.) in pot culture with contaminated soil. Mycorrhiza 5:145–251
Zhu Y, Christie P, Scott Laidlaw A (2001) Uptake of Zn by arbuscular mycorrhizal white clover from Zn-contaminated soil. Chemosphere 42:193–199
Acknowledgements
Authors are grateful to C.A.M. van Gestel (Vrije University, Amsterdam) for useful improvement of an earlier version of the manuscript and to J. Reiczigel (Szent István University, Department of Biomathematics and Informatics) for help with statistical analysis. The authors would like to express their special thanks to Zsuzsa Csaba for revision of the English text.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Seres, A., Bakonyi, G. & Posta, K. Zn uptake by maize under the influence of AM-fungi and Collembola Folsomia candida . Ecol Res 21, 692–697 (2006). https://doi.org/10.1007/s11284-006-0176-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-006-0176-8