Abstract
We studied duet song and vocal duetting behavior in an endemic Taiwanese passerine, Steere’s liocichla (Liocichla steerii). We found that the leading male song in duets was highly individualistic. Also, we found duetting behavior varied significantly across different habitat types. Females were more likely to answer male songs in densely vegetated, steep forest habitat compared to open agricultural habitat. These findings provide quantitative evidence for vocal individuality for a duet song and provide tentative support for the idea that females are duetting to reveal their location to their mates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals engage in antiphonal duets where song produced by one member of a pair is immediately followed by song from an individual’s mate. Antiphonal duets in birds may be so coordinated they may seem to an observer as a single complex song (Thorpe 1972; Farabaugh 1982; Morton 1996; Langmore 1998; Hall 2004). A number of hypotheses attempt to explain the function of duetting behavior (Hall 2004). Many explanations for the function of duet song are based on a conflict of interest between individuals creating the leading song and individuals generating the antiphonal answer (Levin 1996a, b; Hall 2004). Other hypotheses explain duetting in a more cooperative light and recognize the benefit to both sexes in maintaining contact, coordinating parental care, defending the territory and solidifying the pair bond (Thorpe 1972; Hall 2004). Close proximity can be facilitated by coordination of audible signals when visual cues are hindered. A joint effort by males and females to maintain proximity may act as a proximate behavioral mechanism that ultimately plays a role in the maintenance of the pair bond, joint territory defense, coordinating parental activities or facilitating mutually beneficial mate guarding. Duet song under this hypothesis, rather than conflicting with the function of the leading song, acts as a cooperative response to the initiator of the duet and thus serves to reveal a physical location.
Particular habitat characteristics may place limitations on the efficacy of visual cues in maintaining contact between males and females and therefore favor contact maintenance via audible cues such as vocal duetting. We examined the correlation between habitat type and duetting behavior and assessed the degree of vocal individuality in the leading duet songs in an endemic Taiwanese passerine, Steere’s liocichla (Liocichla steerii, Family: Timaliidae). Steere’s liocichla are socially monogamous birds that engage in male lead antiphonal duetting year-round (Lo 1987; Smith and Yu 1992).
Methods
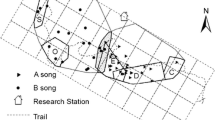
We studied duetting behavior in Steere’s liocichla between July and August 2000 and between May and July 2004 in Nantou County, Taiwan. Our study site (elevation—2,200 m) spans two highly divergent habitat types. At one end is National Taiwan University’s Meifeng Highlands Experiment Farm (MHF) and the other end encompasses the Taiwan Forest Bureau’s Zueyenshi Forest Reserve Area (ZFR). MHF is a comparatively flat, open, human disturbed site with orchards, agricultural fields, research buildings and greenhouses interlaced with forest in varying stages of succession. In contrast, ZFR is situated on a steep slope and consists primarily of dense native forest.
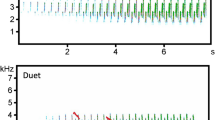
Recordings of male song in this study were made exclusively at MHF during 2000. Singing males were located on their territories and were recorded until they ceased singing. Recordings were made on cassette tape using a Marantz PDM-222 recorder and a Telinga PRO5B microphone with a 150-mm diameter parabolic dish. We used Canary (Charif et al. 1995) to convert our cassette recordings into sonograms using the following settings: clipping level=−90 dB, fast Fourier transformation=512 points, sampling rate=22 kHz and frame length=23 ms. We collected the following descriptive metrics: song length (s), change in frequency from beginning to end of the song, lowest frequency, highest frequency and frequency of maximum amplitude (all frequencies in kilohertz) for 4–28 songs per male across 13 males (Fig. 1). Discriminant function analysis measured individuality of male songs using a jackknifed classification matrix (McGarigal et al. 2000; Terry et al. 2001).
Representative sonogram of a typical two-syllable male song showing song metrics used in this analysis with frequency on the y-axis in kilohertz and time on the x-axis. T1 is time at beginning of song and T2 is time at end of song. T2-T1 equals song length in seconds. F1 is the frequency at the beginning of the song and F2 is the frequency at the end of the song. The difference between F1 and F2 is the change in frequency from the beginning to the end of the song. Fmax is the frequency of maximum amplitude. Fmax is the highest frequency and Fmin is the lowest frequency
For cross-habitat comparisons in duetting behavior between MHF and ZFR we located breeding pairs either by sight or sound and noted all male songs, female songs and the female answer rate during a 20-min period between the hours of 0630 and 1525. Observations between sites were done concurrently with a single observation per pair. During 2000 we also recorded the number of times either one or both members of the pair became visible to the observer. In 2000, our study was conducted near the end of the breeding season with birds at various stages of breeding including nest building, incubation, and nestling and fledgling feeding. We repeated this study during 2004 on six territories at MHF and four territories at ZFR. We compared duetting behavior using non-parametric tests and results are presented as means ± 1 SE. All statistical tests were performed using SYSTAT version 9 (Systat Software Inc., Richmond, CA, USA).
Results and discussion
Males sang a two-note song and females sometimes created the duet by adding their own sex-specific song consisting of three or more buzzy notes either immediately following or overlapping with their partner’s song (Lo 1987; Smith and Yu 1992). Male song was highly individualistic. High frequency was the only variable to be excluded from the model as it did not meet tolerances for inclusion (F-to-enter=2.16, tolerance P=0.001). Based on the remaining song variables discriminant function analysis correctly assigned 86% of all songs to the male that produced them (n=13). Individuality of male songs coupled with the observation that females only answered male songs produced on their territory suggests that individual recognition of the leading song in the duet is likely. These data are consistent with those from other studies demonstrating that birds do use vocal individuality to identify mates (Marzluf 1988; Robertson 1996).
Duetting behavior varied significantly across the two study sites. During 2000 we conducted observations on 20 pairs at MHF and 18 pairs at ZFR. During most observations the focal pair remained out of sight or when focal birds were seen they typically were seen alone. However, we tended to see pairs in close proximity (<5 m) at least once during an observation more often at MHF (4/20 observations) compared to ZFR (2/18 observations), however, the difference was not significant (Binomial test, P=0.17). Males sang more at ZFR (29.11±5.65 songs per observation) compared to MHF (19.45±3.03 songs per observation) but not significantly so (Mann–Whitney U=142.50, P=0.27). However, the rate at which females answered the songs of their mates differed significantly between the two habitat types (Fig. 2). Females answered proportionately more male songs on average at ZFR (mean answer rate 0.29±0.07) compared to MHF (mean answer rate 0.09±0.02; Mann–Whitney U=110.5, P<0.04). We repeated this study during 2004 early in the breeding season and obtained results similar to those obtained late in the breeding season during 2000. We collected data for four pairs at ZFR and six pairs at MHF. Females tended to answer their mate’s song more often at ZFR (mean answer rate 0.42±0.09) compared to MHF (mean answer rate 0.22±0.08), however, the result was statistically non-significant (Mann–Whitney U=3.5, P=0.07).
Male songs/observation and proportion of male songs answered/observation for MHF and ZFR during 2000. Mean number of male songs recorded per observation: (a) for MHF (open) and ZFR (shaded) and mean proportion of male songs answered by females and (b) for MHF (open) and ZFR (shaded) for n=20 pairs for MHF and n=18 pairs for ZFR. ns non-significant, *P<0.05 Fmax
To our knowledge, this is the only study to document a significant correlation between duetting behavior and habitat type and among a few (Grimes 1966; Watson 1969; Bard et al. 2002) to quantitatively examine vocal individuality in an avian duet component. These data suggest that vocal signaling of location is an important function of duetting behavior in Steere’s liocichla. However, we only tested the difference between two adjacent habitat types and our assessment of visual occlusion was qualitative. Differences in the timing of breeding between the two habitats could account for our results. However, we detected no differences in first egg dates between the two habitat types either for nests found during the late breeding season in 2000 (median first egg date MHF=192, n=4; median first egg date ZFR=178.5, n=4; Mann–Whitney U=2.5, P=0.11) or during the early breeding season in 2004 (median first egg date MHF=140, n=11; median first egg date ZFR=134.5, n=4; Mann–Whitney U=13.5, P=0.27). Additionally, we obtained qualitatively similar results for observations across 2 years and two different times during the breeding season and subsequent data revealed no effect of breeding stage on duetting frequency (unpublished data). Other variables we were unable to accurately measure could also account for our results. If habitats differ in quality this could likely influence the degree to which females answer male song especially if forested habitat is more valuable and requires more defense in the form of coordinated vocal advertisement. Barring these and other caveats, our results are consistent with a contact hypothesis where the duet song, in this case produced by the female, acts as a cooperative effort to reveal location between males and females. Other hypotheses concerning conflictory mate guarding (Sonnenschein and Reyer 1983; Levin 1996b), synchronization of breeding (Dilger 1953; Farabaugh 1982), promotion of pair-bond investment (Wickler 1980; Wickler and Seibt 1980; Smith 1994) and territorial defense (Sonnenschein and Reyer 1983; Levin 1996a, b) are not obviously linked to any specific set of habitat characteristics.
Support for contact maintenance hypotheses from other species is mixed. Data from magpie larks (Grallina cyanoleuca) indicate that duetting is not a behavioral mechanism to reveal location (Hall and Magrath 2000). However, in California quail (Lophortyx californicus) duetting behavior only occurs when males and females are separated (Stokes and Williams 1968). Data on the behavior of duetting species is limited so it is unclear how widespread duetting as contact maintenance really is. It is also unclear as to how antiphonal duets could better serve to maintain contact between mates as opposed to other forms of vocal signaling, such as more simple contact calls. We echo the call of Stutchbury and Morton (2001) for more work on the behavioral ecology of tropical birds in general and duetting birds in particular. Elucidating the ecological and social factors underlying the often unique behavioral repertoires of tropical and subtropical birds will lead to broader insights into the evolution of mating systems and male–female conflict and cooperation.
References
Bard SC, Hau M, Wikelski M, Wingfield JC (2002) Vocal distinctiveness and response to conspecific playback in the spotted antbird, a neotropical suboscine. Condor 104:387–394
Charif RA, Mitchell S, Clark CW (1995) Canary 1.2 user’s manual. Cornell Laboratory of Ornithology, Ithaca, NY
Dilger WC (1953) Dueting in the crimson-breasted barbet. Condor 55:220–221
Farabaugh SM (1982) The ecological and social significance of dueting. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, vol 2. Academic, New York, pp 85–124
Grimes L (1966) Antiphonal singing and call notes of Laniarius barbarus. Ibis 108:122–126
Hall ML (2004) A review of hypotheses for the functions of avian duetting. Behav Ecol Sociobiol 55:415–430
Hall ML, Magrath RD (2000) Duetting and mate-guarding in Australian Magpie-Larks (Grallina cyanoleuca). Behav Ecol Sociobiol 47:180–187
Langmore NE (1998) Functions of duet and solo songs of female birds. Trends Ecol Evol 13:136–140
Levin R (1996a) Song behaviour and reproductive strategies in a duetting wren, Thyothorus nigricapillus: I. Removal experiments. Anim Behav 52:1093–1106
Levin R (1996b) Song behaviour and reproductive strategies in a duetting wren, Thyothorus nigricapillus: II. Playback experiments. Anim Behav 52:1107–1117
Lo L-C (1987) The biological study of Liocichla steerii at Chitou area. MS dissertation, National Taiwan University (in Chinese)
Marzluf JM (1988) Vocal recognition of mates by breeding Pinyon Jays, Gymnorhinus cyanocephalus. Anim Behav 36:296–298
McGarigal K, Cushman S, Stafford S (2000) Multivariate statistics for wildlife and ecology research. Springer, Berlin Heidelberg New York
Morton ES (1996) A comparison of vocal behavior among tropical and temperate passerine birds. In: Kroodsma DE, Miller EH (eds) Ecology and evolution of acoustic communication in birds. Academic, New York, pp 258–268
Robertson BC (1996) Vocal mate recognition in a monogamous, flock-forming bird, the Silvereye, Zosterops lateralis. Anim Behav 51:303–311
Smith WJ (1994) Animal duets: forcing a mate to be attentive. J Theor Biol 166:221–223
Smith JI, Yu H-T (1992) The association between vocal characteristics and habitat type in Taiwanese passerines. Zool Sci 9:659–664
Sonnenschein E, Reyer H-U (1983) Mate guarding and other functions of antiphonal duets in the Slate-Coloured Boubou (Laniarius funebris). Zeitschrift fur Tierpsychologie 63:112–140
Stokes AW, Williams HW (1968) Antiphonal calling in quail. Auk 85:83
Stutchbury BJM, Morton ES (2001) Behavioral ecology of tropical birds. Academic, San Diego
Terry AMR, McGregor PK, Peake TM (2001) A comparison of some techniques used to assess vocal individuality. Bioacoustics 11:169–188
Thorpe WH (1972) Dueting and anitphonal singing in birds: Its extent and significance. Behaviour Supplement no. 18
Watson M (1969) Significance of antiphonal song in Eastern Whipbirds, Psophodes olivaceus. Behaviour 35:157–167
Wickler W (1980) Vocal duetting and the pair bond: I. Coyness and partner commitment. A hypothesis. Z Tierpsychol 52:201–209
Wickler W, Seibt U (1980) Vocal dueting and the pair bond: II. Unison dueting in the African Forest Weaver, Symplectes bicolor. Z Tierpsychol 52:217–226
Acknowledgements
Thanks to the staff of National Taiwan University’s Meifeng Highlands Experiment Farm and the Taiwan Forest Bureau. H. Yan allowed the use of recording equipment and P. Nolan and D. Mennill aided in the analysis. Also, thanks to W-T. Liang, Y-T. Liu, S-F. Shen, H-Y. Huang for help in the field. D. Westneat, G. Hill, D. Mennill, D. Logue and two anonymous reviewers provided valuable comments. Funding was provided by National Science Council and Endemic Species Research Institute in Taiwan, and National Science Foundation grant no. 0307421 and National Science Foundation’s East Asia and Pacific Summer Program in the USA.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mays, H.L., Yao, CT. & Yuan, HW. Antiphonal duetting in Steere’s liocichla (Liocichla steerii): male song individuality and correlation between habitat and duetting behavior. Ecol Res 21, 311–314 (2006). https://doi.org/10.1007/s11284-005-0115-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-005-0115-0