Abstract
Objectives
To systematically review the relationship between the fractal dimension (FD) on jaw bones and skeletal bone mineral density (BMD), focusing on the different methodologies and results in the existing literature.
Methods
An electronic literature search was performed to identify articles that evaluated the relationship between the mandibular and maxillary FD and BMD up to February 2014. After selecting the articles, a hierarchical clustering analysis was performed to verify the dissimilarity of the studies. The following variables were grouped into clusters, and assessed for reliability of the cluster distribution: kind of study, method for calculating FD, relationship between FD and low BMD, digital imaging, and kind of dental radiography. The relative frequencies of the categories of variables were calculated and compared.
Results
Only 15 studies were selected and three clusters were obtained. Most articles were in the third cluster, and primarily consisted of clinical studies that used the box-counting method to calculate FD on panoramic radiographs. A significant association was found for the variable relationship between FD and low BMD and the clusters. Most of the clinical studies were performed on trabecular bone, but three studies found differences in FD between individuals with normal mandibular cortex and individuals with cortical porosity.
Conclusions
The kind of study, method for calculating FD, relationship between FD and low BMD, and imaging modality should be better standardized to minimize the controversies found in published studies. The performance of FD on the cortical and trabecular bone for osteoporosis screening deserves further investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fractal analysis, a mathematical method for describing and analyzing complex shapes and structural patterns, has been adapted to the study of various natural structures and biosignals [1]. Fractal analysis is expressed numerically as the fractal dimension (FD). The FD of a process measures its complexity, spatial extent, or space-filling capacity and is related to the shape and dimensionality of the process. If the theoretical FD of a set exceeds its topological dimension, the set is considered to have fractal geometry. The main attraction of fractal geometry stems from its ability to describe the irregular or fragmented shapes of natural features as well as other complex objects that traditional Euclidean geometry fails to analyze. Fractal geometry is widely used in image analysis problems in general and especially in the medical field [2–5].
Trabecular bone has a branching pattern that exhibits fractal properties such as statistical self-similarity and lack of well-defined scale. Because of this phenomenon, application of fractal geometry and measurement of FD can be used to determine the trabecular complexity and bone structure [6–8]. The applicability and relevance of fractal geometry to medical image analysis are justified by the fact that statistical self-similarity can hardly be verified in biological objects imaged with finite resolution [5]. Therefore, algorithms have been developed to quantify the textural properties of an image for evaluating certain bone diseases, such as osteoporosis [9–16].
Osteoporosis is a disease characterized by reduced bone strength and increased susceptibility to fractures per se or secondary to a minor trauma. The bone strength primarily reflects the integration of bone density and quality [17]. Currently, osteoporosis diagnosis and staging are based on the identification of different risk factors, the most important being low bone mineral density (BMD) of the femur or lumbar spine [18].
BMD is routinely determined by dual-energy X-ray absorptiometry (DXA) [19]. Although BMD is a strong determinant of bone strength [20], many patients without densitometric diagnosis are at increased risk of fractures, and a high percentage of fractures occurs in patients with osteopenia or normal BMD T-scores [21]. Consequently, other important factors need to be taken into account for proper assessment of fractures, such as clinical risk factors as well as the macroarchitecture and microarchitecture of bone [22].
One of the most important factors contributing to bone strength is its complex structure [6]. Consequently, fractal analysis of plain radiographs has been employed to assess the trabecular bone structure and biomechanical properties at several bone sites [10–16, 22]. One study found that the relationship between FD analysis and elastic properties of bone was stronger than that with BMD [23]. Other studies demonstrated that inclusion of fractal measures, in addition to BMD, improved the predictions of strength and elastic modulus values [16, 22, 23].
Few studies have analyzed the trabecular pattern on dental radiographs of osteoporotic patients [24–37], following the FD approach. Nevertheless, several previous studies have demonstrated that dental imaging could be strongly positioned as a way to screen for osteoporosis, given that it is applied to a large fraction of the older population annually in private practices and public health clinics [24–40]. These studies revealed reduced mandibular cortical and alveolar BMD, decreased alveolar bone height, increased porosity of mandibular alveolar and inferior cortical bone, erosion and reduced width of the inferior border of the mandible, and altered trabecular patterns in maxillary and mandibular bone in osteoporotic patients.
Although fractal analysis has been proven to be efficient in evaluating bone quality at several bone sites, there is no scientific evidence for application of FD analysis for the same purpose in the jaw bones. This article offers a systematic review and analysis of published information in the area, focusing on the different methodologies and results in the existing literature.
Materials and methods
Literature search and selection of articles
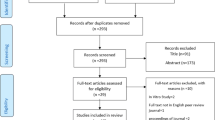
An electronic literature search was performed in the PubMed interface of Medline (National Library of Medicine), Scopus Database, and Web of Science to identify as many articles as possible that applied FD analysis in the assessment of skeletal BMD using dental imaging modalities up to February 2014. The inclusion criteria used for the initial selection of appropriate articles from the published abstracts consisted of combinations of the following keywords: (1) fractal and bone mineral density, (2) fractal and osteoporosis, (3) fractal and jaws, and (4) fractal and mandibular. The initial eligibility of potential studies was determined by reading the title and abstract of each article identified by the search engine using the criteria mentioned above. All articles that appeared to meet the inclusion criteria based on their abstracts were selected and collected. The first electronic search resulted in 204 different abstracts. The final selection was completed after eliminating duplicated articles, reviews of the literature, and articles that were not related to osteoporosis and FD analysis of the jaws. Figure 1 shows a flowchart of the study selection procedure.
Statistical analyses
A meta-analysis of the data in the selected studies was not attempted because of the variations in study design, methodology, imaging modality, and method for calculating FD.
After selecting and reading the articles, a hierarchical clustering analysis according to Ward’s method was performed to verify the dissimilarity of the studies, using the chord distance. For this purpose, five variables were defined for each study: (1) kind of study (in vitro, in vivo with patients, in vivo with animals), (2) method for calculating FD (power spectrum method, caliper method, box-counting method, dilation method), (3) relationship between FD and low BMD (increase in FD, decrease in FD, absence of change/correlation), (4) kind of digital image (direct using digital technologies/receptors, indirect using analog techniques/scanned images), and (5) kind of dental radiography [intraoral, extraoral, intraoral and extraoral, cone-beam computed tomography (CBCT)]. Subsequently, the aforementioned variables were grouped into clusters, and assessed for reliability of the cluster distribution by the cophenetic correlation coefficient. The relative frequencies of the categories of variables were calculated and compared by clusters using the χ2 test and Fisher’s exact test. All statistical analyses were performed with XLStat 2010 software (Addinsoft, New York, NY). Values of p < 0.05 were considered to indicate statistical significance for all tests.
Results
After a complete analysis of the articles, only 14 articles met all of the inclusion criteria [24–37]. One article contained two different experiments, and was thus considered as two different studies [24]. The study designs, objectives, main results, and conclusions related to the FD are shown in Table 1. Table 2 shows the technical parameters of the FD calculations in the selected articles. Reading of the selected articles further demonstrated that the sizes and locations of the regions of interest (ROIs) were not standardized, as can be seen in Fig. 2.
Three different clusters were obtained after testing the dissimilarity of the selected articles. The third cluster was composed of nine articles [28, 30–37], while the remaining articles were divided into the first [24, 26] and second [25, 27, 29] clusters. Figure 3 shows a dendrogram of the dissimilarity of the clusters of the selected articles.
The first cluster was mainly composed of studies that used digitized intraoral radiographs and the power spectrum method for calculating FD, and the FD increased in most of the studies of this cluster. The second cluster was also mainly composed of studies that used digitized intraoral radiographs, but the caliper method was chosen for calculating FD. In most of the studies in the second cluster, the relationship between the FD and BMD was not significant. The third cluster primarily consisted of clinical studies (in vivo) that used the box-counting method to calculate FD on panoramic radiographs.
Figure 4 demonstrates the relationships between the five variables defined for each study and the clusters. Regarding the type of study (Fig. 4a), a higher frequency of in vivo studies in patients was present in the third cluster (88.9 %), but there were no associations between the other categories of studies and the other clusters (p = 0.210).
Relationships between the variables and the clusters. a Kind of study (in vitro, in vivo with patients, in vivo with animals). b Relationship between FD and low BMD (increase in FD, decrease in FD, absence of change/correlation). c Method for calculating FD (power spectrum method, caliper method, box-counting method, dilation method). d Kind of digital image (direct using digital technologies/receptors, indirect using analog techniques/scanned images). e Kind of dental radiography (intraoral, extraoral, intraoral and extraoral, and CBCT)
Regarding the variable relationship between FD and low BMD (Fig. 4b), a significant association was found with the clusters (p = 0.044). An increase in FD was observed in the articles in the first cluster (100 %). An absence of correlation was observed in 67 % of articles in the second cluster. Conversely, FD decreased with low BMD in most of the studies in the third cluster (55.6 %).
As shown in Fig. 4c, there was a strong association (p < 0.001) between the power spectrum method of calculating FD and cluster 1 (100 %), the caliper method and cluster 2 (100 %), and the box-counting method and cluster 3 (88.9 %), but no association was found between the dilation method and the clusters.
No association was found between the kind of digital image (digitized or direct digital images) and the clusters (p = 0.238), although scanned images were used in all articles in the first cluster and 67 % of articles in the second cluster. Conversely, the direct method was used in 67 % of studies in the third cluster (Fig. 4d).
Most of the studies in the first and second clusters used intraoral radiographs, while 67 % of articles in the third cluster were based only on panoramic radiographs (Fig. 4e). However, no significant association was found between the kind of dental radiographs and the clusters (p = 0.127).
Discussion
To the best of our knowledge, the present study is the first systematic review with the aim of discussing the little available evidence about the relationship between mandibular and maxillary FD and skeletal BMD. First, only a few articles related to this subject were found. Of the 204 articles initially found, only 14 met the inclusion criteria [24–37]. As one article had two different experiments (in vitro and in vivo), we decided to consider this article as two different studies for quantitative and qualitative analyses [24].
Some authors have stated that fractal analysis of radiographs at different skeletal sites appears to be a promising tool for assessment of trabecular bone structure, biomechanical properties, and skeletal status, and these measures could play a complementary role in addition to BMD in the examination of osteoporosis and skeletal status [10–16]. Regarding the application of FD analysis to the jaws, a previous study stated that, among several methods developed to estimate the alveolar bone density from dental radiographs, fractal analysis appears to hold the greatest promise as an inexpensive and readily available method [41].
In our systematic review, we made an attempt to collate, compare, and discuss the methodologies and results of different studies that calculated FD on the jaw bones and related it to skeletal BMD. Reading of the selected articles revealed significant heterogeneity. Variations in the study design, methodology, imaging modality, and method for calculating FD precluded a meta-analysis of the data in the selected studies. A comparison of the findings was also difficult because of the significant methodological differences between the selected studies and the conflicting results. To verify the dissimilarity of the studies, we used a hierarchical clustering analysis according to Ward’s method. To more clearly examine this area of investigation, five variables were divided into three clusters.
The first and second clusters were mainly composed of studies that used digitized intraoral radiographs [24–27, 29]. Nevertheless, the methods for calculating FD were different in these clusters, as all articles in the first cluster used the power spectrum method [24, 26] and all articles in the second cluster used the caliper method [25, 27, 29]. A significant association was only found in the method for calculating FD and the first and second clusters. This result may be related to the number of studies with the same authors in these clusters.
In some articles, the FD increased in postmenopausal women [24], osteoporotic patients [26, 28], and patients with a thinner and severely eroded mandibular cortex [26, 32]. Conversely, some previous studies showed a strong correlation between simulated osteoporosis in the maxillae and decreased FD [25], and also for decreased FD with low BMD [33–37]. In addition, we found some articles in which the FD on the jaw bones was not related to the density of the spine, hip, or radius [29], or the FD did not differ significantly between normal and osteoporotic patients [30, 31].
The conflicting results of the clinical studies have been mentioned previously in the literature [42]. However, the small sample sizes in the above three studies that found no relationship between FD and low BMD should be emphasized. Moreover, two of these studies were performed in younger populations [29, 31], while BMD was only measured in the lumbar spine in the other study [30].
Most of the clinical studies were in the third cluster, which primarily consisted of studies that used the box-counting method to calculate FD on panoramic radiographs. In our review, only three studies evaluated FD on the mandibular cortical bone, with different methodologies for selecting the cortex [32, 35, 37]. The first of these studies showed a significant difference in the mean FD of the mandibular cortex for groups of patients classified as C1, C2, and C3 by the MCI index [32]. Nevertheless, BMD was not evaluated in that study. Other authors found a decreased FD on the mandibular cortex in patients with low BMD [36]. A recent study found that the odds of having lower mean values for the mandibular cortical width and FD on the cortical bone were higher in osteoporotic patients [37]. These results may be explained by resorption of the inferior border of the mandibular cortical bone, which is often seen in patients with low BMD [34, 38, 39].
The conflicting data regarding trabecular bone, as suggested by some authors, may be explained by anatomical variations, discrepancies in the imaging methods used to obtain two-dimensional or three-dimensional bone images, differences in selecting the areas to be measured, and/or differences in techniques for measuring FD [42]. Further, a recent study suggested that the performance of FD on the cortical bone was different from that of FD on the trabecular bone in women [37]. The same authors stated that cortical bone measurements might be considered as auxiliary tools to referring patients for DXA examinations, while the FD on the mandibular trabecular bone still cannot be used as an osteoporosis predictor [37]. However, in the most recent studies, a tendency toward a reduction in the FD on trabecular bone was seen in patients with osteoporosis compared with individuals with normal BMD [33–37]. This may explain the significant association found in our systematic review between the variable relationship between FD and low BMD and the clusters. The FD on cortical and trabecular bone should be further investigated with a view to use for osteoporosis screening.
Our systematic review also showed that some clinical studies used different imaging modalities such as intraoral radiography [24, 26, 28–30], panoramic radiography [26, 28, 30–32, 34–37], and CBCT [33]. Therefore, comparison of the results in these studies was difficult, as FD can only be reliably compared when using imaging modalities at the same spatial resolution [43]. Each imaging modality may have its own non-linear artifacts such as sampling frequency, noise, and spatial resolution. A previous study also verified that FD values estimated from digitized films on radiographs of rat bones were significantly greater than those produced by direct digital images [44]. Regarding our sample of studies, digitized forms of conventional dental radiographs (intraoral or panoramic) were generally used. This means that the FD may have been overestimated in most studies. FD analysis of CBCT images warrants further investigation, as only one study used this imaging modality.
Many methods exist for measuring FD, and each method has its own theoretical basis. This fact often leads to the acquisition of different dimensions based on different methods for the same feature [5]. The most commonly used procedure for calculating FD on dental radiographs was the box-counting method, probably because of its simplicity and availability [30–37]. When using the box-counting method for calculating FD, the studies generally used ImageJ software, a public domain program that can be downloaded from http://rsb.info.nih.gov/ij/. In fractal analysis, a box-counting algorithm is mainly used to quantify the trabecular pattern by counting the trabecular bone and bone marrow interface. The method also assesses the boundary of the trabecular bone and bone marrow, and a higher box-counting value indicates a more complex structure [4]. However, the results of the selected studies using the box-counting method were not in line with the above-mentioned statement.
Although fractals are very useful, calculation of quantitative properties like the FD is elusive and challenging. Therefore, several attempts have been made to investigate the reliability of FD estimation with different algorithms applied to different fractal functions [45]. Despite being the most commonly used procedure, the box-counting method has several limitations, such as the difficulty in obtaining error bounds [46], possibility of overestimation or underestimation [45], construction of empty boxes, box-size dependency of the FD computation, grid effect, and process of signal binarization required for this method [5]. The box-counting method may not cover the image surface very well, and hence cannot capture the FD for a rough-textured surface [2]. Further, disadvantages of the box-counting dimension are the choice of initial and final sizes of the magnification factor and the longer time required for computation [47].
Some authors have demonstrated that large systematic errors can occur for a variety of estimator techniques, such as the box-counting method and power spectrum method, over a wide range of input FD values. When applied to Takagi and Brownian motion curves, these authors demonstrated the roles of resolution and quantization as limiting factors in the estimation process [45]. Since no estimators work well universally, the best outcome is to observe and explain the relative discrepancies of all estimators simultaneously to gain insights [42]. Therefore, further radiographic studies should be performed with combinations of different estimators at a standardized resolution to minimize the aforementioned limitations.
Our study has strengths and limitations. Owing to the scarcity and heterogeneity of relevant studies in the literature, it was not possible to perform a meta-analysis of the data in the selected studies. Consequently, it was decided to select the variables that could influence the dissimilarities found in the few published studies. Regarding the choice of areas to be analyzed by FD, the sizes and locations of the ROIs were extremely variable in the studies, and were not included in the hierarchical clustering analysis. Some authors described that absolute ROI placement might not be necessary, since the FD values determined from ROIs on digital radiographic images of alveolar bone were insensitive to small variations in X-ray exposure, beam alignment, and ROI position [48].
In conclusion, this review has highlighted directions in which further research could help to determine the potential of detecting low BMD in patients based on FD analysis of dental radiographs. However, the kind of study, method for calculating FD, relationship between FD and low BMD, and imaging modality should be better standardized to minimize the controversies found in published studies. Future studies may also clarify the differences in performance between FD on the cortical and trabecular bone for osteoporosis screening.
References
Sánchez I, Uzcátegui G. Fractals in dentistry. J Dent. 2011;39:273–92.
Sarkar N, Chaudhuri BB. An efficient approach to estimate fractal dimension of textural images. Pattern Recogn. 1992;25:1035–41.
Naik GR, Arjunan S, Kumar D. Applications of ICA and fractal dimension in sEMG signal processing for subtle movement analysis: a review. Australas Phys Eng Sci Med. 2011;34:179–93.
Caligiuri P, Giger ML, Favus M. Multifractal radiographic analysis of osteoporosis. Med Phys. 1994;21:503–8.
Lopes R, Betrouni N. Fractal and multifractal analysis: a review. Med Image Anal. 2009;13:634–49.
Sanchez-Molina D, Velazquez-Ameijide J, Quintana V, Arregui-Dalmases C, Crandall JR, Subit D, et al. Fractal dimension and mechanical properties of human cortical bone. Med Eng Phys. 2013;35:576–82.
Haire TJ, Hodgskinson R, Ganney PS, Langton CM. A comparison of porosity, fabric and fractal dimension as predictors of the Young’s modulus of equine cancellous bone. Med Eng Phys. 1998;20:588–93.
Geraets WG, van der Stelt P. Fractal properties of bone. Dentomaxillofac Radiol. 2000;29:144–53.
Demirbaş AK, Ergün S, Güneri P, Aktener BO, Boyacioğlu H. Mandibular bone changes in sickle cell anemia: fractal analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:41–8.
Ergün S, Saraçoglu A, Güneri P, Ozpinar B. Application of fractal analysis in hyperparathyroidism. Dentomaxillofac Radiol. 2009;38:281–8.
Pothuaud L, Lespessailles E, Harba R, Jennane R, Royant V, Eynard E, et al. Fractal analysis of trabecular bone texture on radiographs: discriminant value in postmenopausal osteoporosis. Osteoporos Int. 1998;8:618–25.
Lin JC, Grampp S, Link T, Kothari M, Newitt DC, Felsenberg D, et al. Fractal analysis of proximal femur radiographs: correlation with biomechanical properties and bone mineral density. Osteoporos Int. 1999;9:516–24.
Majumdar S, Link TM, Millard J, Lin JC, Augat P, Newitt D. In vivo assessment of trabecular bone structure using fractal analysis of distal radius radiographs. Med Phys. 2000;27:2594–9.
Lespessailles E, Poupon S, Niamane R, Loiseau-Peres S, Derommelaere G, Harba R, et al. Fractal analysis of trabecular bone texture on calcaneus radiographs: effects of age, time since menopause and hormone replacement therapy. Osteoporos Int. 2002;13:366–72.
Lespessailles E, Gadois C, Kousignian I, Neveu JP, Fardellone P, Kolta S, et al. Clinical interest of bone texture analysis in osteoporosis: a case control multicenter study. Osteoporos Int. 2008;19:1019–28.
Le Corroller T, Halgrin J, Pithioux M, Guenoun D, Chabrand P, Champsaur P. Combination of texture analysis and bone mineral density improves the prediction of fracture load in human femurs. Osteoporos Int. 2012;23:163–9.
NIH Consensus Development Panel on Osteoporosis Prevention. Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95.
Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–81.
Grampp S, Genant HK, Mathur A, Lang P, Jergas M, Takada M, et al. Comparisons of noninvasive bone mineral measurements in assessing age-related loss, fracture discrimination, and diagnostic classification. J Bone Miner Res. 1997;12:697–711.
Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9.
Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202.
Kolta S, Paratte S, Amphoux T, Persohn S, Campana S, Skalli W, et al. Bone texture analysis of human femurs using a new device (BMA™) improves failure load prediction. Osteoporos Int. 2012;23:1311–6.
Majumdar S, Lin J, Link T, Millard J, Augat P, Ouyang X, et al. Fractal analysis of radiographs: assessment of trabecular bone structure and prediction of elastic modulus and strength. Med Phys. 1999;26:1330–40.
Ruttimann UE, Webber RL, Hazelrig JB. Fractal dimension from radiographs of peridental alveolar bone. A possible diagnostic indicator of osteoporosis. Oral Surg Oral Med Oral Pathol. 1992;74:98–110.
Southard TE, Southard KA, Jakobsen JR, Hillis SL, Najim CA. Fractal dimension in radiographic analysis of alveolar process bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:569–76.
Law AN, Bollen AM, Chen SK. Detecting osteoporosis using dental radiographs: a comparison of four methods. J Am Dent Assoc. 1996;127:1734–42.
Southard TE, Southard KA, Krizan KE, Hillis SL, Haller JW, Keller J, et al. Mandibular bone density and fractal dimension in rabbits with induced osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:244–9.
Bollen AM, Taguchi A, Hujoel PP, Hollender LG. Fractal dimension on dental radiographs. Dentomaxillofac Radiol. 2001;30:270–5.
Southard TE, Southard KA, Lee A. Alveolar process fractal dimension and postcranial bone density. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:486–91.
Yaşar F, Akgünlü F. The differences in panoramic mandibular indices and fractal dimension between patients with and without spinal osteoporosis. Dentomaxillofac Radiol. 2006;35:1–9.
Tosoni GM, Lurie AG, Cowan AE, Burleson JA. Pixel intensity and fractal analyses: detecting osteoporosis in perimenopausal and postmenopausal women by using digital panoramic images. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:235–41.
Yasar F, Akgunlu F. Evaluating mandibular cortical index quantitatively. Eur J Dent. 2008;2:283–90.
Hua Y, Nackaerts O, Duyck J, Maes F, Jacobs R. Bone quality assessment based on cone beam computed tomography imaging. Clin Oral Implants Res. 2009;20:767–71.
Alman AC, Johnson LR, Calverley DC, Grunwald GK, Lezotte DC, Hokanson JE. Diagnostic capabilities of fractal dimension and mandibular cortical width to identify men and women with decreased bone mineral density. Osteoporos Int. 2012;23:1631–6.
Roberts MG, Graham J, Devlin H. Image texture in dental panoramic radiographs as a potential biomarker of osteoporosis. IEEE Trans Biomed Eng. 2013;60:2384–92.
Oliveira ML, Pedrosa EF, Cruz AD, Haiter-Neto F, Paula FJ, Watanabe PC. Relationship between bone mineral density and trabecular bone pattern in postmenopausal osteoporotic Brazilian women. Clin Oral Investig. 2013;17:1847–53.
Sindeaux R, Figueiredo PT, de Melo NS, Guimarães AT, Lazarte L, Pereira FB, et al. Fractal dimension and mandibular cortical width in normal and osteoporotic men and women. Maturitas. 2014;77:142–8.
Taguchi A, Suei Y, Ohtsuka M, Otani K, Tanimoto K, Ohtaki M. Usefulness of panoramic radiography in the diagnosis of postmenopausal osteoporosis in women. Width and morphology of inferior cortex of the mandible. Dentomaxillofac Radiol. 1996;25:263–7.
Leite AF, Figueiredo PT, Guia CM, Melo NS, Paula AP. Correlations between seven panoramic radiomorphometric indices and bone mineral density in postmenopausal women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:449–56.
White SC, Rudolph DJ. Alterations of the trabecular pattern of the jaws in patients with osteoporosis. Oral Surg Oral Med Oral Pathol. 1999;88:628–35.
Amer ME, Heo MS, Brooks SL, Benavides E. Anatomical variations of trabecular bone structure in intraoral radiographs using fractal and particles count analyses. Imaging Sci Dent. 2012;42:5–12.
Chappard C, Brunet-Imbault B, Lemineur G, Giraudeau B, Basillais A, Harba R, et al. Anisotropy changes in post-menopausal osteoporosis: characterization by a new index applied to trabecular bone radiographic images. Osteoporos Int. 2005;16:1193–202.
Veenland JF, Grashius JL, van der Meer F, Beckers AL, Gelsema ES. Estimation of fractal dimension in radiographs. Med Phys. 1996;23:585–94.
Pornprasertsuk S, Ludlow JB, Webber RL, Tyndall DA, Yamauchi M. Analysis of fractal dimensions of rat bones from film and digital images. Dentomaxillofac Radiol. 2001;30:179–83.
Brewer J, Girolamo LD. Limitations of fractal dimension estimation algorithms with implications for cloud studies. Atmos Res. 2006;82:433–54.
Dubuc B, Dubuc S. Error bounds on the estimation of fractal dimension. Siam J Numer Anal. 1996;33:602–26.
Carlin M. Measuring the complexity of non-fractal shapes by a fractal method. Patt Recog Lett. 2000;21:1013–7.
Shrout MK, Potter BJ, Hildebolt CF. The effect of image variations on fractal dimension calculations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:96–100.
Conflict of interest
André Ferreira Leite, Paulo Tadeu de Souza Figueiredo, Hugo Caracas, Rafael Sindeaux, Ana Tereza Bittencourt Guimarães, Leonardo Lazarte, Ana Patrícia de Paula, and Nilce Santos de Melo declare that they have no conflict of interest.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leite, A.F., de Souza Figueiredo, P.T., Caracas, H. et al. Systematic review with hierarchical clustering analysis for the fractal dimension in assessment of skeletal bone mineral density using dental radiographs. Oral Radiol 31, 1–13 (2015). https://doi.org/10.1007/s11282-014-0188-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11282-014-0188-y