Abstract
Nowadays, the Internet of Bio-NanoThings (IoBNT) is playing an important role to become the leading technique used in healthcare delivery systems. The IoBNT is considered a promising paradigm for efficient communication between nanodevices to deliver therapeutic drug molecules and thus achieve the target concentration to the diseased cell/tissue inside intra-body nanonetworks. However, ignoring the physical architecture of these nanodevices may effect on the delivered concentration when employing the IoBNT paradigm. Therefore, in this paper, we propose a spherical transmitter nanodevice, namely reservoir for controlling the drug molecules to be released and showing the effects of the geometry design of such nanodevice on the concentration arrived inside intra-body nanonetwork. Moreover, we present a pharmacokinetic system comprising of a mathematical model for studying the effects and variance in drug concentration, while taking into consideration the distance from the center of the nanotransmitter to the center of the nanoreceiver. The performance analysis of the proposed IoBNT system is numerically investigated. The performance is evaluated by employing the pharmacokinetic model in terms of bio-cyber interface forward and reverse links. The simulation results reveal that the proposed model is able to achieve a high concentration around the targeted cells and thus decrease side effects around healthy cells compared with the state-of-the-art. Additionally, the results illustrate that proposed reservoir is capable of controlling the emission of the therapeutic drug molecules and thus improving the delivery of the dose at target time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanotechnology is a modern scientific approach that includes materials and equipment capable of manipulating the physical properties of the materials at the particulate and molecular levels of the system [1, 2]. Bionanotechnology is a branch of nanotechnology which uses biological materials, employs biological design or fabrication, and applied in medicine or biotechnology. One of the most important applications of bionanotechnology is the Internet of Bio-Nanothings (IoBNT). The IoBNT expands upon the Internet of Nanothings (IoNT) by using biologically embedded computing nanoscale devices. Moreover, the IoNT and IoBNT are defined as a novel paradigm-shifting concept for communication among nanoscale devices interconnected through the Internet [3]. On the other side, molecular communication (MC) is the branch that attracting the most significant attention in bionanotechnology [4]. MC is a recent technique that uses biochemical signals to achieve information interchange among naturally and artificially created bio-nanoscale machines over short distances.
There are many domains that use MC applications like nanomedicine [5, 6], bioengineering [7, 8], targeted drug delivery (TDD) system [9], and environmental safety [10]. Additionally, there are other applications involve intra-body sensing and actuation, intra-body connectivity and control, and environmental control and cleansing [11]. Many works have focused on applications like targeted drug delivery (TDD) [9] and disease diagnosis, monitoring, and therapy [12], while this study focuses specifically on the TDD system. TDD is an advanced method that is delivering the drugs to the patients where the medicament is selectively targeted site or delivering the only to its site of action or absorption and not to the non-targeted site. Furthermore, TDD is capable of concentrating the medicament in targeted tissues, organs or cells and reducing the relative concentration of the medication in the non-targeted tissues, that is improving efficacy and reducing side effects. It also reduces the frequency of the drug intake and the dosage, while maximizing the therapeutic indexes. The idea behind TDD is to elongate, localize, target and have the protected drug interaction with the infected tissue. In the TDD system, drug particle injection and transportation in human body and delivery to target tissue can be sighted in the context of MC. In the current study, the spherical reservoir nanodevice is the nanotransmitter (Nano-T), smart polymeric drug particles are information carriers, the communication channel is the blood vessel network, and the target tissue is one of the parts of the nanoreceiver (Nano-R). The idea behind the transmitter in this work is first, controlling the releasing drug molecules and, secondly, studying the effects of the geometry design of this transmitter on the concentration of the drug molecules released in target site.
The design of nanodevices depends on the existing biological systems or biochemical molecules. Disease diagnosis, monitoring, and therapy employed nanodevices as analytic and imaging tools, biochemical sensors for observing, and tissue engineering for in vivo tissue reform and reengineering [13]. The dimensions of these nanodevices are minute, and their biocompatible nature makes them powerful tools for manipulating biomaterials at the nanoscale level of organisms and systems. Moreover, this minute of the nanodevices makes easy to control their storage capacity and computational. While cooperation among them is achieving particular tasks [14]. The number of nanodevices in an intra-body nanonetwork is in the range from a few to millions. The challenging and essential task is how to achieve efficient communication between these nanodevices in a given body area network (BAN). For enhancing a given BAN and ensuring smooth coordination of communication between BAN and the nanodevices, the concepts of the Internet of Bio-NanoThings (IoBNT) is derived. On the other hand, nanodevices can be connected by other networks such as another BAN or macro-scale network like the Internet. These nanodevices will perform some assignments like detecting, releasing, capturing, synthesizing molecules, storing, and terminating functional decomposition through the IoBNT [15].
There are many defies that meet engineering and the realization of a practical IoBNT. These defies involve the physical architecture (geometry design) and the development of nanodevices, the coordination of molecular communication within the BAN, and the interface connection between the BAN and the internet in employing the IoBNT. Most of the previous studies are considered the nanotransmiter as an ideal point and ignored the design effects of this transmitter on releasing the drug molecules in the blood vessel channel. These studies focus only on communication efficiency between the nanodevices in a given BAN. Furthermore, there are other challenges such as storage rate, storage capacity, and how to control this process in a hybrid device. Nanodevice tracking and localization and network security are challenging issues need extensive research. The mentioned challenges are still open issues in nanocommunication research.
Our work focuses on the IoBNT and its application in healthcare monitoring and targeted drug delivery system. We propose a spherical transmitter nanodevice, namely reservoir for controlling the drug molecules to be released and showing the effects of the geometry design of such nanodevice on the concentration arrived inside intra-body nanonetwork. Moreover, we present a pharmacokinetic system comprising of a mathematical model for studying the effects and variance in drug concentration, while taking into consideration the distance from the center of the nanotransmitter (Nano-T) to the center of the nanoreceiver (Nano-R). The main contribution of this work can be summarized as follows:
-
1.
Modeling a spherical reservoir nanodevice to mimic the Nano-T in an IoBNT paradigm-based MC system, its function is controlling the released drug molecules inside the blood vessel channel.
-
2.
Modeling a nanoreceiver Nano-R with specific ligand and a high affinity constant for binding to the receptors on target cells. Its function is improving the effective therapies and increasing the absorption of the drug molecules by the receptors at the surface of the target cells.
-
3.
Evaluating the performance of the proposed model in the forward and reverse links by using a mathematical model of the pharmacokinetic model.
The rest of this work is organized as follows. Section 2 presents the related work. Section 3 introduces the system model. Section 4 presents the proposed approach for reservoir Nano-Transmitter. In Sect. 5, Nano-T and Nano-R model are derived. Section 6 introduces forward and reverses links based on the pharmacokinetic model. Finally, the numerical results and discussion are presented in Sect. 7.
2 Related work
In recent times, drug delivery systems (DDs) has a lot of attention because of the limitations of conventional administration drugs. As we mentioned in Section I, TDDs are a therapeutic technique aiming to achieve therapeutic medicaments, control the rate of drug released, and decrease side effects around healthy cells at the target tissue. TDDs can be managed by internal or external devices or materials that simplify the management of medicaments to a patient. The authors [16] have focused on the internal reservoir-based systems for controlling the drug released. The benefit of this system is how to deliver the therapeutic medicaments to the targeted area, especially if this area is difficult to reach by traditional systems or the drugs have a toxic or may spend a long-term track of dosing. In [17], the authors have represented the mathematical modelling of diffusion-controlled drug delivery, based on the geometry design devices which includes reservoir systems. Moreover, they have explained how to decide the type of mathematical equation which applied on the model by depending on the geometry device used. Even though the proposed method can achieve TDD systems, however the methods of how to represent its models in a realistic way based the physics models of drug release, transfer, and take-up and the complexity of human body are absent.
On the other side, MC can address the TDDs problems as it owns principles of multi-scale molecular and biological phenomena. MC can address these challenges by using mathematical tools like stochastic analysis, communication theory, and control theory as illustrated in [18]. The authors in [1] have injected nanotransmitter and nanoreceiver devices in targeted body’s system blood network to present MC-based TDD for delivering the therapeutic drug molecules to numerous sites. The authors have used compartmental model to achieve the effectiveness of TDD at the diseased sites. The efficacy of this mathematical model has been evaluated based on important parameters such as the diffusion characteristics, the receiver radius, the total nanotransmitter volume capacity, and the concentration of the enzyme on surface of targeted tissue. The authors in [19] have presented MC-based advanced drug delivery systems (ADDs) to convey information through molecules to identify the unique properties of antibodies and their possibilities. This presented model focused on solving some of the pharmacokinetics model issues. While, this model does not have enough temporal and spatial accuracy to apply ADDs efficiently to address some diseases like tumors, which are highly localized and proliferate. In [20], the authors have focused on the propagation channel, which is represented in the performance of MC system by providing the analysis of the physical processes implied MC by applying the information-theoretic. The goal of this work was to provide a mathematical framework for each block of MC supported by a general model for chemical kinetics. As well as they provided the statistical mechanisms to estimate the information capacity for communication channel. Moreover, molecular propagation is classified according to the application, which is used as follows: random walk, drifted random walk, and active transport. The chapter entitled [21] has focused on the development of the nanomaterials design of smart DDS as a nanocarriers. The issue of how to select targeted diseased cells by delivering the controlled amount of drug upon an external stimulus and saving the healthy cells from side effects. The authors have validated the proposed systems by using experimental and theoretical modeling. Additionally, they have reported the challenges facing the multivalent models such as ligand and receptor densities, the stability of the ligand and receptor complexes, or the ligand flexibility. On the other hand, they have introduced the benefits of DNA-based nanotechnology as a smart nanocarrier to solve the problem of controlling nanomaterials at the molecular level.
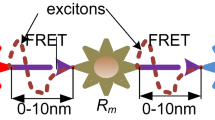
Even though the MC solves the gab of TDDs, but it is still facing a challenge of how to control, modify or reengineer for the transmission the molecular information from the natural. Hence, the IoBNT paradigm is applied for monitoring and controlling the nanodevices in body-area network (BAN) by employing Internet technology [22]. Wherein, it is capable of interconnecting the conventional network with the intra-body nanonetwork to interact with each other by exchanging different types of information like synchronization signals, and sets of commands and instructions through bio-cyber interface device. At this end, we can say that the TDDs, which presents the health monitoring and information technology communication (ICT), presents the Internet of biological nanothings (IoBNT) are joined to enhance the health tech. Subsequently, the authors [23] have presented a descriptive scenario and system model of the IoBNT. The authors applied the proposed model in an advanced healthcare delivery system. The idea behind this proposed model is to interconnect bio-chemical signal-based bionanonetwork with the traditional electromagnetic based Internet to remedy one of the main challenges of the IoBNT. The analysis of this work concentered on the system design which includes the bio-cyber interface and the propagation of molecular information in universal communication from medical personal to nanodevices in an intra-body nanonetwork. In the same direction, the authors [24] have presented a MolCom system for the uplink and downlink bio-cyber interface for IoBNT paradigm by employing the Fluorescence Resonance Energy Transfer (FRET) nanocommunications. The work entitled [3] has presented another architecture of the IoBNT model which focused on self-annihilation. Wherein, the deterministic model, made as a communication interface between the nanotrasnmitter and the nanodevices in an intra-body nanonetwork. The idea of the proposed model is making the nanotransmitter responsible for sending the death command to other nanodevices in an intra-body nanonetwork for eliminating the process of the dose when it is required through the Internet by using natural cells. The authors in [22] have described the source of the IoBNT paradigm which is from a combination of synthetic biology and nanotechnology. The engineering of computing devices can cooperate with biological cells and their functionalities with biochemical domains for helping on sensing and actuation in an intra-body and environmental control of toxic agents and pollution.
To the best of our knowledge, most of the above literatures have discussed IoBNT and molecular communications by considering the transmitter as an ideal point and ignoring the effects of the geometry (physical) design of this nanotransmitter on releasing the therapeutic drug molecules inside blood vessel channel. Further, these studies focused only on the efficient communication between nanodevices in a given targeted human body area nanonetwork and ignoring side effects that may occur in healthy cells in the same site. On the other hand, other studies focused on delivering therapeutic drug molecules to diseased cells inside a targeted site. Even, these studied did not use IoBNT for controlling and monitoring the diffusion of nanocarriers inside human body area nanonetwork (BAN). Thus, the aim of the proposed approach is to achieve desired therapeutic medicaments, to control the concentration of the drug molecules inside targeted site, and hence to decrease side effects around healthy cells on the same site. By taking into consideration the effects of the geometry design of Nano-T on this concentration, improving the pharmacokinetic system, and controlling the nanodevices in intra-body nanonetwork, we introduce an efficiently IoBNT paradigm comparing with the state-of-the-art.
3 System model
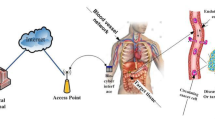
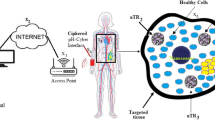
The block diagram of the proposed system model-based IoBNT paradigm is illustrated in Fig. 1. The system model consists of the following seven units: Internet, access point, wireless channel, bio-cyber interface device, blood vessel channel, extracellular channel, and targeted cells. Inspired by the classical communication scenario, those units are modeled as the time-varying impulse response h1, h2, h3, h4, h5, h6, and h7, sequentially. In the first step, the medical personnel send unique binary code orders through the Internet. The order function includes releasing drug molecules, synthesizing, and sensing proper drug molecules. The conventional Internet of things (IoTs) can be modeled as h1(t) and h2(t). Without loss of generality, to simplify the analysis of the system, the effects of the delay and error occurring owing to the Internet and access point are ignored. In this study, we focus on modeling the end-to-end system from the bio-cyber interface to target tissue. Our main objective is to achieve the desired effects of therapeutic medicament while taking into consideration the geometry of the transmitter device. In addition to decrease side effects in healthy cells of the body, we aim to study the issue of fast absorption and elimination of the target dose. To this end, we clarify how our system works, beginning from medical personnel until the delivery drug reaches the target cell site. The medical personal transmits the suitable command information that is forwarded by the access point. Subsequently, the communication between h1(t), h2(t) and h3(t) can be modeled as a wireless channel and its output as follows:
where, \( m^{\left( n \right)} \left( t \right) \) acts as input signals and * mentioned to the convolution operator. The superscript (n) denotes the direction of the communication, whereas (f) represents the forward link from the medical personal to the nanodevices in the target site and (r) represents the reverse link from nanosensor to medical personal.
As illustrated in Fig. 1, target tissue has three nanodevices reservoir Nano-T, Nano-R and Nano-E, and only one nanosensor. The reservoir Nano-T is injected into the vascular system that includes smart polymers as nanocarriers and has some tasks such as stimuli-responsive [25, 26]. The reservoir Nano-T is a transceiver in the blood vessel to release the encapsulated drug molecules to the target site in the diseased cells after influenced by external stimuli such as PH, temperature, and light as in [25,26,27].
Inspired by the biological system, the reservoir Nano-T may need these external stimuli to release the drug molecules to the target cell site after leaving the blood vessel wall through the endothelial gap on the surface of the wall indicated to the fenestrate. This model has one stimulus, which depends on the external application of hyperthermia that is used as a trigger to encourage changes of the polymer structure to release the drug molecules in the blood vessel.
The Nano-R is also a transceiver which synthesizes and emanates the specific therapeutic drug. In this study, the Nano-R is designed as the receiver of the nanocarriers. The receiver Nano-R for the nanocarriers is designed with specific ligand with high-affinity binding to the receptors on the surface of the diseased cells at the target site. After the reservoir, Nano-T releases the nanocarriers, reaches at the target tissue, and hence binds to the receptors on the surface of Nano-R and thus liberates the therapeutic drug molecules. The propagation of the drug molecules from the reservoir Nano-T to the space of Nano-R are activated by receptor-mediation or freely diffusion.
Whenever the drug delivery process has been finished, it is time to eliminate the nanodevices in the intra-body area network. It means that Nano-E received a command of death to elicit all the nanodevices in intra-body area network. On the other hand, according to [28], the Nano-E is responsible for releasing molecules to fabricate death-initiating molecules for artificial cells. In [3], another method for elimination process called self-annihilation, i.e. the Nano-E broadcasts a death signal to other nanodevices to perform the elimination process.
The nanosensor device is a bionanosensor that owns high sensitivity for realizing the existence of the nanocarriers in the blood vessel; it replying by releasing its molecules. The nanosensor according to [28, 29], is designed by utilizing the entire cell or sensor molecule like in [30, 31]. To construct the mathematical model for the nanosensor while taking into consideration the biochemical kinetics and its operation. In this work, the information drug molecules from the bio-cyber interface to target tissue and vice versa pass through the cardiovascular system. The propagation model of the molecular signals through the cardiovascular channel is based on the geometry design of the transmitter [17, 32], compartmental model [33, 34], and conceptual reception space [1]. The next section presents the design’s details of the proposed reservoir Nano-transmitter and its effects on the drug molecules inside blood vessel network and the pharmacokinetics system for the blood vessel network and target site and vice versa. Table 1 describes each part of our proposed system model.
4 Reservoir nano-transmitter model
The reservoir is a spherical nanodevice with constant activity source which contains the smart polymers as nanocarriers for the drug. As illustrated in Fig. 2, inside Nano-T, the drug core is surrounded by a polymer film. This drug is emitted through the rate-controlling porous polymeric membrane [16]. The properties of the coating thickness and the physicochemical properties of the enclosed drug-like drug particle size and molecular weight have been discussed in [17, 35]. As shown in Fig. 2, smart polymeric nanocarriers are used to deliver drugs. These nanocarriers start to release their cargo in response to external stimuli. We considered the stimuli represented by the change in temperature, namely thermal stimuli. Additionally, whenever there is a change in temperature, it starts to change their shape, solubility, or hydrophilic and hydrophobic balance [9].
In this work, we employed the Nano-T as a reservoir nanodevice; thereby, the drug concentration in inner membrane surface is constant. According to [36], the release process of the drug molecules encapsulated by smart polymeric nanocarriers, can be calculated at the time instant as follows:
where MT is the cumulative concentration of the released drug molecules, D is the diffusion coefficient of the drug within the membrane, Ro and Ri are the outer and inner radius of the Nano-T, respectively. k is the partition coefficient of the drug between the Nano-T and membrane. Cnano-T is the concentration of the drug in the core of the Nano-T. In our work, we focus on the concentration of the drug molecules inside the system and thus we can express it by the following derivative function:
where b is the thermal responsive of the released drug molecules rate, it is equal to the first-order rate constant. In this study, the released drug molecules are achieved by thermal responsive. In this case, the thermo-responsive is a system that is ruled by a nonlinear sharp change in the properties of a nanocarrier material with temperature. The released of the drug molecules is responsive to the external trigger and following the diversity in the surrounding temperature. The concentration of the drug molecules released to the system; it also means the output of bio-cyber interface device is given by:
where t is the period of the time from injection to the time starts releasing the drug.
5 Nano-T and nano-R model
The Nano-R is modeled as primarily composes of lipopeptide membrane occupied by target-ligand for targeting. The Nano-R has ligands on the surface, which activated by receptor-mediated or free diffusion. Wherein the released drug molecules are propagated through the tissue channel to Nano-R binds to these ligands according to [35]. As depicted at Fig. 3, we assume that the Nano-T has a position r0 = (x0, y0) with an outer radius R0, and Nano-R has a position r1 = (x1, y1) with reception radius space Rr around it. Subsequently, we can determine the distance, d between the Nano-T and Nano-R as follows:
Therefore, the concentration of the drug molecules that is received by a conceptual reception space around Nano-R based on the following:
where D is the diffusion channel coefficient, and w (t, r1) is the diffusion of the emitted concentration.
The diffusion of information drug molecules from Nano-T can be considered as the distribution of these molecules around Nano-R at time t evenly. This distribution depends on the distance between them. Thus, the initial condition can be described as follows:
where A is the amplitude of wR (0, d). Thus, the concentration of the active drug molecules, g that is received in the reception space Rr is considered as follows:
6 Pharmacokinetic model for forward and reverse link
We apply the compartmental model in [30, 31] to present the concentration of the diffusion of the drug molecules that finally reach from the blood vessel to the target site after being injected. This model is represented in Fig. 4. As the movement of the molecules, rate follows a first-order reaction law, which confirms some biophysical phenomena like diffusion. This means that the distribution of the molecules through the compartments is homogenous. Additionally, we consider the nanocarriers are not influenced by the chemical reactions that occur inside the channel on their link to the target site as well as, the compartment’s volumes do not vary with time. Finally, the elimination process of the drug from the body is fully executed through the blood compartments.
As illustrated in Fig. 4, the central compartment between the bio-cyber interface and target site is a blood vessel network, while in forwarding link f1(t) denotes the concentration of drug molecules in the blood vessel network and f2(t) represents the information molecules in the target site. vE(t) denotes the concentration function of eliminated molecules over time, along with the elimination rate k10. The liver is responsible here for eliminating this process. Furthermore, all the molecules affected by the reaction process, phagocytosis, and the adhesion process are absorbed by the non-targeted tissue inside the concentration. k12,f, and k21,f are the first-order constant rates inside and outside the compartments of target site, respectively, in the forward link. The mathematical equations for forwarding link as shown in Fig. 4 are represented as follows:
Now, we discuss the reverse link as shown in Fig. 4. while v1(t) represents the concentration molecules in a blood vessel and v2(t) is the concentration of the information molecules which arrived at the target site. Furthermore, k12, r, and k21, r are the kinetic rate constants, which are the reverse of the k12, f, and k21, f. kl is the ligand-receptor binding constant rate. The equations for the biological signals in the reverse link can be expressed as follows:
where c0 is the total concentration of the released drug molecules.
7 Numerical results and discussion
In this section, we present the numerical results of the proposed model-based IoBNT paradigm. The aim of this section is to evaluate the performance of the proposed model in terms of the concentration of the therapeutic drug molecules at the targeted site, and hence we study the influence of system parameters on the performance. The effectiveness of our proposed model from the medical personal to target site depends on the simulation parameters that are reordered in Table 2. We confirm our results by using MATLAB as well as we made use of the parameters in the previous experiments [17, 23, 35, 37].
7.1 Effects of drug release concentration in forward link
In this section, we discuss the effects of various parameters on the concentration of the drug molecules released from reservoir Nano-T to a target site. The results obtained according to the numerical solutions of Eqs. (2), (3), (4), and (9). Figure 5 shows the concentration of the drug molecules released from reservoir Nano-T against the period of releasing time with different values of diffusion coefficient, D, while the variation of concentration comes from the exponential term in the equations. As we can observe, increasing the diffusion coefficient result in increasing the concentration of drug release. This is due to, increasing drug molecules cargo makes a stress on the inner membrane of the polymer, leads to increase the release of the drug molecules cargo through a porous polymeric membrane of the target site. As a result, increasing the amount of the drug molecules received by Nano-R, raising the absorption of those molecules by infected cells followed by decreasing side effects around healthy cells in target site.
The influence of varying inner radius of the reservoir Nano-T into the drug release concentration is illustrated in Fig. 6. As we can observe, increasing the value of the inner radius, is meeting a reduction in the delay time of liberating drug molecules cargo from inner to outer surface of reservoir Nano-T, i.e., decreases the initial time that drug molecules cargo takes to start the emission from inner membrane. As a result, drug molecules cargo reaches rapidly to steady-state. On the other hand, it increases the cumulative amount of the releasing drug molecules which helping for speeding the diffusion of these molecules into target site. Besides, growing value of inner radius makes the distance between inner and external radius inside Nano-T small, and hence increasing the concentration of the emitting drug molecules from Nano-T to infected cells inside target site.
The influence of varying outer radius of the reservoir Nano-T into the drug release concentration is illustrated in Fig. 7. As we can see, the concentration of the released drug molecules from reservoir Nano-T decreases, when applying a high value of the outer radius of reservoir Nano-T. In other words, it increases the initial time that these drug molecules take till reach steady-state, results in slowing the diffusion of the emitting drug molecules from reservoir Nano-T inside the target site. Besides, it makes the distance between inner and outer radius large, thus decreasing the concentration of the released drug molecules on the surface of reservoir Nano-T leads to losing drug molecules.
7.2 Effects of the drug concentration at target site in reverse link
This section illustrates the effects of various values of the geometry design parameters on the concentration of the drug molecules in the target site, specifically around Nano-R, i.e. the influence of this concentration onto diseased cells in the target site. Numerical solution of Eqs. (5)–(11) are used to obtain these results. According to the illustration in Fig. 8, the concentration of the drug molecules increases at target site, as the radius of the reception space around Nano-R increases. Additionally, this great area attracts more drug molecules for binding to the receptors on the surface of Nano-R. Thereby, when the size of this area increases, raising the chance of existing more concentration of the drug molecules around diseased cells inside target site. Moreover, it leads to fast absorption of these drug molecules by infected cells and decreases side effects around healthy cells inside target site. In a consequence, it supports finishing the dose delivery efficiently.
It can be seen in Fig. 9, growing the value of outer reservoir Nano-T radius, is meeting increasing of the distance between reservoir Nano-T and Nano-R and thus decreasing the concentration of the drug molecules in the reception space around Nano-R (diseased cells). This configuration causes of losing some molecules by degradation due to spending much time in a moist environment till reach Nano-R (diseased cells). As a result, it causes of decreasing the absorption of these molecules by diseased cells owing to a little molecule binding to the receptors on the surface of Nano-R. Thereby increasing side effects around healthy cells in the same site. Therefore, according to our proposed model, we recommend to choose a small radius of reservoir Nano-T as nanotechnology permits.
Here, we would like to see the communication response of the nanosensor in the target site. As shown in Fig. 10, the higher value of c0, the higher concentration of the drug molecules inside the target site occurs, which means that more information molecules are received by the bio-cyber interface device. Therefore, the nanosensor in the target site is capable of releasing a high concentration of information. It can conclude that a good communication between exists nanosensor inside target site and bio-cyber interface device for increasing total capacity.
7.3 Performance comparison
Figure 11(a) illuminates the performance comparison for the concentration of the therapeutic drug molecules released from nanotransmitter in blood vessel channel between our proposed model and the previous work [23]. The previous work has considered the Nano-T as a point source for molecule emission and employed the constant parameters of the proposed compartmental model [23], while the proposed model considers the physical design structure of reservoir Nano-T as described previously.
Performance comparison between the proposed model and pervious work [23]
As we can see in Fig. 11(a), the proposed model verifies the same behavior of the previous work; however, the proposed model achieves better performance. This is owing to the proposed model achieves more concentration for the therapeutic drug molecules released from reservoir Nano-T comparing with the emission point in the previous work. Additionally, the proposed approach is capable of controlling and managing the therapeutic drug molecules by taking into account the geometry design of reservoir Nano-T. Subsequently, the duration time of drug molecules emission into the blood vessel channel to reach the infected cells is reduced. This result in reducing side effects around healthy cells and a fast dose occurred, compared with the previous work.
Figure 11(b) shows the performance comparison of the concentration of the therapeutic drug molecules at the targeted site, i.e., the concentration of the drug molecules received by the reception space around infected cells for the proposed model and previous work. The simulation parameters used for this comparison are listed inside the plot. As we can observe, the proposed approach is capable of achieving a high concentration for the therapeutic drug molecules around diseased cells by taking into consideration the radius of the reception space, the distance between Nano-T and Nano-R, and the total concentration of the drug molecules comparing with the previous work. Owing to the proposed approach can achieve fast absorption by applying the high affinity of the receptors on the surface of Nano-R. Therefore, the proposed approach based on the IoBNT is able to achieve the desired therapeutic medicaments for targeted drug delivery by improving the pharmacokinetic system model in forward and reverse links comparing with the previous work.
We can conclude from the above analysis that the performance of the proposed model is superior to the related work. The proposed model is also taking into consideration the distance between nanotransmitter and nanoreceiver and thus is able to manage the concentration of the therapeutic drug molecules. While the proposed model is able control and manages the released of the therapeutic drug molecules from Nano-T, the concentration of the therapeutic drug molecules inside the target site is not considered. In order to make the proposed model is more efficient, the proposed model should study the biological effect of the therapeutic drug molecules inside infected cells and hence the system will be more realistic.
8 Conclusion
In this paper, an efficient approach for the nanotransmitter and nanoreceiver, namely, reservoir Nano-T and Nano-R for targeted drug delivery systems in the promising IoBNT paradigm, is proposed. We presented a pharmacokinetic model involved separation distance between reservoir Nano-T and Nano-R, and the geometry design of nanotransmitter and nanoreceiver. The proposed approach is able to control the releasing from reservoir Nano-T and at the same the delivery of drug concentration around diseased cells inside intra-body network. The analysis and numerical results of the proposed approach are verified by the simulation campaign. The results reveal that the proposed approach is able to achieve the desired therapeutic medicaments at the targeted cell by improving the pharmacokinetic system model in forward and reverse links comparing with the previous work.
Additionally, in our future research, we shall study the geometry design of nanotransmitter and nanoreceiver in the existing of stochastic noise from perspective of localization and tracking [32]. On the other hand, the challenges which are still facing the employment of the promising IoBNT-based MC paradigm such as high latency, low selectivity, unknown biocompatibility and unsecured of the bio-cyber interfaces are still opening issues in the future research [22].
References
Chude-Okonkwo, U. A., Malekian, R., & Maharaj, B. S. (2016). Molecular communication model for targeted drug delivery in multiple disease sites with diversely expressed enzymes. IEEE Transactions on Nanobioscience,15(3), 230–245.
Fakruddin, M., Hossain, Z., & Afroz, H. (2012). Prospects and applications of nanobiotechnology: A medical perspective. Journal of Nanobiotechnology,10(1), 31.
Chude-Okonkwo, U. A., Malekian, R., Maharaj, B. et al. Bio-inspired approach for eliminating redundant nanodevices in Internet of Bio-Nano Things, pp. 1–6.
Akyildiz, I. F., Brunetti, F., & Blázquez, C. (2008). Nanonetworks: A new communication paradigm. Computer Networks,52(12), 2260–2279.
Freitas, R. A. (2005). Current status of nanomedicine and medical nanorobotics. Journal of Computational and Theoretical Nanoscience,2(1), 1–25.
Silva, C. O., Pinho, J. O., Lopes, J. M., et al. (2019). Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics,11(1), 22.
Griffith, L. G., & Naughton, G. (2002). Tissue engineering—Current challenges and expanding opportunities. Science,295(5557), 1009–1014.
Singh, A., Bivalacqua, T. J., & Sopko, N. (2018). Urinary tissue engineering: Challenges and opportunities. Sexual Medicine Reviews,6(1), 35–44.
Gandhi, A., Paul, A., Sen, S. O., et al. (2015). Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian Journal of Pharmaceutical Sciences,10(2), 99–107.
Byrne, R., & Diamond, D. (2006). Chemo/bio-sensor networks. Nature Materials,5(6), 421.
Kuscu, M., & Akan, O. B. Modeling and analysis of SiNW bioFET as molecular antenna for bio-cyber interfaces towards the Internet of Bio-Nanothings, pp. 669–674.
Atakan, B., Akan, O. B., & Balasubramaniam, S. (2012). Body area nanonetworks with molecular communications in nanomedicine. IEEE Communications Magazine,50(1), 28–34.
Nel, A. E., Lutz, M. D., Darrell, V., et al. (2009). Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials,8(7), 543.
Nakano, T., Moore, M., Okaie, Y. et al. Swarming biological nanomachines through molecular communication for targeted drug delivery, pp. 2317–2320.
Nakano, T., Suda, T., Okaie, Y., et al. (2014). Molecular communication among biological nanomachines: A layered architecture and research issues. IEEE Transactions on Nanobioscience,13(3), 169–197.
Yang, W.-W., & Pierstorff, E. (2012). Reservoir-based polymer drug delivery systems. Journal of Laboratory Automation,17(1), 50–58.
Siepmann, J., & Siepmann, F. (2012). Modeling of diffusion controlled drug delivery. Journal of Controlled Release,161(2), 351–362.
Chahibi, Y. (2017). Molecular communication for drug delivery systems: A survey. Nano Communication Networks,11, 90–102.
Chahibi, Y., Akyildiz, I. F., Balasubramaniam, S., et al. (2015). Molecular communication modeling of antibody-mediated drug delivery systems. IEEE Transactions on Biomedical Engineering,62(7), 1683–1695.
Akyildiz, I. F., Pierobon, M., & Balasubramaniam, S. (2019). An information theoretic framework to analyze molecular communication systems based on statistical mechanics. Proceedings of the IEEE,107(7), 1230–1255.
Lanfranco, R., Mognetti, B. M., & Bruylants, G. (2019). Achieving selective targeting using engineered nanomaterials. In Thermodynamics and biophysics of biomedical nanosystems, Springer, pp. 147–182.
Akyildiz, I. F., Pierobon, M., Balasubramaniam, S., et al. (2015). The internet of Bio-Nano Things. IEEE Communications Magazine,53(3), 32–40.
Chude-Okonkwo, U. A., Malekian, R., & Maharaj, B. (2016). Biologically inspired bio-cyber interface architecture and model for Internet of bio-nanothings applications. IEEE Transactions on Communications,64(8), 3444–3455.
AbdEl-atty, S. M., Bidar, R., & El-Rabaie, E. S. M. (2019). MolCom system with downlink/uplink biocyber interface for Internet of Bio-NanoThings. International Journal of Communication Systems,33, e4171.
James, H. P., John, R., Alex, A., et al. (2014). Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharmaceutica Sinica B,4(2), 120–127.
Torchilin, V. (2009). Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. European Journal of Pharmaceutics and Biopharmaceutics,71(3), 431–444.
Larson, N., & Ghandehari, H. (2012). Polymeric conjugates for drug delivery. Chemistry of Materials,24(5), 840–853.
Dollard, M.-A., & Billard, P. (2003). Whole-cell bacterial sensors for the monitoring of phosphate bioavailability. Journal of Microbiological Methods,55(1), 221–229.
Monson, E., Brasuel, M., Philbert, M., et al. (2003). PEBBLE nanosensors for in vitro bioanalysis. Biomedical Photonics Handbook,9, 1–14.
Dhillon, S., & Kostrzewski, A. (2006). Clinical pharmacokinetics. London: Pharmaceutical Press.
Miah, M. K., Shaik, I. H., Feturi, F. G. et al. (2019). Clinical pharmacokinetics. In Clinical pharmacy education, practice and research, Elsevier, pp. 409–424.
Kuscu, M., Dinc, E., Bilgin, B. A. et al. (2019). Transmitter and receiver architectures for molecular communications: A survey on physical design with modulation, coding, and detection techniques. In Proceedings of the IEEE.
Klein, B. G. (2019). Cunningham’s textbook of veterinary physiology: Elsevier Health Sciences. Amsterdam: Elsevier.
Bourne, D. A. (2018). Mathematical modeling of pharmacokinetic data. Abingdon: Routledge.
Freiberg, S., & Zhu, X. (2004). Polymer microspheres for controlled drug release. International Journal of Pharmaceutics,282(1–2), 1–18.
Hossen, S., Hossain, M. K., Basher, M., et al. (2018). Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. Journal of Advanced Research,15, 1–18.
Cascone, S., Lamberti, G., Titomanlio, G., et al. (2013). Pharmacokinetics of remifentanil: A three-compartmental modeling approach. Translational Medicine@ UniSa,7, 18.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Fatyany, A., Wang, H. & Abd El-atty, S.M. On mixing reservoir targeted drug delivery Modeling-based Internet of Bio-NanoThings. Wireless Netw 26, 3701–3713 (2020). https://doi.org/10.1007/s11276-020-02294-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11276-020-02294-3