Abstract
Rhizosphere microbial communities are dynamic and play a crucial role in diverse biochemical processes and nutrient cycling. Soil type and cultivar modulate the composition of rhizosphere microbial communities. Changes in the community composition significantly alter microbial function and ecological process. We examined the influence of soil type on eubacterial and diazotrophic community abundance and microbial metabolic potential in chickpea (cv. BG 372 and cv. BG 256) rhizosphere. The total eubacterial and diazotrophic community as estimated through 16 S rDNA and nifH gene copy numbers using qPCR showed the soil type influence with clear rhizosphere effect on gene abundance. PLFA study has shown the variation in microbial community structure with different soil types. Differential influence of soil types and cultivar on the ratio of Gram positive to Gram negative bacteria was observed with most rhizosphere soils corresponding to higher ratios than bulk soil. The rhizosphere microbial activities (urease, dehydrogenase, alkaline phosphatase and beta-glucosidase) were also assessed as an indicator of microbial metabolic diversity. Principal component analysis and K-means non-hierarchical cluster mapping grouped soils into three categories, each having different soil enzyme activity or edaphic drivers. Soil type and cultivar influence on average substrate utilization pattern analyzed through community level physiological profiling (CLPP) was higher for rhizosphere soils than bulk soils. The soil nutrient studies revealed that both soil type and cultivar influenced the available N, P, K and organic carbon content of rhizosphere soil. Our study signifies that soil type and cultivar jointly influenced soil microbial community abundance and their metabolic potential in chickpea rhizosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil is the home for prodigious number of living organisms and as far as the plant growth and soil properties are concerned, microbial communities inhabiting rhizosphere region play a crucial role in diverse biochemical processes and nutrient cycling. The rhizosphere, as a focus of soil microbial communities is dynamic, and plant-microbe interactions are frequent here. The living plant roots secrete large quantity of organic compounds that in turn stimulate a more abundance, higher microbial diversity and enzyme activities in rhizosphere (Smalla et al. 2001). The bulk samples, soil outside rhizosphere, is not penetrated by plant roots and shows low rates of nutrient transformation and microbial activity compared to active rhizosphere (Ai et al. 2012). Rhizosphere microflora can improve the soil quality and crop performance. Many studies have focused on traditional culturable techniques to study the rhizosphere microbial diversity and function, ‘great plate count anomaly’ states that a greater fraction (95–99 %) of microbial community involved in rhizosphere process is unculturable (Nichols 2007). Advanced analytical techniques such as Amplified Ribosomal DNA Restriction Analysis (ARDRA, Gich et al. 2000), BOX PCR (Satyaprakash and Annapurna 2006), Denaturing Gradient Gel Electrophoresis (DGGE, Miller et al. 1999), Quantitative PCR (Fierer et al. 2005), phospholipid fatty acid (PLFA, Guckert and White 1986), and community level physiological profiling (CLPP, Preston-Mafham et al. 2002) are used to examine the diversity of fingerprints allowing for more detailed analyses of abundance and activities of soil microbial communities.
Chickpea (Cicer arietinum L.) popularly known as gram or Bengal gram is an annual legume and mostly grown under rainfed conditions in arid and semi-arid regions in India. The world production of chickpea accounted for 14.24 million tonnes, with India alone accounting for 69.7% (9.93 million tonnes) of total production (FAOSTAT 2019). Globally, chickpea has yield levels of about 920 kg ha−1. Chickpea being a leguminous crop replenishes soil fertility through Biological Nitrogen Fixation (BNF) by forming symbiotic association with effective Mesorhizobium strains. Under favorable conditions, the symbiotic nitrogen fixation can supply up to 85% of the N required by chickpea crop (Walley et al. 2005). The crop can also assemble root-associated microbial communities primarily derived from the rhizosphere soil (Reinhold-Hurek et al. 2015). These microbes could improve plant growth and health as this crop is generally grown under low chemical input. The beneficial function of rhizosphere microbial communities includes improved nutrient uptake, plant hormone production, imparting disease resistance and stress tolerance (Mendes et al. 2011; Kwak et al. 2018; Swarnalakshmi et al. 2020). The microbial abundance and function are known to be impacted by various ecological as well as plant factors (Pii et al. 2016; Liu et al. 2019). The various root exudate compounds secreted by the different cultivars were reported to influence the diversity and function of root associated microbial communities in rhizosphere soil ( Lundberg et al. 2018; Sasse et al. 2018). Soil characteristics such as organic carbon and nutrient level have pronounced effect on microbial abundance and performance (Hu et al. 2014; Zhu et al. 2015).
A number of methods are employed to study and quantify microbial diversity and their function ranging from physiological to molecular techniques. It is essential to use a combination of approaches for accurate results because no single approach can provide neither a complete picture of the type of microbes present nor their relative abundance within each of those types. The soil type mediated metabolic diversity of microbial communities associated with chickpea rhizosphere has never been explored. Hence, the present investigation is focused to study the influence of cultivarsand soil types on the nutrient availability, metabolic potential and microbial abundance associated with chickpea rhizosphere. We applied analyses of PLFA and CLPP in order to evaluate the microbial community structure and their functional diversity of soils collected from different regions across the country where chickpea is grown as part of cropping system. The influence of soil types on the eubacterial and diazotrophic abundance was determined using qPCR assays. In addition, soil microbial enzyme activities and nutrient content in the rhizosphere soil were assessed to estimate the rhizosphere microbial function.
Materials and methods
Experimental design
A pot experiment was carried out at National Phytotron facility, ICAR-Indian Agricultural Research Institute (IARI), New Delhi during rabi 2018–19. Soils were collected from chickpea growing regions of different agro-climatic zones of India. The 4” size pots were filled with finely ground soil samples of different locations. Location details along with chemical and enzyme activities of initial soil samples are given in the Table 1. The available N content of the collected soil samples was found to be low (< 280 kgha−1). The available K at three locations viz Dharwad, New Delhi site II and Pune Site I was low and soils collected from Jharkhand, New Delhi site I, Haryana, Varanasi, Pune site II and Kanpur showed medium range. Organic C content of all soils was in the low range. pH of the soils varied from neutral to alkaline. Two chickpea cultivars viz., BG-256 and BG-372 were grown in all soil types and the influence of cultivarwas compared with control pots without plants (Bulk soil). Thus a total of 27 treatments (9 × 3) were replicated three times (completely randomized design-CRD). Each pot was sown with 3 seeds with equal spacing and irrigation was given to maintain 60% water holding capacity. The rhizosphere (soil particles adhering to root surface) as well as bulk soil (control) were collected by destructive sampling at vegetative stage (60 days after sowing). The soil samples were homogenized and passed through 2 mm sieve. The soil enzyme analysis was carried out in fresh samples and sub-samples were stored at − 80 °C for microbial PLFA, CLPP and qPCR analysis, while another set of sub-samples were air-dried and passed through 0.2 mm sieve for analysis of soil nutrient content.
Soil nutrient analysis
Available Nitrogen was estimated using alkaline permanganate procedure described by Subbiah and Asija (1956). The soil was treated with alkaline KMnO4 and distilled. The organic matter present in the soil was oxidized by the nascent oxygen liberated by KMnO4 in the presence of NaOH and thus ammonia released was distilled and absorbed in a known volume of boric acid. The contents were titrated against standard sulphuric acid (0.02 N) using mixed indicator (0.07 g of methyl red and 0.1 g of bromocresol green in 95% ethanol). Available P was estimated by standard method described by Olsen et al. (1954). The available potassium content in the soil was detected using flame photometer against the known standards (Jackson 1973). Soil organic carbon was determined by Walkley and Black’s method (1934). EC and pH were determined by method as described by Smith and Doran (1996).
Soil enzyme analysis
The activities of four soil enzymes viz. dehydrogenase (Casida 1977), urease (Tabatabai and Bremner 1972), alkaline phosphatase (Tabatabai and Bremner, 1969) and β-glucosidase (Eivazi and Tabatabai 1988) activities were measured as per the standard procedure described. The dehydrogenase activity was analyzed by 2-3-5 Triphenyl tetrazolium chloride (TTC) reduction technique by adding 1 mL 3% TTC to 6.0 g soil sample followed by methanol extraction after 24 h of incubation at 37 °C in dark. The aliquot containing TPF was measured at 485 nm.Urease activity was assayed by weighing 5 g soil sample followed by addition of 2.5 mL urea solution (0.5%). The samples were incubated at 37 °C for 2 h and followed by KCl (1 M) extraction. The contents were filtered and 5 mL sodium salicylate and 2 mL sodium dichloro-isocyanide solution was added to the filtrate followed by incubation for 30 min. The green color developed was measured at 690 nm. Alkaline phosphatase activity was measured by homogenizing the pre-weighed (2.0 g) soil with 4 mL MUB (Modified Universal Buffer, pH 11) solution and 1ml p-nitro phenyl phosphate (0.025 M). The samples were incubated at 37 °C for 1 h and 1 mL of 0.5 M CaCl2 and 4 mL of 0.5 M NaOH were added. The contents were filtered and the yellow color developed was measured at 400 nm. The β-glucosidase activity was measured by mixing the known amount of soil with 4 mL MUB (pH 6) solution and 1 mL of p-nitrophenyl β-D-glucopyranoside (25mM) solution to known amount of soil followed by extracting the content with 0.1 M Tris buffer (pH 12). The yellow color developed was measured at 400nm (Eivazi and Tabatabai 1988). Microbial biomass carbon (MBC) was estimated by chloroform fumigation extraction method (Vance et al. 1987). Known amount of soil samples were fumigated with chloroform, non-fumigated samples served as control. Both fumigated and non-fumigated samples were extracted with 0.5 M K2SO4 and organic carbon extracted was measured by a dichromate digestion procedure. Microbial biomass carbon was calculated using the formula: MBC = 2.64 X [OCF–OCUF] and expressed as µg g−1 soil.
Structural diversity of microbiota by PLFA profiling
Lipid extraction and PLFA analyses of soil samples were performed using the modified Bligh and Dyer-method (Bligh and Dyer 1959; Frostegard et al. 1991). Briefly, 2.0 g of freeze-dried soil was extracted with a chloroform-methanol-citrate buffer mixture (1:2:0.8), and the phospholipids were separated on a silicic acid column. The phospholipids were subjected to a mild alkaline methanolysis and the resulting fatty acid methyl esters were prepared according to the MIDI protocol. The total PLFA concentration was expressed as nmol g−1 soil.
Eubacterial and diazotrophic abundance by RTPCR
SYBR green technology for quantitative-PCR (C1000 CFX 96 Real-Time PCR System, Biorad) was used to quantify the number of eubacterial (16 S rDNA) and diazotrophic (nifH) gene copies (Gupta et al. 2019) present in the community DNA extracts of soil samples. Total DNA from bulk and chickpea rhizosphere soil was extracted using Power Soil DNA kit (MoBio). All extracts were stored at − 80 °C until use. DNA quality was checked by running samples in Qubit fluorometer. The primer pair CAACGCGAAGAACCTTAC (F)/CGGTGTGTACAAGGCCCGGGAACG(R) and AAAGGYGGWATCGGYAARTCCACC(F)/TTGTTSGCSGCRTACATSGCCATCAT(R) were used for specific amplification of the bacterial 16 S rDNA (Fierer et al. 2005) and nifH (Poly et al. 2001) genes respectively. The qPCR reaction mixture for 16 S rDNA amplification consisted of 7.5 µL master mix containing 1X SYBER green, 0.3 µL of each primer (10 µM), 3 µL DNA and 3.9 µL water. The qPCR program for bacterial 16 S rDNA gene began with an initial step of 15 min at 95 °C, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 54 °C for 30 s, extension at 72 °C 1 min followed by 1 cycle of extension at 72 °C for 5 min and denaturation at 95 °C for 1 min, annealing at 54 °C for 30 s and final extension at 95 °C for 30 s. The nifH gene qPCR reaction mixture consisted of 12.5 µL master mix containing 1X SYBER green, 0.25 µL of each primer (10 µM), 5 µL DNA and 7 µL water. The nifH gene copy number was amplified with an initial step of 15 min at 95 °C, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 45 s, extension at 72 °C for 45 s followed by 1 cycle of final extension at 72 °C for 10 min, annealing at 68 °C for 5 s and denaturation at 95 °C for 50 s. Fluorescence data were recorded during the qPCR run and a cycle threshold (Ct) was determined automatically with the SDS software package (Biorad). Real-time amplification plots of the products were monitored for each reaction for quality control. To generate standard curves for the transformation of Ct values into absolute units (total number of gene copies), 10-fold serial dilution (101 to 108) of known copy numbers of pGM-T easy (Promega) cloned template for 16 S rDNA and nifH gene was generated. The standard curves were run simultaneously and the log-linear correlation coefficients, R2 between the number of 16 S rDNA and nifH gene copies and Ct values were > 0.98 in all standard curves.
Functional potential of microbiota using CLPP analysis
The commercially available EcoPlates of BIOLOG Inc. were used to study the metabolic diversity of soil microbial communities using C-substrate utilization. Each BIOLOG plate contained 31 C substrates with one control in triplicates. Bulk and rhizosphere soil suspension was prepared for each sample by adding 10 g of homogenized soil in 250 mL flasks with 90 mL of sterile 0.85% NaCl solution. The flasks were shaken for 30 min on rotary shaker at 120 rpm. The NaCl extracts were allowed to settle for 20 min to clear the supernatant, which was serially diluted to 10−³. Ecoplates were inoculated with 125 µL per well with 10−3 dilution and were incubated at 30 °C. The color change was measured at 590 nm at 72 h using BIOLOG Micro-Station reader. Microbial activity of BIOLOG Ecoplates was expressed as Average Well Color Development (AWCD) (Garland and Mills 1991), which was derived from the mean difference among the OD values of the 31 response wells (R) and the OD value of control well (C). The AWCD was calculated using the formula: AWCD = Σ (R−C) /31. The functional diversity of microbial communities was calculated by Shannon-diversity (H) and McIntosh evenness (U). Shannon-diversity (H) was quantified by the formula: H=−Σpi(lnpi). Where, pi = proportional color development of the well over total color development of all wells of a plate. McIntosh Evenness (U) was calculated by the formula: U=√Σ(ni)2, where n represents therelative absorbance value for each C source (Garland 1996).
Plant growth and nutrient uptake
The chickpea plants were uprooted at vegetative stage. The root and shoot tissues were dried at 65 °C until complete removal of moisture. The dried shoots and roots were weighed using a weighing balance and the dry weight was expressed in milligram per plant. The dried plant tissues were finely ground for nitrogen and phosphorus analysis. N content was determined by a micro Kjeldahl method (Yuen and Pollard 1953) and P content was estimated by vanado-molybdate method (Jackson 1973).
Statistical analysis
Data for soil nutrient status, enzyme activities and plant growth attributes were subjected to ANOVA and two factor analysis. For analyzing the parameters between different locations, data was subjected to combined analysis and principal component analysis (PCA) using SAS software 9.4. Data for qPCR, PLFA and CLPP were subjected to agglomerative hierarchical clustering techniquesand principal component analysis (PCA) using R version 3.4.4 (2018-03-15), Platform: x86_64-w64-mingw32/x64 (64-bit).
Results
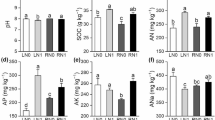
The mean values of soil chemical and enzyme properties for different locations is given in Table 2. Though the chickpea was grown under controlled conditions at New Delhi, our results showed different soil type had varied chemical and biological properties. Among the locations, available N content at New Delhi site II (235.55 kg ha−1) showed higher mean value and lowest value was recorded with New Delhisite I (96.17 kg ha−1) in which rice and maize were the previous crop respectively. Available P content was more in Dharwad soil (28.87 kg ha−1) and lowest value was recorded in soils collected from Kanpur location (9.46 kg ha−1). Varanasi location (512.04 kg ha−1) showed highest available K whereas lowest reading was recorded with Rajnandgaon (351.05 kg ha−1). Organic C was found to be higher in Kanpur (12.57 g kg−1) and Pune siteII (12.49 g kg−1) and Rajnandgaon (7.75 g kg−1) recorded lowest value. The soil pH varied from 7.81 (Gangwa) to 8.16 (Kanpur) and was neutral to alkaline. The electrical conductivity of different soils varied from 0.34 dSm−1 (New Delhi site I) to 0.81 dS m−1 (New Delhi site II). The Dehydrogenase activity of soil washighest in Dharwad soil (71.67 µg TPF g−1 soil h−1) and least in Gangwa soil (27.99 µg TPF g−1 soil h−1). Urease enzyme was higher in New Delhi site I (36.8 µg NH4−N g−1 soil h−1) and lowest in Varanasi soil (14.948 µg NH4−N g−1 soil h−1). New Delhi site I showed higher alkaline phosphatase activity (190.87 µg PNP g−1 soil h−1) and lowest in Kanpur soil (73.66 µg PNP g−1 soil h−1). Highest β-glucosidase activity is recorded with New Delhi site II (23.2 µg PNP g−1 soil h−1) and least with New Delhi site I (8.25 µg PNP g−1 soil h−1). Among the different locations, the maximum microbial biomass carbon was recorded with New Delhi site 1 (105.65 µg g−1 soil day−1) whereas Pune site 1 recorded least value i.e. 47.74 µg g−1soil day−1.
The cultivar influence on soil chemical and biological properties is given in Table 3. It was found that the available N was higher in rhizosphere soils of both BG 372 and BG 256 cultivarsover the bulk soils from all the locations. Available P content was higher in rhizosphere soils of BG 256. On the other hand, the available K and organic carbon was higher in rhizosphere soil of BG 372. The soil pH of rhizosphere and bulk soil is near alkaline. EC content of rhizosphere soil of BG 256 is slightly higher (0.76 dS m−1). The dehydrogenase, urease and alkaline phosphatase activity was higher in rhizosphere soil of BG 256 whereas β-glucosidase activity was higher in rhizosphere soil of BG 372. Our results clearly indicate the cultivar influence on soil chemical and enzyme activities. On the contrary, microbial biomass carbon was higher in bulk soil.
The biomass content of soils varied from as low as 49.55 nmoles g−1 soil in BG 256 rhizosphere soil of Pune site 2 to as high as 1948.18 nmoles g−1 soil in New Delhi site II rhizosphere soil of BG 372 (Fig. 1). It was observed that the BG 372 cultivar had a more pronounced effect on biomass content in rhizosphere soils of Dharwad (1132.44 nmoles g−1 soil), Jharkhand (1391 nmoles g−1 soil), New Delhi site II (1948 nmoles g−1 soil), Pune site I (404.26 nmoles g−1 soil) and Pune site II (608.25 nmoles g−1 soil) as compared to BG 256 rhizosphere soils from same locations. Exception to this were BG 256 rhizosphere soils of New Delhi site I (1616.15 nmoles g−1 soil) and Gangwa (1048 nmoles g−1 soil). The ratio of Gram positive to Gram negative bacteria varied from a lower ratio of 0.67 in bulk soil of Jharkhand site to a higher ratio of 4.87 in rhizosphere soil of Dharwad with BG 372 cultivar (Fig. 2). The ratio of Gram positive to Gram negative bacteria was found to be influenced both by soil types and cultivarswith most rhizosphere soils corresponding to higher ratios. On the whole, the Gram positive to Gram negative ratio was higher in bulk soils from New Delhi site II (2.31) and Varanasi (3.53) but the maximum impact was found in BG 256 rhizosphere soil of Jharkhand (4.76).
The quantification of16S rDNA and nifH gene copy numbers using qPCR in the present study estimated the abundance of total bacterial and the diazotroph communities in bulk and rhizosphere soils (Table 4). The 16 S eubacterial rRNA gene copy numbers were around 5.26 × 106 g−1 soil where as nifH copy numbers was around 1.36 × 105 g−1 soil. The 16 S rDNA and nifH gene copy number varied significantly across all locations. The New Delhi site I significantly differed from other sites in 16 S (Fig. 3)and nifH copy number (Fig. 4). The 16 S rDNA and gene copy numbers were in general found higher in bulk soils (Supplementary Table 1). The nifH copy number was not influenced by cultivarat New Delhi Site I, Gangwa, Pune Site I and Pune Site II whereas at New Delhi Site II and Kanpur soils, the cultivar BG 372 influence on nifH copy number is evident. The PCA analysis showed the cultivar BG256 (63.94% variation) to be the most significant attribute to determine eubacterial abundance whereas BG 372 (60.42% variation) was the most influential attribute of diazotrophic abundance (Supplementary Tables 2 & 3). The average ratio of nifH to eubacteria 16 S gene copy numbers also varied with cultivar and soil type (Table 5). Rhizosphere soil bacterial community of BG 256 at Pune site II and Kanpur showed highest ratio. The rhizosphere soil of cultivar BG 372 showed 10-fold decrease in nifH/16S ratio at New Delhi site I, New Delhi site II, Gangwa, Pune site I, and Kanpur site where as BG 256 rhizosphere soil showed 10-fold decline in Rajnandgaon, Gangwa, Pune Site I.
Average Well Color Development (AWCD), an indicator of carbon substrate utilization pattern was found to be higher for rhizosphere soils than bulk soils (Table 6). The measurement of AWCD at 72 h displayed that the BG 372 rhizosphere soil of Dharwad was found to have highest value whereas the bulk soil of Jharkhand showed the lowest. The BG 256 rhizosphere soil exhibited consistently higher AWCD in most of the soils as compared to bulk and BG 372 rhizosphere soils except at Dharwad soil. The carbon substrate utilization pattern was found to be lower in bulk soils except for Pune site I, Varanasi and Kanpur bulk soils where they exhibited higher substrate utilization pattern. The metabolic activity of bulk and rhizosphere soils of different locations is depicted in heatmap (Fig. 5). Of the 31 C-substrates, 26 were utilized by the microbial communities of bulk soils of Varanasi and Kanpur. In the rhizosphere soils of the two cultivars, C-substrate utilization ranged from 22 to 29 (BG 372) and 27 to 29 (BG 256). AWCD and McIntosh index at 72 h was higher in rhizosphere soils of BG 372 at all soil types except Pune site I (bulk soil showed higher index) however, Shannon diversity was more or less similar in bulk and rhizosphere soil of all locations.
The dry weight of plant biomass varied from 143 mg plant−1in BG 372 cultivargrown in Haryana soil to 1287.33 mg plant−1 in same cultivar grown in Pune site I soil (Table 7). Further, in relation to plant dry weight both BG 372 and BG 256 cultivars performed equally in all locations, except for BG 256 grown in Haryana soil (967.67 mgplant−1) and BG 372 grown in Pune site 1 (1287.33 mg plant−1) as well as Kanpur (575.33 mg plant−1) soils showed higher plant biomass with respect to one another. Among the different soils, highest biomass was accumulated with Pune site I (936.33 mg plant−1) and least was recorded at New Delhi site I (329.33 mg plant−1). The total plant N content in both the cultivarsranged from 2.34% to 372 plants grown in Gangwa soil and 5.85% BG 256 plants grown in Pune site I soil. The plant N content (Table 7) was higher in BG 256 cultivar grown among Gangwa (3.21%) and Pune site I (5.85%) soils whereas BG 372 showed higher plant N content when grown in New Delhi site II (5.62%) and Kanpur (4.85%) soils. Though the cultivar alone was found non-significant with respect to plant N content, soil type has significantly affected the plant N. The chickpea plants grown with Gangwa soil recorded lowest N content (2.77%) which is at par with Jharkhand location (2.98%). Higher N content was observed with the Pune site II (5.53%). The total plant P content (Table 7) estimated in both the cultivars ranged from a lower value of 0.75% in BG 256 plants grown in Kanpur soil to a higher value of 2.43% in BG 372 plants grown in same Kanpur soil. The plant P content was found to be higher in BG 372 cultivar when grown in Pune site I (1.57%) and Kanpur (2.43%) soils. Also, it was higher in BG 256 cultivar when grown in New Delhi site 2 (2.02%), Gangwa (1.52%) and Pune site II (2.21%) soils. Among the different locations, Pune site I recorded highest P content (1.89%) whereas lowest P content was observed with New Delhi site I (1.18%).
Discussion
Soil microbial communities are key players for the maintenance of soil function, health and fertility as they are involved in vital processes such as organic matter decomposition, soil structure formation and cycling of nutrients in the environment. Together with an increasing emphasis on eco-friendly and low input agricultural practices; there is also a rising interest to study soil microbial communities for enhancing crop growth and health. In the present study,we analyzed the impact of soil types and chickpea cultivars on rhizosphere microbial community function and abundance using CLPP and qPCR. The cultivars and soil types have been shown to influence the microbial diversity, microbial metabolic potential and abundance in chickpea rhizosphere, as evidenced by the soil type and plant species dependent variation in the rhizosphere-associated microbial communities, and their activities (Inceoglu et al. 2012; Qiao et al. 2017). Our earlier study has shown genotype mediated variation in rhizobial symbiosis in soybean (Naik et al. 2017). Microbial community composition differs among the rhizosphere compartments, with diversity of these communities increasing from root towards bulk soil (Donn et al. 2015). Rhizosphere is a biological hotspot differs substantially from the surrounding bulk soil and microbial community structure and their metabolic activities differ significantly in both niches. This marked difference in micro environments of rhizosphere and bulk soil is due to rhizodeposition from root exudations. The carbon-rich rhizosphere selects specific microbial populations and supports the establishment of microorganisms, and the root exudate components vary with soil type and cultivar (Jones et al. 2009).
PLFA is a culture-independent method used to determine the microbial community composition by analyzing signature molecules present in the cell membranes of the microorganisms (Frostegard et al. 1991). PLFAs can be used as indicators of microbial stress and are used to track changes in soil microbial community composition (White et al. 1996; Kaur et al. 2005). This technique can determine microbial biomass, shift in microbial community structure and activities and physiological status of the sample. PLFA technique can differentiate various taxonomic groups of soil microorganisms and eliminate the bias associated with cultivation-dependent method. Bacterial biomarkers include specifically, 15:0 (Pentadecanoic acid), 16:0 (Hexadecanoic acid), 17:0 (Heptadecanoic acid), 16:1 w7c ((9Z)-9-hexadecenoic acid), 18:1 w7c ((11Z)-11-Octadecenoic acid), a15:0 (12-methyltetradecanoic acid), a17:0 (14-methylhexadecanoic acid), i14:0 (11-Methyltridecanoic acid), i15:0 (13-methyltetradecanoic acid), i16:0 (14-methylpentadecanoic acid), and i17:0 (15-methylhexadecanoic acid). Furthermore, i14:0 (11-methyltridecanoic acid), i15:0 (13-methyltetradecanoic acid), i16:0 (14-methylpentadecanoic acid), i17:0 (15-methylhexadecanoic acid), a15:0 (12-methyltetradecanoic acid) and a17:0 (14-methylhexadecanoic acid) were Gram-positive (G+) bacteria biomarkers and 16:1 w7c ((9Z)-9-hexadecenoic acid), 18:1 w7c ((11Z)-11-octadecenoic acid), cy17:0 (methylenehexadecanoic acid), cy19:0 (methyleneoctadecanoic acid) were Gram-negative (G−) bacteria biomarkers (Zhao 2016). In the present study, the microbial biomass estimated as total PLFA biomarkers also showed difference among the soil types and cultivars. A marked increase in the total concentration and composition of PLFAs was seen in rhizosphere soils as compared to the bulk soils which can be attributed to root induced shifts in microbial activity (Neumann and Romheld 2002). Except for Dharwad and Rajnandgaon sites, the ratio of Gram + to Gram − bacteria was higher in the bulk soil than rhizosphere which confirms the studies on root exudates enriched rhizosphere soil favor the Gram − bacteria, leading to their increased growth in plant rhizosphere (Chen et al. 2016). A previous study also showed that Gram − bacteria is positively correlated in the presence of higher soil N and organic residues (Buyer et al.2010; Bray et al. 2012).
Quantification of bacterial communities using qPCR technique is a robust method which provides an estimate of the abundance of the bacterial community in a sample without any culturable discrepancies and bias. Eubacterial and diazotrophic population densities of bulk and rhizosphere soils of chickpea cultivarsgrown in different soil types were analyzed through qPCR. 16 S rRNA gene is a taxonomic unit for bacteria and nifH is the structural gene coding for nitrogenase enzyme present in diazotrophs (Newton 2013). The diazotrophic communities are key players of nitrogen cycle particularly with legume crops as they are grown under low N. Different soil types are thought to harbor specific microbial communities (Fierer and Jackson 2006) and nifH copy number can detect shifts in diazotroph community structure (Pereira-e-Silva et al. 2011). We evidenced differential distribution of nifH copy number across the soil types which probably is due to changes in the physicochemical characteristics such as pH, EC, organic carbon and available nitrogen content. It has been shown that the nifH copy number varies with organic carbon content and soil chemistry (Collavino et al. 2014) and significant difference in the abundance and composition of diazotroph community among different soils can lead to the variation in rate of biological nitrogen fixation (Chen et al. 2019). The influence of cultivar on relative density of diazotrophic abundance was observed as the plant genotype also affect the indigenous functional microbial guilds of rhizosphere (Liu et al. 2019). Our earlier study also showed a clear-cut evidence that microbial populations in the spatial compartments of field grown chickpea are distinct (Swarnalakshmi and Annapurna 2019) and diazotrophic abundance was more in rhizosphere soil than bulk soil (Sharma et al. 2020).
The CLPP was proposed (Garland and Mills 1991) as a rapid screening means to characterize microbial communities from different habitats using BIOLOG plate and is being used to assess microbial community function and functional diversity (Preston-Mafham et al. 2002; Rutgers et al. 2016; Al-Dhabaan and Bakhali 2017). In the present study, there was clear discrimination between the carbon utilization patterns between bulk and rhizosphere soils evaluated using BIOLOG assay. The lower AWCD and McIntosh evenness in bulk soil is presumably due to the rhizosphere influence on microbial community function. Further, microbial activities between bulk and rhizosphere soils of two chickpea cultivar showed significant difference. This difference may be due to presence of diverse microbial communities and their metabolic capabilities. Further carbohydrates, amino acids, carboxylic acids were the substrates chiefly accountable for this distinction as plants may differ in exudation of these compounds (Grayston 1997).
In our study, both soil type and cultivar influenced the available N, P, K and organic carbon content of the soil. The nutrient content was higher in rhizosphere soil in contrast to bulk soil. This could be due to root-induced organic matter decomposition and mineral weathering and this result was consistent with the findings of Ai et al. (2012). The available N, P, K in the soil increased the activity of soil microorganisms (Ge et al. 2008) but Treseder (2008) has demonstrated a contrast result where soil nutrient status had little or no effect on either soil microbial activity or diversity. Soil pH and EC of rhizosphere and bulk soil was not significantly different, which was in contrast to results obtained by Fan (2017).
The microbial metabolic activity measured in terms of soil enzymes also varied significantly with cultivar and soil type. The extracellular enzyme secreted by the soil microorganisms are the negotiators of organic matter formation and decomposition. Our results indicated that both soil types as well as soil fractions (rhizosphere and bulk soil) differed significantly for the enzyme activities and were not equally distributed between them. Variation in enzyme activities in different soil fraction was reported by Ai et al. (2012). In our study, all enzyme activities varied with soil types as well as cultivar, and at New Delhi location, site I and II indicates significant influence of previous crop (rice and maize) on soil biological activities. We observed higher dehydrogenase and β-glucosidase enzyme activity in rhizosphere soils which may be due to rhizosphere effect. Dehydrogenase activity denotes over all microbial activity in the soil whereas microbial glucosidase activity is involved in C cycle. The later hydrolyze complex sugars and carbohydrates in the rhizosphere region and is considered as one of the soil quality indicators (Utobo and Tewari 2015). In our study, alkaline phosphatase and urease activity significantly differed with cultivar. Soil phosphatases (phosphoric monoester hydrolases) are classified as acid phosphatase (ACP) or alkaline phosphatase (ALP) by their optimum pH. Plant roots are the major producers of ACP whereas ALP is produced mainly by soil microbes and considered as a key driver of microbial P transformation (Nannipieri et al. 2011). Soil urease activity is important in N mineralization of applied fertilizer and regulate the N availability for plant growth (Piotrowska-Dlugosz and Charzynski 2015). Plant cultivar significantly influenced soil microbial activities. The genetic variation in plant cultivar may attribute differences in root exudation which in turn caused this observed changes in rhizosphere microbial community function (Monchgesang et al. 2016).
Principal component analysis (Fig. 6) and K-means non-hierarchical cluster mapping (Fig. 7) showed three groups of locations (soil types) in which group 1 consist of New Delhi site I, group 2 comprised Pune Site I, Pune Site II and New Delhi site II and group 3 had Kanpur, Varanasi, Haryana, Dharwad and Jharkhand locations. In New Delhi site I (group 1), the most important contributors are available nitrogen, available phosphorus and alkaline phosphatase. In group 2, pH is the significant contributor followed by available K. Dehydrogenase and urease activities influenced the group 3 locations.Collectively, our results suggest the presence of distinct microbial composition and distribution patterns among the various bulk and rhizosphere soils which may result in potential function differentiation (Fan 2017). Soil type (Zachowet al. 2008) as well as cultivar (Graneret al. 2003) have a considerable influence on the structure and function of microbial communities inhabiting the chickpea rhizosphere. Hence, there is no general conclusion about the key player involved, both soil type (Da Silva et al. 2003) and cultivar (Milling et al. 2004) can dominate depending on the biotic and abiotic conditions of the system. From the previous studies, it can be established that the microbial community composition in the rhizosphere is influenced by a complex interaction between soil type, plant type and root zone location (Marschner 2001).
The plant biomass and plant N content were insignificant with cultivar however soil types and interactive effect of soil and cultivar showed significant influence. Plant P content was influenced by both cultivarand soil types. The plant nutrient was found to vary significantly with soil types whereas within locations the nutrient content was less affected by the cultivars. Chekanai (2018) showed that the cultivareffect was not significant with plant N and P content of biomass. The two cultivars showed little or no response with regard to plant dry weight, N and P content in same soil type. However, between the soil types the chickpea growth and nutrient status was found to differ, indicating soil type to be the key factor to influence the chickpea growth across various locations. The study signifies the interaction between soil type and cultivar together has more impact on soil microbial activities rather than the soil type and cultivar alone.
References
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 174(2):330–338
Al-Dhabaan FAM, Bakhali AH (2017) Analysis of the bacterial strains using Biolog plates in the contaminated soil from Riyadh community. Saudi J Bio Sci 24(4):901–906
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. CanJ Biochem Physiol 37(8):911–917
Bray SR, Kitajima K, Mack MC (2012) Temporal dynamics of microbial communities on decomposing leaf litter of 10 plant species in relation to decomposition rate. Soil Biol Biochem 49:30–37
Buyer JS, Teasdale JR, Roberts DP, Zasada IA, Maul JE (2010) Factors affecting soil microbial community structure in tomato cropping systems. Soil Biol Biochem 42:831–841
Casida LE (1977) Microbial metabolic activity in soil as measured by dehydrogenase determinations. Soil Sci 34(6):630–636
Chekanai V, Chikowoa R, Vanlauwe B (2018) Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric Ecosyst Environ 266:167–173
Chen ZJ, Tian YH, Zhang Y, Song BR, Li HC, Chen ZH (2016) Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol Eng 92:243–250
Chen J, Shen W, Xu H, Li Y, Luo T (2019) The Composition of nitrogen-fixing microorganisms correlates with soil nitrogen content during reforestation: a comparison between legume and non-legume plantations. Front Microbiol 10:508
Collavino MM, Tripp HJ, Frank IE, Vidoz ML, Calderoli PA, Donato M, Zehr JP, Aguilar OM (2014) nifH pyrosequencing reveals the potential for location-specific soil chemistry to influence N2‐fixing community dynamics. Environ Microbiol 16(10):3211–3223
Da Silva KRS, Salles JF, Seldin L, d Van Elsas JD, (2003) Application of a novel Paenibacillus-specific PCR-DGGE method and sequence analysis to assess the diversity of Paenibacillus spp. in the maize rhizosphere. J Microbiol Methods 54:213–231
Donn S, Kirkegaard JA, Perera G, Richardson AE, Watt M (2015) Evolution of bacterial communities in the wheat crop rhizosphere. Environ Microbiol 17(3):610–621
Eivazi F, Tabatabai MA (1988) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606
Fan K, Cardona C, Li Y, Shi Y, Xiang X, Shen C, Wang H, Gilbert JA, Chu H (2017) Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol Biochem 113:275–284
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. PNAS 103(3):626–631
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71(7):4117–4120
Frostegard A, Tunlid A, Baath E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14:151–163
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57(8):2351–2359
Ge Y, Zhang JB, Zhang LM, Yang M, He JZ (2008) Long-term fertilization regimes affect bacterial community structure and diversity of an agricultural soil in northern China. J Soils Sediment 8(1):43–50
Gich FB, Amer E, Figueras JB, Abella CA, Balaguer MD, Poch M (2000) Assessment of microbial community structure changes by amplified ribosomal DNA restriction analysis (ARDRA). Internatl Microbiol 3:103–106
Graner G, Persson P, Meijer J, Alstrom S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 29:269–276
Grayston SJ, Vaughan D, Jones D (1997) Rhizosphere carbon flow in trees, in comparison with annual plants: the importance of root exudation and its impact on microbial activity and nutrient availability. Appl Soil Ecol 5(1):29–56
Guckert JB, White DC (1986) Phospholipid ester-linked fatty acid analysis in microbial ecology. In: Megusar F, Kantar G (eds) Perspectives in microbial ecology, Proc Fourth Int Symp Microb Ecol Ljubljana. American Society for Microbiology, Washington, pp 455–459
Gupta VSR, Zhang B, Penton CR, Yu J, Tiedje JM (2019) Diazotroph Diversity and Nitrogen Fixation in Summer Active Perennial Grasses in a Mediterranean Region Agricultural Soil. FrontMolBioSci 6:115. https://doi.org/10.3389/fmolb.2019.00115
Hu Y, Xiang D, Veresoglou SD, Chen F, Chen Y, Hao Z, Zhang X, Chen B (2014) Soil organic carbon and soil structure are driving microbial abundance and community composition across the arid and semi-arid grasslands in northern China. Soil Biol Biochem 77:51–57
Inceoglu O, Salles JF, van Elsas JD (2012) Soil and cultivar type shape the bacterial community in the potato rhizosphere. Microbial Ecol 63(2):460–470
Jackson ML (1973) Soil Chemical Analysis, 1st edn. Prentice Hall of India Private Limited, New Delhi
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Kaur A, Chaudhary A, Kaur A, Choudhary R, Kaushik R (2005) Phospholipid fatty acid a bio indicator of environment monitoring and assessment in soil ecosystem. Curr Sci 89:1103–1112
Kwak MJ, Kong HG, Choi K, Kwon SK, Song JY, Lee J … Kim JF (2018) Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat Biotechnol 36(11):1100–1109
Liu F, Hewezi T, Lebeis SL, Pantalone V, Grewal PS, Staton ME (2019) Soil indigenous microbiome and plant genotypes cooperatively modify soybean rhizosphere microbiome assembly. BMC Microbiol 19(1):1–19
Lundberg DS, Teixeira PJ (2018) Root-exuded coumarin shapes the root microbiome. PNAS 115(22):5629–5631
Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem 33:1437–1445
Mendes R, Kruijt M, De Bruijn I, Dekkers E, van der Voort M, Schneider JH, … Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332(6033):1097–1100
Miller KM, Ming TJ, Schulze AD, Withler RE (1999) Denaturing gradient gel electrophoresis (DGGE): a rapid and sensitive technique to screen nucleotide sequence variation in populations. BioTechniques 27:1016–1030
Milling A, Smalla K, Xaver F, Maidl K, Schloter M, Munch JC (2004) Effects of transgenic potatoes with an altered starch composition on the diversity of soil and rhizosphere bacteria and fungi. Plant Soil 266:23–39
Monchgesang S, Strehmel N, Schmidt S, Westphal L, Taruttis F, Muller E, Herklotz S, Newmann S, Scheel D (2016) Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. Sci Rep 6:29033
Naik SKS, Annapurna K, Kumari A, Vithal L, Reddy KK, Swarnalakshmi K (2017) Soybean (Glycine Max) genotype-mediated variation in the symbiotic performance of Rhizobium. Indian J Agric Sci 87(8):1051–1054
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Phosphorus in action. Springer, Berlin, pp 215–243
Neumann G, Romheld V (2002) Root-induced changes in the availability of nutrients in the rhizosphere. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, third edn. Marcel Dekker, Inc, New York, pp 617–649
Newton PCD (2013) A reduced fraction of plant N derived from atmospheric N (%Ndfa) and reduced rhizobial nifH gene numbers indicate a lower capacity for nitrogen fixation in nodules of white clover exposed to long-term CO2 enrichment. Biogeosci 10:8269–8281
Nichols D (2007) Cultivation gives context to the microbial ecologist. FEMS Microbiol Ecol 60(3):351–357
Olsen S, Cole C, Watanabe F, Dean L (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circular Nr 939. US Gov. Print. Office, Washington
Pereira-e-Silva MC, Semenov AV, van Elsas JD, Salles JF (2011) Seasonal variation in diversity and abundance of diazotrophic communities across soils. FEMS Microbiol Ecol 77:57–68
Pii Y, Borruso L, Brusetti L, Crecchio C, Cesco S, Mimmo T (2016) The interaction between iron nutrition, plant species and soil type shapes the rhizosphere microbiome. Plant Physiol Biochem 99:39–48
Piotrowska-Dlugosz A, Charzynski P (2015) The impact of the soil sealing degree on microbial biomass, enzymatic activity, and physicochemical properties in the ekranic technosols of toruń (poland). J Soils Sediments 15:47–59
Poly F, Monrozier LJ, Bally R (2001) Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103
Preston-Mafham J, Boddy L, Randerson PF (2002) Analysis of microbial community functional diversity using sole-carbon-source utilization profiles-a critique. FEMS Microbiol Ecol 42:1–14
Qiao Q, Wang F, Zhang J, Chen Y, Zhang C, Liu G, … Zhang J (2017) The variation in the rhizosphere microbiome of cotton with soil type, genotype and developmental stage. Sci Rep 7(1):1–10
Reinhold-Hurek B, Bünger W, Burbano CS, Sabale M, Hurek T (2015) Roots shaping their microbiome: global hotspots for microbial activity. Ann Rev Phytopathol 53:403–424
Rutgers M, Wouterse M, Drost SM, Breure AM, Mulder C, Stone D (2016) Monitoring soil bacteria with community-level physiological profiles using Biolog ECO-plates in the Netherlands and Europe. Appl Soil Ecol 97:23–35
Sasse J, Martinoia E, Northen T (2018) Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci 23(1):25–41
SatyaPrakash C, Annapurna K (2006) Diversity of soybean bradyrhizobial population adapted to an Indian soil. J Plant Bio chem Biotechnol 15:27–32
Sharma R, Pooniya V, Bisaria VS, Swarnalakshmi K, Sharma S (2020) Bioinoculants play a significant role in shaping the rhizospheric microbial community: a field study with Cajanus cajan. World J Microbiol Biotechnol 36(3):1–17
Smalla K, Wieland G, Buchner A, Zock A, Parzy J, Kaiser S, Roskot N, Heuer H, Berg G (2001) Bulk and rhizosphere soil bacterial communities studied by denaturing gradient gel electrophoresis: Plant-dependent enrichment and seasonal shifts revealed. Appl Environ Microbiol 67:4742–4751
Smith JL, Doran JW (1996) Measurement and use of pH and electrical conductivity for soil quality analysis. In: Doran JW, Jones AJ (eds) Methods for assessing soil quality. Soil Science Society of America Inc. Madison, Wisconsin, USA, pp 169–186
Subbiah B, Asija G (1956) A rapid procedure for the estimation of available nitrogen in soils. Curr Sci 25:259–260
Swarnalakshmi K, Annapurna K (2019) Compositional changes of bacterial communities associated with field grown chickpea. Regional Young Investigators’ meeting held at NIPGR, New Delhi during August 6–7, 2019
Swarnalakshmi K, Yadav V, Tyagi D, Dhar DW, Kannepalli A, Kumar S (2020) Significance of plant growth promoting rhizobacteria in grain legumes: growth promotion and crop production. Plants 9(11):1596
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soils. Soil Biol Biochem 4:479–487
Treseder KK (2008) Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol Lett 11(10):1111–1120
Utobo EB, Tewari L (2015) Soil enzymes as bioindicators of soil ecosystem status. ApplEcol Environ Res 13:147–169
Vance ED, Brookes PC, Jenkinson DS (1987) An Extraction method for measuring soil microbial biomass carbon. Soil Biol Biochem 19:703–704
Walkley AJ, Black IA (1934) Estimation of soil organic carbon by the chromic acid titration method. Soil Sci 37:29–38
Walley FL, Boahen SK, Hnatowich G, Stevenson C (2005) Nitrogen and phosphorous fertility management for desi and kabuli chickpea. Can J Plant Sci 85:73–79
White DC, Stair JO, Ringelberg DB (1996) Quantitative comparisons of in situ microbial biodiversity by signature biomarker analysis. J Ind Microbiol Biotechnol 17:185–196
Yuen SH, Pollard AG (1953) Determination of nitrogen in soil and plant materials: Use of boric acid in the micro-kjeldahl method. J Sci Food Agric 4:490–496
Zachow C, Tilcher R, Berg G (2008) Sugar beet-associated bacterial and fungal communities show a high indigenous antagonistic potential against plant pathogens. Microbial Ecol 55(1):119–129
Zhao S, Lic K, Zhoua W, Qiua S, Huanga S, Hea P (2016) Changes in soil microbial community, enzyme activities and organic matter fractions under long-term straw return in north-central China. Agric Ecosyst Environ 216:82–88
Zhu X, Zhu B (2015) Diversity and abundance of soil fauna as influenced by long-term fertilization in cropland of purple soil, China. Soil Tillage Res 146:39–46
Acknowledgements
We acknowledge the Indian Council of Agricultural Research (ICAR) funded projects ICAR-AINP Research Program on Soil diversity and biofertilizer and ICAR-BNF project for sponsoring this research. SG gratefully acknowledges the help for BIOLOG facility provided by Dr. Devyani Tipre, Department of Microbiology & Biotechnology, Gujarat University, India.
Funding
This article was funded by Indian Council of Agricultural Research with Grant Nos. 21-47 and 21-30.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The authors declare no ethical conflicts.
Informed consent
Authors declare that they have consented to participate in the manuscript and publish it.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sneha, G.R., Swarnalakshmi, K., Sharma, M. et al. Soil type influence nutrient availability, microbial metabolic diversity, eubacterial and diazotroph abundance in chickpea rhizosphere. World J Microbiol Biotechnol 37, 167 (2021). https://doi.org/10.1007/s11274-021-03132-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03132-0