Abstract
The Role of microorganisms in the petroleum industry is wide-ranging. To understand the role of microorganisms in hydrocarbon transformation, identification of such microorganisms is vital, especially the ones capable of in situ degradation. Microorganisms play a pivotal role in the degradation of hydrocarbons and remediation of heavy metals. Anaerobic microorganisms such as Sulphate Reducing Bacteria (SRB), responsible for the production of hydrogen sulphide (H2S) within the reservoir, reduces the oil quality by causing reservoir souring and reduction in oil viscosity. This paper reviews the diversity of SRB, methanogens, Nitrogen Reducing Bacteria (NRB), and fermentative bacteria present in oil reservoirs. It also reviews the extensive diversity of these microorganisms, their applications in petroleum industries, characteristics and adaptability to survive in different conditions, the potential to alter the petroleum hydrocarbons properties, the propensity to petroleum hydrocarbon degradation, and remediation of metals.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An oil reservoir contains thousands of complex mixtures of petroleum hydrocarbons and other organic compounds (Cooney et al. 1985). Petroleum is a heterogeneous mixture of hydrocarbons, including aliphatic (n-alkanes), alicyclic and complex hydrocarbons which vary in compositional and physical properties according to the reservoir depth and geological locations (Peixoto et al. 2011; Li et al. 2020). Complex hydrocarbon such as monocyclic aromatic compounds like Benzene, Toluene, Ethylbenzene and Xylene (BTEX), and Polycyclic Aromatic Hydrocarbons (PAHs) are the most often encountered surface and subsurface contaminants (Holliger et al. 1997). PAHs are predominantly mutagenic, cytotoxic, teratogenic, and carcinogenic hydrocarbons (Peixoto et al. 2011; Qian et al. 2021). Degradation of petroleum hydrocarbon and change in crude oil quality is mostly caused by anaerobic microorganisms which are present in oil reservoirs. Oil reservoirs contain rich species of indigenous microbes and species of active surfactants production, methanogenesis, and hydrocarbon degradation functions (Lin et al. 2014). Ecologically, hydrocarbon-metabolizing microorganisms are widely distributed in an oil reservoir (Van Hamme et al. 2003). The anaerobic microorganisms can either degrade or produce hydrocarbons depending on the presence of certain metabolic pathways and environmental conditions (Peixoto et al. 2011). Numerous anaerobic microorganisms such as SRB, methanogens, NRB, and fermentative bacteria can exist in an oil reservoir (Youssef et al. 2009). Few microorganisms found are Archaeoglobus fulgidus, Desulfacinum infernum, Desulfovibrio longus, Desulfovibrio gobonensis, Methanobacterium bryantii, Methanobacterium ivanovii, Methanobacterium thermoaggregans, Methanobacterium thermoautrophicum, Thauera phenylacetica, Pseudomonas stutzeri, Geobacillus subterraneus, Geobacillus uzenensis, Geotoga petraea, Anaerobaculum thermoterranum, Acetoanaerobium romashkovii, Geotoga subterranean (Magot et al. 2000; Sierra-Garcia and de Oliveira 2013). These microorganisms have several roles in petroleum exploration and production activities. Bass (1999), studied the importance of microorganism in the petroleum industry and stated that petroleum microbiology can be divided into six categories, starting with the diagenesis of organic component in sediments and subsequent oleogenesis, degradation of hydrocarbons, and remediation of metals, Enhanced Oil Recovery (EOR) from oil reservoir, modification of hydrocarbon products either in the formation or past production, mitigation of its effect of organisms during production and bioremediation of escaped products either crude or processed (Bass 1999). Anaerobic microorganisms are injected into depleted oil reservoirs for EOR, removal of sulphur by bio desulphurization methods without degradation of associated carbon moieties. Microorganisms are also used for the removal of nitrogen (N) from crude oil leading to a reduction of emissions of nitric oxide (NO). Recently bacterial biosensors have also been used to analyze petroleum-contaminated environments (Van Hamme et al. 2003). Several microbial products, hydrocarbon metabolism, biosurfactants, biopolymers, solvents, acids, gases and emulsifiers are used for various applications in petroleum industries. A few such applications are removal of paraffin deposits, oil degradation, oil emulsification, Interfacial Tension (IFT) reduction, viscosity reduction and wettability alteration.
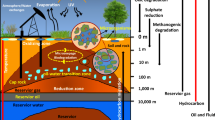
Anaerobic microorganisms can be isolated from both non-water flooded and water flooded oil reservoirs. Microorganisms species such as Desulfotomaculum halophilim, Desulfotomaculum thermocisternum and Desulfovibrio longus have been isolated from non-water flooded oil reservoirs (Magot et al. 2000; Sierra-Garcia and de Oliveira 2013). These microorganisms can belong to the indigenous microbial communities. Many novel species of microorganisms such as Desulfacinum subterraneum, Desulfobacter vibrioformis, Desulfobacterium cetonicum, Desulfobulbus rhabdoformis, Desulfovibrio gabonensis, Desulfovibrio vietnamensis, and Thermodesulfobacterium norvegicus were isolated from the water flooded oil reservoirs like (Lien et al. 1998; Magot et al. 2000; Rozanova et al. 2001; Sierra-Garcia and de Oliveira 2013). Hence, it cannot be concluded that these species are indigenous microbial communities to the oil reservoir. Rather these microorganisms are considered to be growing in the upper parts of the well or originating from injection waters. A sketch of a typical petroleum reservoir with various microbial inhabitants is shown in Fig. 1. Most of the microorganisms are found in the oil-water transition zone and microbial degradation in oil reservoirs is believed to be taken place in this zone (Pannekens et al. 2020).
The survival and activities of anaerobic microorganisms depend on several environmental factors. The existence and survival of various microorganisms in oil reservoirs were studied by Pannekens et al. (2019), which was performed in the different environmental conditions of high toxicity, temperature, pressure, salinity, hydrophobicity, and low water activity (Pannekens et al. 2019). This study revealed that the survival conditions of various microorganisms differ from each other. Temperature is considered the most influential factor for the growth of microorganisms. The microorganisms can survive up to 80–90°C inside an oil reservoir. The other influenced factors are pH, the activity of water, salinity, oxygen availability, nutrient availability, permeability (Al-Hawash et al. 2018; Rajbongshi and Gogoi 2020).
Significance of the study
Anaerobic microorganisms have become prominent in the petroleum exploration and production industry because of their diversity and their wide applications in the petroleum industry. Many anaerobic microorganisms have been isolated from different kinds of petroleum reservoirs of varying depths and geographic locations of varying temperatures and pressures. Several studies have been performed on these microorganisms, but still knowledge on these microorganisms is limited. Therefore, extensive and collective reviews are required to understand the diversity of these microorganisms, their applications in the petroleum industry, their characteristics and adaptability to survive in different conditions, their potential to alter the petroleum hydrocarbons properties, the propensity to petroleum hydrocarbon degradation as well as remediation of metals. This review intends to compile information on the diversity of various anaerobic microorganisms and their applications in the petroleum industry.
Anaerobic microorganisms in oil reservoirs
Sulphate reducing bacteria
SRB grow on environmental contaminants of petroleum hydrocarbon constituents such as BTEX, PAHs, and alkanes (Novelli 1944; Ensley and Suflita 1995). SRB are responsible for the production of H2S within the reservoir as shown in reaction no (1), which can reduce the oil quality by causing reservoir souring, reduction of oil viscosity, degradation of hydrocarbons (both aliphatic and aromatic), corrosion of steel, reduction of the injectivity of water injection wells by precipitation of amorphous iron sulphide (FeS), reduction of permeability of oil-bearing rock pore spaces (plugging). SRB can lead to inefficient secondary oil recovery and can affect workers’ health due to its high toxicity (Castro et al. 1997; Bass et al. 1998; Magot et al. 2000; Barton and Fauque 2009; Al-Sulaimani et al. 2011; Song et al. 2014). SRB are extensively studied microorganisms in oilfields because of their reservoir souring problems. It uses sulphate (SO42−) as a final electron acceptor instead of oxygen (O2) for respiration (Al Zuhair et al. 2008). The troublesome and most common SRB belong to the genus Desulfovibrio and these species have been isolated from the deep subsurface Formation Water (FW) of oil reservoirs (Basso et al. 2005). They get energy from the organic compounds available in the well as in reaction (1).
Bastin et al. (1926), has reported the presence of SRB in oil reservoirs and stated that these microorganisms can belong to the indigenous microbial communities to the subsurface oil reservoirs (Bastin et al. 1926). SRB have been isolated from a variety of environments such as fresh to saline and hyper saline waters or FW (Risatti et al. 1994; Nilsen et al. 1996a; Nilsen et al. 1996b; Bahr et al. 2005), either from surface or subsurface habitats (Kovacik Jr et al. 2006), marine or lake sediments (Sass et al. 1998; Ravenschlag et al. 2000; Mußmann et al. 2005; Webster et al. 2006), hydrothermal vents (Jeanthon et al. 2002), hydrocarbon seeps and mud volcanoes (Knittel et al. 2003; Stadnitskaia et al. 2005; Kniemeyer et al. 2007), rhizosphere of plants (Hines et al. 1999).
Diversity of sulphate reducing bacteria
SRB have been determined by the use of 16 S rRNA or dsrAB (dissimilatory sulfite reductase) gene molecular techniques (Dar et al. 2007). For the rapid determination of SRB diversity in different environments, the dsrAB gene fingerprinting methods t-RFLP, DGGE, and gel-retardation analyses have been widely used in the last few decades. Microbial communities can also be characterized by SEM-EDS (Daghio et al. 2018). The 16 S rRNA gene sequence can be aligned with the related sequences from GenBank and EzBioCloud databases and the phylogenetic tree can be constructed using MEGA software (Wang et al. 2020). More than 220 SRB species of 60 genera have been described till now (Barton and Fauque 2009). Varieties of SRB have been isolated from oilfields around the world like Canada, France, USA, North Sea, Vietnam, Congo, Paris, California. Many researchers isolated SRB from FW of different oilfields such as Desulfotomaculum nigrificans (Nazina and Rozanova 1978), Desulfotomaculum kuznetsovii (Nazina et al. 1988), Desulfacinum subterraneum (Rozanova et al. 2001), Desulfobacter cetonicum (Galushko and Rozanova 1991), Desulfovibrio gracillis and Desulfovibrio longus (Magot et al. 1992, 2004), Thermodesulforhabdus norvegicus and Archaeoglobus fulgidus (Beeder et al. 1994, 1995), Thermodesulfobacterium commune (l’Haridon et al. 1995), Desulfacinum infernum (Rees et al. 1995), Desulfovibrio vietnamensis (Dang et al. 1996), Desulfotomaculum thermocisternum (Nilsen et al. 1996b), Desulfovibrio gobonensis and Desulfotomaculum halophilum (Tardy-Jacquenod et al. 1996b, 1998), Desulfobacter vibrioformis (Lien and Beeder 1997), Desulfobulbus rhabdoformis (Lien et al. 1998), Desulfovibrio alaskensis (Feio et al. 2004), Desulfovermiculus halophilus (Belyakova et al. 2006), Desulfotignum toluenicum (Ommedal and Torsvik 2007), Desulfonauticus autotrophicus (Mayilraj et al. 2009). The most frequently isolated SRB from FW belong to deltaproteobacteria. They comprise of mesophilic members of the genera Desulfovibrio, Desulfobulbus, Desulfobacterium, Desulfobacter and thermophilic members of the genera Thermodesulforhabdus and Desulfacinum. Desulfovibrio sp. are the most frequently isolated microorganisms (Tardy-Jacquenod et al. 1996a). Desulfomicrobium and Desulfovibrio species have been found in high-temperature oil-bearing formations (Orphan et al. 2000; Watanabe et al. 2002). Many microbiological and molecular studies revealed the presence of Desulfotomaculum species of Gram-positive group in oil reservoir (Rozanova and Nazina 1979; Rosnes et al. 1991; Christensen et al. 1992; Nilsen et al. 1996b; Watanabe et al. 2002). The Desulfotomaculum species were also isolated from a high-temperature North Sea oil well and a continental high-temperature oil reservoir in Western Siberia, Russia (Nilsen et al. 1996a; Bonch-Osmolovskaya et al. 2003). Thermodesulfobacterium species are generally considered as the most thermophilic sulphate-reducing microorganisms of the domain bacteria, with an upper limit temperature around 80 °C for growth (Christensen et al. 1992; l’Haridon et al. 1995). These species are considered as indigenous community of deep subsurface ecosystem of an oil reservoir (Bonch-Osmolovskaya et al. 2003). Thermophilic and mesophilic species like Thermodesulfobacterium thermophilum, Thermodesulforhabdus norvegicus, Archaeoglobus fulgidus, Desulfacinum infernum, Desulfotomaculum, Desulfobulbus rhabdoformis and Desulfomicrobium from North Sea reservoir were isolated by many researchers (Rosnes et al. 1991; Christensen et al. 1992; Beeder et al. 1995; Rees et al. 1995; Lien et al. 1998; Leu et al. 1999). Desulfotomaculum halophilum, Desulfovibrio longus, Desulfovibrio putealis, Archaeoglobus fulgidus and Thermotoga elfii were isolated from oilfields of Paris basin while, Desulfovibrio vietnamensis, Desulfacinum subterraneum were isolated from Vietnam oilfields (Magot et al. 1992; Dang et al. 1996; Tardy-Jacquenod et al. 1998; Rozanova et al. 2001; Basso et al. 2005; Fardeau et al. 2009). Some SRB that were isolated from various oil reservoirs are given in Table 1.
Role of sulphate-reducing bacteria in the enhanced oil recovery process
Microbial Enhanced Oil Recovery (MEOR) processes use microbial technology to improve the recovery of crude oil from oil reservoirs (Sen 2008). MEOR technology is used to harness the indigenous microorganism resources present in the reservoirs. MEOR is considered to be a cost-effective and environment-friendly EOR process (Lin et al. 2014). MEOR technologies have been approved universally as cost-effective and eco-friendly to improve oil production because their products are biodegradable and have low toxicity (Sarkar et al. 1989; Lazar et al. 2007; Suthar et al. 2008; Banat et al. 2010; Al-Bahry et al. 2013). MEOR depends on an adequate understanding of the relationship between the microbial community structure and oil reservoir conditions (Lin et al. 2014). Therefore, several studies on the microorganisms in oil reservoirs have been performed (Basso et al. 2005; Grabowski et al. 2005; Nazina et al. 2005; Li et al. 2007; Song et al. 2010; Xiu et al. 2010). Several books on MEOR have been published (Zajic et al. 1983; Yen 1986; Donaldson et al. 1989). The diversity of SRB is widely distributed in global oil reservoirs. Therefore, special emphasis has been given on SRB for EOR by researchers and reviewers (Champagne et al. 1996; Callbeck et al. 2013; Song et al. 2014). Metabolites of SRB can reduce both Surface Tension (ST) and IFT between gas/liquid and oil/liquid respectively and form a stable emulsion system with crude oil in an oil reservoir (Song et al. 2014). Many studies reported that metabolites produced by a variety of microbes can act as biosurfactants to reduce IFT between oil and water (Singer and Finnerty 1988; Banat 1993; Singh et al. 2007). Oil viscosity plays an important role in EOR and SRB metabolite/oil emulsion and biodegradation has contributed partially to the reduction of oil viscosity (Al-Sulaimani et al. 2011; Ghosh and Al Shalabi 2011; Homayuni et al. 2011; Song et al. 2014). The degradation of hydrocarbons by microorganisms is important for MEOR because of its role in increasing the mobility of crude oil (Li et al. 2020). Zobell (1946), has used the method of injection of microorganisms into the depleted oil reservoir to enhance the oil recovery rate (Zobell 1946). Bass (1999), mentioned that Zobell in 1970, isolated a strain of Desulfovibrio that can grow on salt solution which tolerated relatively high temperature. These microorganisms can enhance the oil production from a depleted oil reservoir by producing biosurfactants. The direct action of biosurfactant on petroleum hydrocarbon may increase hydrocarbon production by reducing the IFT of unsaturated hydrocarbons such as long-chain hydrocarbon and shorter chain hydrocarbon which strongly hold on rock surface (Bass 1999). Song et al. (2014), reported that the oil recovery rate of SRB from a saturated oil reservoir was 39.2 % (Song et al. 2014). A field-wide test was done in the Mink Unit of Delaware-Childers field in Nowata County, Oklahoma to measure the production rate in a mature waterflood field by MEOR process and it was found that the production was increased by 13 % in that year while water/oil ratios at the producing wells dropped to nearly 35 % (Bryant et al. 1990). The efficacy of some biosurfactants used in the MEOR process is given in Table 2.
The action of indigenous microorganisms during the MEOR process in an oil reservoir is shown in Fig. 2. In the first phase, the microorganisms utilize oil as a nutrient (carbon source) and produce natural surfactant (biosurfactant), and oil is released from the trapped rock pores. In the second phase, the microorganisms multiply and after some time, blocks the usual water flow paths. This opens a new path for water that pushes the oil out and allows the microorganisms to reach the trapped oil. In the third phase, once the trapped oil is released, the microbes disperse because there is no carbon source (nutrient) for the microbes. For this reason, the blocked water pathways reopen. Bryant reviewed the MEOR field tests in the US and reported that the oil production increased by 20 to 200 %, depending on a variety of factors including initial oil saturation, temperature, salinity, permeability, microorganism, nutrients employed, and injection procedures (Bryant 1987; Bryant and Douglas 1987). Some applications of microbial products in petroleum exploration and production industries are given in Table 3.
Role of sulphate-reducing bacteria in biodegradation of petroleum hydrocarbon
Biodegradation of crude oil in subsurface oil reservoirs is an important alteration process with major economic consequences. Biodegradation of crude oil in subsurface oil reservoirs has adversely affected the majority of the world’s oil, making recovery and refining of the oil more costly (Meyer 1987). Anaerobic hydrocarbon degradation is a common process in biodegraded subsurface oil reservoirs which dominate subsurface sedimentary environments (Head et al. 2003; Aitken et al. 2004). The hydrocarbon-degrading microorganisms produce biosurfactants of diverse chemical nature and molecular size. These surface-active materials increase the surface area of hydrophobic water-insoluble substrates and increase their bioavailability, thereby enhancing the growth of microorganisms and the rate of bioremediation (Ron and Rosenberg 2002). At water flooded oilfield, the most active microbial processes of oil degradation occur near the bottom zone of an injection well and in deep subsurface oil (Nazina et al. 2017). Oil degradation probably occurs via methanogenesis in the biodegraded reservoir and it is the dominant terminal process for hydrocarbon degradation driven by anaerobic biodegradation (Silva et al. 2013). The anaerobic microorganisms belonging to the microbial diversity of bacteria have the potential to metabolize hydrocarbon and inorganic compounds (Silva et al. 2013). Hydrocarbons such as toluene (Edwards et al. 1992; Langenhoff et al. 1997; Elshahed and McInerney 2001), alkylbenzenes including m-, o- and p-xylene and trimethylbenzenes (Ball and Reinhard 1996; Chen and Taylor 1997; Haner et al. 1997; Phelps and Young 1999), benzene (Kazumi et al. 1997; Burland and Edwards 1999; Rooney-Varga et al. 1999), naphthalene and phenanthrene (Coates et al. 1996; Bedessem et al. 1997; Zhang and Young 1997; Meckenstock et al. 2000), methylnaphthalene and tetralin (Annweiler et al. 2000, 2002), n-alkanes (Caldwell et al. 1998; Anderson and Lovley 2000; Ehrenreich et al. 2000; So and Young 2001), branched alkanes (Bregnard et al. 1996, 1997), organic acids (Devereux et al. 1992; Ensley and Suflita 1995; Karr et al. 2005) and hydrocarbon mixtures (Grishchenkov et al. 2000) can be metabolized by bacteria under anaerobic conditions. All SRB genera preferentially degrade certain organic acids and cannot degrade others. Only a few SRB genera are known to readily degrade a wide range of organic acids. E.g., Desulforhabdus amnigenus can degrade lactate, acetate, butyrate, and propionate (Elferink et al. 1995). Recent research on SRB revealed that they can also degrade long-chain alkanes, alkenes, and short-chain alkanes (Davidova et al. 2006; Grossi et al. 2007; Kniemeyer et al. 2007; Fullerton et al. 2013; Kleindienst et al. 2014; Herath et al. 2016). Song et al. (2014), mentioned that SRB could be directly used for anaerobic biodegradation of oil components (C11- C13 n-alkanes) (Song et al. 2014). Zhao et al. (2018), performed in situ bioremediation and reported more than 50 % of oil spilled pollutants n-alkanes (C12-C27) and PAHs were degraded within 70 days of bioremediation (Zhao et al. 2018). Ayangbenro et al. (2018), mentioned that complex organic compounds such as carboxylic acids (acetate, fumarate, butyrate, malate), amino acids (alanine, glycine, serine), alcohols, and aromatic compounds can be degraded by SRB in anaerobic condition (Ayangbenro et al. 2018). The biological technique of SRB generates H2S under anaerobic conditions in a reaction between elemental sulphur (S) with an electron donor organic compound (acetic acid) as in reaction (2).
SRB are important regulators of various processes in wetland soils, including organic matter turnover, biodegradation of chlorinated aromatic pollutants in anaerobic soils, and sediment sand mercury methylation (Miller and Wakerley 1966; Plugge et al. 2011). SRB cannot degrade substrates like cellulose, fats, nucleic acids, proteins, starch, polymeric organic compounds directly but depends on other microorganisms to degrade these substrates (Muyzer and Stams 2008).
Role of sulphate-reducing bacteria in remediation of metals
Heavy metal pollution caused by exploration and production activities of the oil & gas industry and other mining industries has induced an adverse impact on the environment. Heavy metal causes pollution because of its high toxicity and non-biodegradability. This has threatened the health of human beings and the stability of the ecological system. The remediation and detoxification of active heavy metal ions in the natural environment can be achieved through microbial activities (Yin et al. 2019). SRB are good in the detoxification of active heavy metal ions which have been successfully applied on the industrial scale for the remediation of metals from various wastewater. SRB are the most studied biosulfidogens which have immense potential of remediating metal-rich wastewater. They also can reduce SO42−, thiosulphate (S2O32−), S and takes part in the breakdown process of sulphur-containing amino acids in proteins to produce sulphide (S2−) (Hussain et al. 2016). The remediation of metals by SRB has proved to be effective in metal removal and also in controlling environmental pollution. This process is a very cost-effective technique in the elimination of heavy metals like arsenic (As), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), mercury (Hg), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), selenium (Se) and zinc (Zn) (Ayangbenro et al. 2018; Yin et al. 2019). Many researchers have declared that chemical treatment methods are environmentally non-compatible, has low treatment efficiency, high operational cost, complicated in operation with a possible generation of secondary pollutant (Rocha et al. 2009; Abbas et al. 2016).
The most recent approach for remediation of metallic waste is the precipitation of metal ions in the form of their respective sulphides. A fruitful way to know the efficiency of a microorganism in the remediation of heavy metals is the study of the adsorption isotherm model. Mathematical isotherm models help to verify their suitability for describing biosorption of heavy metals by microorganisms as well as to understand the sorption isotherm phenomenon between the biomass surface and the metal molecules (Brouers and Al-Musawi 2015). Yin et al. (2019), reviewed the adsorption isotherm model for microorganisms like Pesudomonas aeruginosa, Bacillus sp., Arthrobacter viscosus, Eichhornia sp., Brevibacterium sp., Rhodobacter capsulatus, Ochrobactrum sp. during removal of heavy metal ions like Hg (II), Pb (II), Cr (VI), Cu (II), Zn (II), Cd (II) where the best-fitted isotherm model was Langmuir isotherm (Yin et al. 2019).
Methanogens
Methanogens are a unique group of anaerobic archaea that are more metabolically diverse. Methanogenic archaea are an important group of microorganisms present in oil reservoirs (Magot et al. 2000). Methanogens can generate only methane (CH4) by coupling the oxidation of products formed by fermentative bacteria with the reduction of carbon dioxide (CO2) (Holmes and Smith 2016). The activity of the methanogenic archaea is affected by physical and chemical factors such as temperature, salt content, and pH (Parthipan et al. 2017). Many methanogens thrive in neutral pH, low salinity, and temperate environments. Most methanogens are mesophilic, some are extremophiles e.g. Methanopyrus kandleri and Methanococcus vulcanicus which survive at temperatures of 110oC (Kurr et al. 1991). Several thermophilic species having the ability to oxidize hydrogen have been identified and reported from various onshore and offshore reservoirs. Examples of such species are Methanothermococcus and Methanothermobacter. Anaerobic methanogenic archaea can also be isolated from a saline environment (Elias et al. 1999). Methanogenic microorganisms isolated from oil reservoirs belong to species of Methanosarcinales, Methanomicrobiales, Methanobacteriales, and Methanococcales (Parthipan et al. 2017). An incumbent process occurring in oil reservoirs and also the contaminated aquifers is methanogenic biodegradation of crude. Biodegradation of fuel components by the anaerobic electron-accepting process has also been reported in various hydrocarbon-impacted subsurface environments (Widdel et al. 2010). However, when available electron acceptors are depleted in such environments, hydrocarbon biodegradation has to proceed via methanogenesis (Berdugo-Clavijo and Gieg 2014). The biodegradation of hydrocarbons under methanogenic conditions have been widely investigated for crude oil and its components such as n-alkanes, benzene, toluene and polycyclic aromatic hydrocarbons (Grbić-Galić and Vogel 1987; Godsy et al. 1992; Edwards and Grbić-Galić 1994; Zengler et al. 1999; Anderson and Lovley 2000; Townsend et al. 2003; Ulrich and Edwards 2003; Chang et al. 2006; Jones et al. 2008; Gieg et al. 2010; Berdugo-Clavijo et al. 2012; Zhang et al. 2012). An iron corroding methanogen, Methanococcus maripaludis strain KA1, isolated from a crude-oil storage tank produces CH4. This strain can oxidize iron much faster than Methanococcus maripaludis, strain JJT (Uchiyama et al. 2010). Dinh et al. (2004), isolated a methanogen (strain IM1) that produces CH4 more rapidly than Methanococcus maripaludis (Dinh et al. 2004). Methanogen can be used in biogas production. Methanobacterium which is found in anaerobic sludge can be used as an anaerobic bacteria feed on cellulose which generates large amounts of CH4 along with CO2 and H2. Some anaerobic methanogens that were isolated from various oil reservoirs are given in Table 4.
Nitrate reducing bacteria
Nitrate reducing microorganisms isolated from oil reservoirs is currently the need of the hour due to in situ use of nitrate by oil companies to decrease S2− concentration in oilfields (Davidova et al. 2001). In most surface or subsurface environments N and phosphorus (P) are often the main limiting nutrients. Nitrate is reduced to ammonium. Dissimilatory Nitrate Reduction to Ammonium (DNRA) can proceed through chemoautrophy or a fermentative pathway as in reaction (3) and (4) (Tobias and Neubauer 2019).
Autotropic DNRA
Fermentative DNRA
However, if N is limited in the reservoirs, the abundant ammonium ions (NH4+) present there can be used as the primary N source for in situ bacterial activity (Head et al. 2003). In oilfields, N is also available as dinitrogen (N2) gas or as heterocyclic aromatic nitrogen compounds in petroleum. Researchers have revealed that nitrate-reducing bacteria may inhabit oilfield reservoir ecosystems (Greene et al. 1997; Ollivier and Cayol 2005). A thermophilic heterotrophic bacterium, Garciella nitratireducens which reduces nitrate to ammonium was isolated from an oil well located in the Gulf of Mexico (Miranda-Tello et al. 2003). The activities of NRB can remove existing S2− from oilfield FW and also inhibit the production of S2− (Davidova et al. 2001). Nitrate serves as an electron acceptor for the reoxidation of S2− to SO42− or S by sulfur-oxidizing chemolithotrophic bacteria as shown in reaction (5) (Sublette et al. 1994; Telang et al. 1999). Nitrite produced by NRB can react with dissolved sulphide to produce S as shown in reaction (6) (Jenneman et al. 1996).
A chemoautotroph, Thiobacillus denitrificans can convert S to SO42− as in reaction (7) (Doelle 2014). The production of SO42− from nitrite is shown in the reaction (8) (Zhang et al. 2019).
Some anaerobic NRB that were isolated from various oil reservoirs are given in Table 5.
Fermentative bacteria
Besides SRB, methanogens, NRB, fermentative microorganisms have also been frequently isolated from the FW of the oilfields. Stetter et al. (1993), first provided evidence of the presence of Thermotoga strains in oilfields FW (Stetter et al. 1993). Fermentative microorganisms isolated from various oilfield environments have been reviewed by Ollivier and Cayol (2005), (Ollivier and Cayol 2005). A wide range of mesophilic and thermophilic fermentative bacteria have been identified from oil reservoirs. Most of them belong to the domain bacteria (Halanaerobium and Thermotoga), however, only a few hyperthermophiles belong to the domain Archaea (Thermococcus sp. and Pyrococcus sp.) (Ollivier and Cayol 2005). Researchers have also isolated fermentative microorganisms from oilfield environments like Thermotoga elfii, Thermotoga subterranean, Thermotoga hypogeal, Thermotoga petrophila, Thermotoga naphthophila, Thermosipho geolei, Petrotoga sibiria, Petrotoga olearia, Geotoga petraea, Geotoga subterranean, Petrotoga miotherma, Petrotoga mexicana, Thermotoga brockii, Caldanaerobacter subterraneus, Anaerobaculum thermoterranum at different temperature and salinity (Davey et al. 1993; Cayol et al. 1995; Jeanthon et al. 1995; Ravot et al. 1995; Fardeau et al. 1997, 2004; Rees et al. 1997; Haridon et al. 2001; Takahata et al. 2001; l’Haridon et al. 2002; Miranda-Tello et al. 2003). Some fermentative bacteria which were isolated from various oil reservoirs are given in Table 6.
Environmental impact on anaerobic bacteria
The activities of anaerobic microorganisms can be affected by several environmental factors such as temperature, pH, the activity of water, salinity, oxygen availability, nutrient availability, the permeability of the reservoir (Al-Hawash et al. 2018; Rajbongshi and Gogoi 2020). The optimal growth condition of temperature, pH, salinity, and nutrients for some anaerobic microorganisms are given in Table 7.
Temperature
The possibility of living organisms surviving in oilfield environments depends on the physical characteristics and chemical composition of the ecosystem. Temperature is the most influenced factor that affects the rate of degradation of crude oil in oil reservoirs and microbial community structures (Chen et al. 2010). The largest microbial diversity occurs at moderate temperatures up to 55 °C, where higher metabolic activity increases the abundance of genes involved in carbon cycling and the degradation of aromatic and other organic compounds. Approximately above 80 °C, oil reservoirs are considered to be sterile (Pannekens et al. 2019). In situ oil degradation was never observed in reservoirs where the temperature exceeded 82°C and maximum biodegradation occurred below 80 °C (Philippi 1977; Barth 1991). Since, temperature increases with depth at a mean rate of 3 °C per 100 m (but regional geothermal gradients may be significantly different), deep oil reservoirs which attain in situ temperature exceeding 130–150°C cannot sustain bacterial growth (Magot et al. 2000). This temperature range is considered the highest theoretical limit for growth due to the thermal instability of biological molecules (Stetter et al. 1993). In a microbiological study, hyperthermophilic bacteria could not be isolated from 100 oilfield water samples whose reservoir temperature was higher than 82 °C (Bernard et al. 1992). Researchers have also stated that the presence of indigenous bacteria in oil reservoirs are limited to a threshold temperature between 80–90 °C. But hyperthermophilic microorganisms, growing at temperatures as high as 103 °C, have been isolated from some reservoirs (Stetter et al. 1993).
pH
Change in pH affects the growth of microorganisms and biodegradation of hydrocarbons as different microorganisms have different pH range for optimal functioning. Microorganisms that grow optimally at a pH less than 5 are called acidophiles, within pH 5–8 are neutrophiles and above pH 8 are called alkaliphiles (Jin and Kirk 2018; Keenleyside 2019). The highest microbial growth and biodegradation rates are generally observed at neutral pH. Extreme pH affects the structure of all macromolecules. It also affects the thermodynamics and kinetics of microbial respiration, which then helps to shape the composition and function of microbial communities. It controls the energy yields of common redox reactions in anoxic environments, such as syntrophic oxidation, iron reduction, sulphate reduction, and methanogenesis (Jin and Kirk 2018).
Activity of water
Availability of water directly affects the movement and growth of microorganisms (Rajbongshi and Gogoi 2020). The biodegradation rates of hydrocarbons in terrestrial ecosystems may be reduced because of the water available for the metabolism and growth of microbes.
Salinity
Salinity is one of the important factors which influence the activities of bioremediation and biodegradation as well as microbial growth and diversity. A higher concentration of salt inhibits microbial degradation. Hydrocarbon degradation in saline environments also depends upon the types of microorganisms (Rajbongshi and Gogoi 2020). Ward and Brock (1978), reported a decline in microbial metabolic rates with an increase in salinity level (Ward and Brock 1978). In high saline reservoirs, anaerobic and denitrifying bacteria are not the predominant species. Lin et al. (2014), mentioned that the content of halophilic bacteria was 30 % in an oil reservoir with salinity up to 10,000 mg/L (Lin et al. 2014). Achromobacter bacteria is one of the halophilic bacteria which can be found in high salinity oil reservoirs (Lin et al. 2014).
Oxygen availability
Aerobic treatment in the subsurface is difficult due to the limited availability of oxygen where its low solubility restricts not only the respiration process but also the degradation (Holliger et al. 1997). It is an important parameter for aerobic bacteria to activate and cleave the aromatic ring by the action of oxygenases but anaerobes cannot grow in the presence of oxygen. It is toxic for them and they must therefore depend on other substances such as electron acceptors (Hentges 2011). Wilkinson (1983), studied the prevention of growth rate of SRB in water and oil by aeration method. Eventually, this method reduced the growth rate of SRB in water but unfortunately, he found no way to prevent the formation of H2S in the oil storage system (Wilkinson 1983).
Nutrient availability
Nutrients play a vital role in successful biodegradation, including N, iron (Fe), and P in some cases which enhance the degradation process (Rajbongshi and Gogoi 2020). Other nutrients such as potassium (K), S, Cu, Zn, aluminum (Al), Co, and Ni are also necessary for the activities of the microorganisms (Mathir 2013). Carbon source is another factor that affects the growth of anaerobic microorganisms. The hydrocarbon compounds in the oil reservoirs can satisfy this nutrient requirement for biodegradation to CH4 and CO2. Whereas, dissolved CO2 is the carbon source for autotrophic methanogens (Mathir 2013). The addition of surplus nutrients can stimulate bacterial activities and increase the metabolism of products (Zhou et al. 2015). Atlas (1985), reported that the excess concentration of nutrients can inhibit the activity of biodegradation (Atlas 1985). Adequate contact time between microbial consortia and the substrate plays an important role in the biodegradation of hydrocarbons (Mathir 2013). SRB is generally recognized as a hazard in the production process of oilfields (Castro et al. 1997). It is responsible for H2S production and the ability of H2S to precipitate FeS that could plug the reservoir rock pores is of utmost concern. A study on SRB was carried out by Wilkinson (1983), where he observed that the growth rate of SRB in stagnant seawater and the production rate of H2S was 10 times greater when oil was added as a carbon source (Wilkinson 1983).
Permeability
Permeability plays an important role in the activity of microorganisms. High permeability viscous oil reservoirs after a long period of water injection resulted in a significant increase of microbial diversity by doubling the species and genera number of microorganisms (Lin et al. 2014).
Conclusions
Anaerobic microorganisms such as SRB, methanogens, NRB, and fermentative bacteria are widely distributed in oil reservoirs. They have become prominent in petroleum industries for their wide applications during exploration and production activities. Their major functions are oil degradation, viscosity alteration, reduction of IFT, wettability alteration, and remediation of toxic heavy metals. The review attributes to the diversity of these microorganisms in oil reservoirs, their applications in petroleum exploration and production activities, and growth in different environments. The study on SRB revealed their association with reservoir souring that causes reservoir plugging and corrosion of associated equipment. While the diversity of SRB showed that most of these belong to the taxonomical group of deltaproteobacteria, methanogens belonged to archaea and euryarchaeota. It is notable that with the MEOR process, the oil recovery rate could be as high as 90% while that from primary and secondary processes is only 10 % and 15–60 % respectively. The metabolites or biosurfactants produced from these microorganisms can reduce both ST and IFT between oil/gas or gas/liquid that will aid in the EOR process by increasing the oil mobility. NRB can remove sulphide existing from oilfield FW and also inhibit its production. The review gives a deep insight into the role of microorganisms in petroleum industries which would help researchers with in-depth knowledge on the role of microorganisms in oil field applications.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbas A, Al-Amer AM, Laoui T et al (2016) Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol 157:141–161
Aitken CM, Jones DM, Larter S (2004) Anaerobic hydrocarbon biodegradation in deep subsurface oil reservoirs. Nature 431:291–294
Al Zuhair S, El-Naas MH, Al Hassani H (2008) Sulfate inhibition effect on sulfate-reducing bacteria. J Biochem Technol 1:39–44
Al-Bahry S, Al-Wahaibi Y, Elsha A, Al-Bemani AS, Joshi SJ, Al-Makhmari HS, Al-Sulaimani HS (2013) Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int Biodeterior Biodegrad 81:141–146
Al-Hawash AB, Dragh MA, Li S et al (2018) Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquat Res 44:71–76
Al-Sulaimani H, Joshi S, Al-Wahaibi Y et al (2011) Microbial biotechnology for enhancing oil recovery: current developments and future prospects. Biotechnol Bioinf Bioeng 1:147–158
Anderson RT, Lovley DR (2000) Hexadecane decay by methanogenesis. Nature 404:722–723
Annweiler E, Materna A, Safinowski M et al (2000) Anaerobic degradation of 2-methylnaphthalene by a sulfate-reducing enrichment culture. Appl Environ Microbiol 66:5329–5333
Annweiler E, Michaelis W, Meckenstock RU (2002) Identical ring cleavage products during anaerobic degradation of naphthalene, 2-methylnaphthalene, and tetralin indicate a new metabolic pathway. Appl Environ Microbiol 68:852–858
Arora P, Kshirsagar P, Rana DP, Dhakephalkar P (2019) Hyperthermophilic Clostridium sp. N-4 produced a glycoprotein biosurfactant that enhanced recovery of residual oil at 96°C in lab studies. Colloids Surf B Biointerfaces 182:110372
Atlas RM (1985) Effects of hydrocarbons on microorganisms and petroleum biodegradation in arctic ecosystems. Pet Eff Arct Environ 63–100
Ayangbenro AS, Olanrewaju OS, Babalola OO (2018) Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front Microbiol 9:1986
Bahr M, Crump BC, Klepac-Ceraj V et al (2005) Molecular characterization of sulfate‐reducing bacteria in a New England salt marsh. Environ Microbiol 7:1175–1185
Ball HA, Reinhard M (1996) Monoaromatic hydrocarbon transformation under anaerobic conditions at Seal Beach, California: laboratory studies. Environ Toxicol Chem Int J 15:114–122
Banat IM (1993) The isolation of a thermophilic biosurfactant producing Bacillus sp. Biotechnol Lett 15:591–594
Banat IM, Franzetti A, Gandolfi I et al (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87:427–444
Barth T (1991) Organic acids and inorganic ions in waters from petroleum reservoirs, Norwegian continental shelf: a multivariate statistical analysis and comparison with American reservoir formation waters. Appl Geochem 6:1–15
Barton LL, Fauque GD (2009) Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv Appl Microbiol 68:41–98
Bass C (1999) ZoBell’s contribution to petroleum microbiology. In Proceedings of the 8th international symposium on microbial ecology. Microbial biosystems: New Frontiers. Atlantic Canada Society for Microbial Ecology, Halifax, Canada
Bass C, Sanders P, Lappin-Scott H (1998) Study of biofilms of sulfidogens from North Sea oil production facilities using continuous-flow apparatus. Geomicrobiol J 15:101–120
Basso O, Caumette P, Magot M (2005) Desulfovibrio putealis sp. nov., a novel sulfate-reducing bacterium isolated from a deep subsurface aquifer. Int J Syst Evol Microbiol 55:101–104
Bastin ES, Greer FE, Merritt C, Moulton G (1926) The presence of sulphate reducing bacteria in oil field waters. Science 63:21–24
Bedessem ME, Swoboda-Colberg NG, Colberg PJ (1997) Naphthalene mineralization coupled to sulfate reduction in aquifer-derived enrichments. FEMS Microbiol Lett 152:213–218
Beeder J, Nilsen RK, Rosnes JT et al (1994) Archaeoglobus fulgidus isolated from hot North Sea oil field waters. Appl Environ Microbiol 60:1227–1231
Beeder J, Torsvik T, Lien T (1995) Thermodesulforhabdus norvegicus gen. nov., sp. nov., a novel thermophilic sulfate-reducing bacterium from oil field water. Arch Microbiol 164:331–336
Belyakova E, Rozanova E, Borzenkov I et al (2006) The new facultatively chemolithoautotrophic, moderately halophilic, sulfate-reducing bacterium Desulfovermiculus halophilus gen. nov., sp. nov., isolated from an oil field. Microbiology 75:161–171
Berdugo-Clavijo C, Dong X, Soh J et al (2012) Methanogenic biodegradation of two-ringed polycyclic aromatic hydrocarbons. FEMS Microbiol Ecol 81:124–133
Berdugo-Clavijo C, Gieg LM (2014) Conversion of crude oil to methane by a microbial consortium enriched from oil reservoir production waters. Front Microbiol 5:197
Bernard F, Connan J, Magot M (1992) Indigenous microorganisms in connate water of many oil fields: a new tool in exploration and production techniques. Society of Petroleum Engineers
Bonch-Osmolovskaya EA, Miroshnichenko ML, Lebedinsky AV et al (2003) Radioisotopic, culture-based, and oligonucleotide microchip analyses of thermophilic microbial communities in a continental high-temperature petroleum reservoir. Appl Environ Microbiol 69:6143–6151
Bregnard T, Haner A, Hohener P, Zeyer J (1997) Anaerobic degradation of pristane in nitrate-reducing microcosms and enrichment cultures. Appl Environ Microbiol 63:2077–2081
Bregnard TP, Höhener P, Häner A, Zeyer J (1996) Degradation of weathered diesel fuel by microorganisms from a contaminated aquifer in aerobic and anaerobic microcosms. Environ Toxicol Chem Int J 15:299–307
Brouers F, Al-Musawi TJ (2015) On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J Mol Liq 212:46–51
Bryant R, Douglas J (1987) Evaluation of microbial systems in porous media for enhanced oil recovery. In SPE International symposium on oilfield chemistry, pp 449–456
Bryant RS (1987) Potential uses of microorganisms in petroleum recovery technology. Proceedings of the Oklahoma Academy of Science 67:97–104
Bryant RS, Burchfield TE, Dennis D, Hitzman D (1990) Microbial-enhanced waterflooding: Mink Unit project. SPE Reserv Eng 5:9–13
Burggraf S, Fricke H, Neuner A et al (1990a) Methanococcus igneus sp. nov., a novel hyperthermophilic methanogen from a shallow submarine hydrothermal system. Syst Appl Microbiol 13:263–269
Burggraf S, Jannasch HW, Nicolaus B, Stetter KO (1990b) Archaeoglobus profundus sp. nov., represents a new species within the sulfate-reducing archaebacteria. Syst Appl Microbiol 13:24–28
Burland SM, Edwards EA (1999) Anaerobic benzene biodegradation linked to nitrate reduction. Appl Environ Microbiol 65:529–533
Caldwell ME, Garrett RM, Prince RC, Suflita JM (1998) Anaerobic biodegradation of long-chain n-alkanes under sulfate-reducing conditions. Environ Sci Technol 32:2191–2195
Callbeck CM, Agrawal A, Voordouw G (2013) Acetate production from oil under sulfate-reducing conditions in bioreactors injected with sulfate and nitrate. Appl Environ Microbiol 79:5059–5068
Castro H, Teixeira P, Kirby R (1997) Evidence of membrane damage in Lactobacillus bulgaricus following freeze drying. J Appl Microbiol 82:87–94
Cayol J-L, Ollivier B, Patel B et al (1995) Description of Thermoanaerobacter brockii subsp. lactiethylicus subsp nov, isolated from a deep subsurface French oil well, a proposal to reclassify Thermoanaerobacter finnii as Thermoanaerobacter brockii subsp. finnii comb. nov., and an emended description of Thermoanaerobacter brockii. Int J Syst Evol Microbiol 45:783–789
Champagne CP, Mondou F, Raymond Y, Roy D (1996) Effect of polymers and storage temperature on the stability of freeze-dried lactic acid bacteria. Food Res Int 29:555–562
Chang W, Um Y, Holoman TRP (2006) Polycyclic aromatic hydrocarbon (PAH) degradation coupled to methanogenesis. Biotechnol Lett 28:425–430
Chen CI, Taylor R (1997) Thermophilic biodegradation of BTEX by two consortia of anaerobic bacteria. Appl Microbiol Biotechnol 48:121–128
Chen Q, Liu Z, Peng Q et al (2010) Diversity of halophilic and halotolerant bacteria isolated from non-saline soil collected from Xiaoxi National Natural Reserve, Hunan Province. Wei Sheng Wu Xue Bao 50:1452–1459
Christensen B, Torsvik T, Lien T (1992) Immunomagnetically captured thermophilic sulfate-reducing bacteria from North Sea oil field waters. Appl Environ Microbiol 58:1244–1248
Coates JD, Anderson RT, Lovley DR (1996) Oxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions. Appl Environ Microbiol 62:1099–1101
Cooney J, Silver S, Beck E (1985) Factors influencing hydrocarbon degradation in three freshwater lakes. Microb Ecol 11:127–137
Daghio M, Vaiopoulou E, Aulenta F et al (2018) Anode potential selection for sulfide removal in contaminated marine sediments. J Hazard Mater 360:498–503
Dang PN, Dang TCH, Lai TH, Stan-Lotter H (1996) Desulfovibrio vietnamensis sp. nov., a halophilic sulfate-reducing bacterium from Vietnamese oil fields. Anaerobe 2:385–392
Dar SA, Yao L, van Dongen U et al (2007) Analysis of diversity and activity of sulfate-reducing bacterial communities in sulfidogenic bioreactors using 16S rRNA and dsrB genes as molecular markers. Appl Environ Microbiol 73:594–604
Davey ME, Wood WA, KEy R et al (1993) Isolation of three species of Geotoga and Petrotoga: two new genera, representing a new lineage in the bacterial line of descent distantly related to the “Thermotogales”. Syst Appl Microbiol 16:191–200
Davidova I, Hicks M, Fedorak P, Suflita J (2001) The influence of nitrate on microbial processes in oil industry production waters. J Ind Microbiol Biotechnol 27:80–86
Davidova IA, Duncan KE, Choi OK, Suflita JM (2006) Desulfoglaeba alkanexedens gen. nov., sp. nov., an n-alkane-degrading, sulfate-reducing bacterium. Int J Syst Evol Microbiol 56:2737–2742
Devereux R, Kane MD, Winfrey J, Stahl DA (1992) Genus-and group-specific hybridization probes for determinative and environmental studies of sulfate-reducing bacteria. Syst Appl Microbiol 15:601–609
Dinh HT, Kuever J, Mußmann M et al (2004) Iron corrosion by novel anaerobic microorganisms. Nature 427:829–832
Doelle HW (2014) Bacterial metabolism. 2nd edition, Academic Press, New York
Donaldson EC, Chilingarian GV, Yen TF (1989) Microbial enhanced oil recovery. Developments in petroleum science 22:1–227
Edwards E, Wills L, Reinhard M, Grbić-Galić D (1992) Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Appl Environ Microbiol 58:794–800
Edwards EA, Grbić-Galić D (1994) Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl Environ Microbiol 60:313–322
Ehrenreich P, Behrends A, Harder J, Widdel F (2000) Anaerobic oxidation of alkanes by newly isolated denitrifying bacteria. Arch Microbiol 173:58–64
Elferink SJO, Maas RN, Harmsen HJ, Stams AJ (1995) Desulforhabdus amnigenus gen. nov. sp. nov., a sulfate reducer isolated from anaerobic granular sludge. Arch Microbiol 164:119–124
Elias DA, Krumholz LR, Tanner RS, Suflita JM (1999) Estimation of methanogen biomass by quantitation of coenzyme M. Appl Environ Microbiol 65:5541–5545
Elshahed MS, McInerney MJ (2001) Is interspecies hydrogen transfer needed for toluene degradation under sulfate-reducing conditions? FEMS Microbiol Ecol 35:163–169
Ensley BD, Suflita JM (1995) Metabolism of environmental contaminants by mixed and pure cultures of sulfate-reducing bacteria. In: Sulfate-reducing bacteria. Springer, pp 293–332
Fardeau M-L, Goulhen F, Bruschi M et al (2009) Archaeoglobus fulgidus and Thermotoga elfii, thermophilic isolates from deep geothermal water of the Paris Basin. Geomicrobiol J 26:119–130
Fardeau M-L, Ollivier B, Patel B et al (1997) Thermotoga hypogea sp. nov., a xylanolytic, thermophilic bacterium from an oil-producing well. Int J Syst Evol Microbiol 47:1013–1019
Fardeau M-L, Salinas MB, l’Haridon S, et al (2004) Isolation from oil reservoirs of novel thermophilic anaerobes phylogenetically related to Thermoanaerobacter subterraneus: reassignment of T subterraneus, Thermoanaerobacter yonseiensis, Thermoanaerobacter tengcongensis and Carboxydibrachium pacificum to Caldanaerobacter subterraneus gen. nov., sp. nov., comb. nov. as four novel subspecies. Int J Syst Evol Microbiol 54:467–474
Feio MJ, Zinkevich V, Beech IB et al (2004) Desulfovibrio alaskensis sp. nov., a sulphate-reducing bacterium from a soured oil reservoir. Int J Syst Evol Microbiol 54:1747–1752
Fiala G, Stetter KO, Jannasch HW et al (1986) Staphylothermus marinus sp. nov. represents a novel genus of extremely thermophilic submarine heterotrophic archaebacteria growing up to 98 C. Syst Appl Microbiol 8:106–113
Fischer F, Zillig W, Stetter K, Schreiber G (1983) Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature 301:511–513
Fullerton H, Crawford M, Bakenne A et al (2013) Anaerobic oxidation of ethene coupled to sulfate reduction in microcosms and enrichment cultures. Environ Sci Technol 47:12374–12381
Galushko A, Rozanova E (1991) Desulfobacterium cetonicum sp. nov., a sulfate-reducing bacterium which oxidizes fatty acids and ketones. Microbiol Mikrobiol 60:742–746
Ghosh B, Al Shalabi E (2011) Solvent induced oil viscosity reduction and its effect on waterflood recovery efficiency. Adv Pet Explor Dev 2:24–31
Gieg LM, Davidova IA, Duncan KE, Suflita JM (2010) Methanogenesis, sulfate reduction and crude oil biodegradation in hot Alaskan oilfields. Environ Microbiol 12:3074–3086
Godsy EM, Goerlitz DF, Grbic-Galic D (1992) Methanogenic biodegradation of creosote contaminants in natural and simulated ground‐water ecosystems. Groundwater 30:232–242
Grabowski A, Nercessian O, Fayolle F et al (2005) Microbial diversity in production waters of a low-temperature biodegraded oil reservoir. FEMS Microbiol Ecol 54:427–443
Grbić-Galić D, Vogel TM (1987) Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol 53:254–260
Greene AC, Patel BK, Sheehy AJ (1997) Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese-and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Evol Microbiol 47:505–509
Grishchenkov V, Townsend R, McDonald T et al (2000) Degradation of petroleum hydrocarbons by facultative anaerobic bacteria under aerobic and anaerobic conditions. Process Biochem 35:889–896
Grossi V, Cravo-Laureau C, Méou A et al (2007) Anaerobic 1-alkene metabolism by the alkane-and alkene-degrading sulfate reducer Desulfatibacillum aliphaticivorans strain CV2803T. Appl Environ Microbiol 73:7882–7890
Haner A, Hohener P, Zeyer J (1997) Degradation of Trimethylbenzene Isomers by an Enrichment Culture under N (inf2) O-Reducing Conditions. Appl Environ Microbiol 63:1171–1174
Haridon S, Miroshnichenko M, Hippe H et al (2001) Thermosipho geolei sp. nov., a thermophilic bacterium isolated from a continental petroleum reservoir in Western Siberia. Int J Syst Evol Microbiol 51:1327–1334
Head IM, Jones DM, Larter SR (2003) Biological activity in the deep subsurface and the origin of heavy oil. Nature 426:344–352
Hentges DJ (2011) Anaerobes: general characteristics. In: Medical Microbiology, 4th edn. University of Texas Medical Branch at Galveston, Galveston (TX)
Herath A, Wawrik B, Qin Y et al (2016) Transcriptional response of Desulfatibacillum alkenivorans AK-01 to growth on alkanes: insights from RT-qPCR and microarray analyses. FEMS Microbiol Ecol 92:fiw062
Hines ME, Evans RS, Genthner BRS et al (1999) Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol 65:2209–2216
Holliger C, Gaspard S, Glod G et al (1997) Contaminated environments in the subsurface and bioremediation: organic contaminants. FEMS Microbiol Rev 20:517–523
Holmes D, Smith J (2016) Biologically produced methane as a renewable energy source. Adv Appl Microbiol 97:1–61
Homayuni F, Hamidi A, Vatani A et al (2011) Viscosity reduction of heavy and extra heavy crude oils by pulsed electric field. Pet Sci Technol 29:2052–2060
Huber R, Langworthy TA, König H et al (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90oC. Arch Microbiol 144:324–333
Huber R, Woese C, Langworthy TA et al (1989) Thermosipho africanus gen. nov., represents a new genus of thermophilic eubacteria within the “Thermotogales”. Syst Appl Microbiol 12:32–37
Hussain A, Hasan A, Javid A, Qazi JI (2016) Exploited application of sulfate-reducing bacteria for concomitant treatment of metallic and non-metallic wastes: a mini review. 3 Biotech 6:1–10
Jeanthon C, l’Haridon S, Cueff V et al (2002) Thermodesulfobacterium hydrogeniphilum sp. nov., a thermophilic, chemolithoautotrophic, sulfate-reducing bacterium isolated from a deep-sea hydrothermal vent at Guaymas Basin, and emendation of the genus Thermodesulfobacterium. Int J Syst Evol Microbiol 52:765–772
Jeanthon C, Reysenbach A-L, l’Haridon S et al (1995) Thermotoga subterranea sp. nov., a new thermophilic bacterium isolated from a continental oil reservoir. Arch Microbiol 164:91–97
Jenneman G, Gevertz D, Wright M (1996) Sulfide bioscavenging of sour produced water by natural microbial populations. In Proceedings of the 3rd International petroleum environmental conference, pp 693–704
Jin Q, Kirk MF (2018) pH as a primary control in environmental microbiology: 1. thermodynamic perspective. Front Environ Sci 6:21
Jones D, Head I, Gray N et al (2008) Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 451:176–180
Jones W, Leigh J, Mayer F et al (1983) Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol 136:254–261
Karr EA, Sattley WM, Rice MR et al (2005) Diversity and distribution of sulfate-reducing bacteria in permanently frozen Lake Fryxell, McMurdo Dry Valleys, Antarctica. Appl Environ Microbiol 71:6353–6359
Kazumi J, Caldwell M, Suflita J et al (1997) Anaerobic degradation of benzene in diverse anoxic environments. Environ Sci Technol 31:813–818
Keenleyside W (2019) Microbiology: Canadian Edition. Simple Book Publishing, New York, NY
Kleindienst S, Herbst F-A, Stagars M et al (2014) Diverse sulfate-reducing bacteria of the Desulfosarcina/Desulfococcus clade are the key alkane degraders at marine seeps. ISME J 8:2029–2044
Kniemeyer O, Musat F, Sievert SM et al (2007) Anaerobic oxidation of short-chain hydrocarbons by marine sulphate-reducing bacteria. Nature 449:898–901
Knittel K, Boetius A, Lemke A et al (2003) Activity, distribution, and diversity of sulfate reducers and other bacteria in sediments above gas hydrate (Cascadia Margin, Oregon). Geomicrobiol J 20:269–294
Kovacik WP Jr, Takai K, Mormile MR et al (2006) Molecular analysis of deep subsurface Cretaceous rock indicates abundant Fe (III)-and S°‐reducing bacteria in a sulfate‐rich environment. Environ Microbiol 8:141–155
Kryachko Y, Dong X, Sensen CW, Voordouw G (2012) Compositions of microbial communities associated with oil and water in a mesothermic oil field. Antonie Van Leeuwenhoek 101:493–506
Kurr M, Huber R, König H et al (1991) Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110oC. Arch Microbiol 156:239–247
l’Haridon S, Miroshnichenko M, Hippe H et al (2002) Petrotoga olearia sp. nov. and Petrotoga sibirica sp. nov., two thermophilic bacteria isolated from a continental petroleum reservoir in Western Siberia. Int J Syst Evol Microbiol 52:1715–1722
l’Haridon S, Reysenbacht A-L, Glenat P et al (1995) Hot subterranean biosphere in a continental oil reservoir. Nature 377:223–224
Langenhoff AA, Brouwers-Ceiler DL, Engelberting JH et al (1997) Microbial reduction of manganese coupled to toluene oxidation. FEMS Microbiol Ecol 22:119–127
Lazar I, Petrisor I, Yen T (2007) Microbial enhanced oil recovery MEOR. Pet Sci Technol 25:1353–1366
Leu J, McGovern-Traa C, Porter A, Hamilton W (1999) The same species of sulphate‐reducing Desulfomicrobium occur in different oil field environments in the North Sea. Lett Appl Microbiol 29:246–252
Li D, Midgley DJ, Ross JP et al (2012) Microbial biodiversity in a Malaysian oil field and a systematic comparison with oil reservoirs worldwide. Arch Microbiol 194:513–523
Li G, McInerney MJ (2017) Use of Biosurfactants in Oil Recovery. In: Lee SY (ed) Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals. Springer International Publishing, Cham, pp 1–16
Li H, Lai R, Jin Y et al (2020) Directional culture of petroleum hydrocarbon degrading bacteria for enhancing crude oil recovery. J Hazard Mater 390:122160
Li H, Yang S-Z, Mu B-Z et al (2007) Molecular phylogenetic diversity of the microbial community associated with a high-temperature petroleum reservoir at an offshore oilfield. FEMS Microbiol Ecol 60:74–84
Lien T, Beeder J (1997) Desulfobacter vibrioformis sp. nov., a sulfate reducer from a water-oil separation system. Int J Syst Evol Microbiol 47:1124–1128
Lien T, Madsen M, Steen IH, Gjerdevik K (1998) Desulfobulbus rhabdoformis sp. nov., a sulfate reducer from a water-oil separation system. Int J Syst Evol Microbiol 48:469–474
Lin J, Hao B, Cao G et al (2014) A study on the microbial community structure in oil reservoirs developed by water flooding. J Pet Sci Eng 122:354–359
Magot M, Basso O, Tardy-Jacquenod C, Caumette P (2004) Desulfovibrio bastinii sp. nov. and Desulfovibrio gracilis sp. nov., moderately halophilic, sulfate-reducing bacteria isolated from deep subsurface oilfield water. Int J Syst Evol Microbiol 54:1693–1697
Magot M, Caumette P, Desperrier J et al (1992) Desulfovibrio longus sp. nov., a sulfate-reducing bacterium isolated from an oil-producing well. Int J Syst Evol Microbiol 42:398–402
Magot M, Ollivier B, Patel BK (2000) Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 77:103–116
Mathir A (2013) Analysis of nutrient requirements for the anaerobic digestion of Fischer-Tropsch reaction water. PhD Thesis, University of Kwazulu-Natal
Mayilraj S, Kaksonen AH, Cord-Ruwisch R et al (2009) Desulfonauticus autotrophicus sp. nov., a novel thermophilic sulfate-reducing bacterium isolated from oil-production water and emended description of the genus Desulfonauticus. Extremophiles 13:247–255
Mayumi D, Mochimaru H, Yoshioka H et al (2011) Evidence for syntrophic acetate oxidation coupled to hydrogenotrophic methanogenesis in the high-temperature petroleum reservoir of Yabase oil field (Japan). Environ Microbiol 13:1995–2006
Meckenstock RU, Annweiler E, Michaelis W et al (2000) Anaerobic naphthalene degradation by a sulfate-reducing enrichment culture. Appl Environ Microbiol 66:2743–2747
Meyer RF (1987) Exploration for heavy crude oil and natural bitumen. United States
Miller J, Wakerley D (1966) Growth of sulphate-reducing bacteria by fumarate dismutation. Microbiology 43:101–107
Miranda-Tello E, Fardeau M-L, Sepulveda J et al (2003) Garciella nitratireducens gen. nov., sp. nov., an anaerobic, thermophilic, nitrate-and thiosulfate-reducing bacterium isolated from an oilfield separator in the Gulf of Mexico. J Med Microbiol 53:1509–1514
Miroshnichenko M, Bonch-Osmolovskaya E, Neuner A et al (1989) Thermococcus stetteri sp. nov., a new extremely thermophilic marine sulfur-metabolizing archaebacterium. Syst Appl Microbiol 12:257–262
Mußmann M, Ishii K, Rabus R, Amann R (2005) Diversity and vertical distribution of cultured and uncultured Deltaproteobacteria in an intertidal mud flat of the Wadden Sea. Environ Microbiol 7:405–418
Muyzer G, Stams AJ (2008) The ecology and biotechnology of sulphate-reducing bacteria. Nat Rev Microbiol 6:441–454
Myhr S, Torsvik T (2000) Denitrovibrio acetiphilus, a novel genus and species of dissimilatory nitrate-reducing bacterium isolated from an oil reservoir model column. Int J Syst Evol Microbiol 50:1611–1619
Nazina T, Ivanova A, Kanchaveli L, Rozanova E (1988) Desulfotomaculum kuznetsovii sp. nov., a new spore-forming, thermophilic, methylotrophic, sulfate-reducing bacterium. Mikrobiologiya 57:823–827
Nazina T, Rozanova E (1978) Thermophillic sulfate-reducing bacteria from oil-bearing strata. Mikrobiologiia 47:142–148
Nazina T, Sokolova DS, Shestakova N et al (2005) The phylogenetic diversity of aerobic organotrophic bacteria from the Dagang high-temperature oil field. Microbiology 74:343–351
Nazina TN, Shestakova NM, Semenova EM et al (2017) Diversity of metabolically active bacteria in water-flooded high-temperature heavy oil reservoir. Front Microbiol 8:707
Neuner A, Jannasch HW, Belkin S, Stetter KO (1990) Thermococcus litoralis sp. nov.: a new species of extremely thermophilic marine archaebacteria. Arch Microbiol 153:205–207
Nikolova C, Gutierrez T (2020) Use of microorganisms in the recovery of oil from recalcitrant oil reservoirs: Current state of knowledge, technological advances and future perspectives. Front Microbiol 10:2996
Nilsen RK, Beeder J, Thorstenson T, Torsvik T (1996) Distribution of thermophilic marine sulfate reducers in north sea oil field waters and oil reservoirs. Appl Environ Microbiol 62:1793–1798
Nilsen RK, Torsvik T (1996a) Methanococcus thermolithotrophicus isolated from North Sea oil field reservoir water. Appl Environ Microbiol 62:728–731
Nilsen RK, Torsvik T, Lien T (1996b) Desulfotomaculum thermocisternum sp. nov., a sulfate reducer isolated from a hot North Sea oil reservoir. Int J Syst Evol Microbiol 46:397–402
Novelli G (1944) Assimilation of petroleum hydrocarbons by sulfate-reducing bacteria. J Bacteriol 7:47–48
Ollivier B, Cayol J (2005) Fermentative, iron-reducing, and nitrate‐reducing microorganisms. Pet Microbiol 71–88
Ommedal H, Torsvik T (2007) Desulfotignum toluenicum sp. nov., a novel toluene-degrading, sulphate-reducing bacterium isolated from an oil-reservoir model column. Int J Syst Evol Microbiol 57:2865–2869
Orphan V, Taylor L, Hafenbradl D, Delong E (2000) Culture-dependent and culture-independent characterization of microbial assemblages associated with high-temperature petroleum reservoirs. Appl Environ Microbiol 66:700–711
Pannekens M, Kroll L, Müller H et al (2019) Oil reservoirs, an exceptional habitat for microorganisms. New Biotechnol 49:1–9
Pannekens M, Voskuhl L, Meier A et al (2020) Densely populated water droplets in heavy-oil seeps. Appl Environ Microbiol. https://doi.org/10.1128/AEM.00164-20
Parthipan P, Elumalai P, Karthikeyan OP et al (2017) A review on biodegradation of hydrocarbon and their influence on corrosion of carbon steel with special reference to petroleum industry. J Environ Biotechnol Res 6:12–33
Peixoto R, Vermelho A, Rosado A (2011) Petroleum-degrading enzymes: bioremediation and new prospects. Enzyme Res. https://doi.org/10.4061/2011/475193
Pham VD, Hnatow LL, Zhang S et al (2009) Characterizing microbial diversity in production water from an Alaskan mesothermic petroleum reservoir with two independent molecular methods. Environ Microbiol 11:176–187
Phelps CD, Young L (1999) Anaerobic biodegradation of BTEX and gasoline in various aquatic sediments. Biodegradation 10:15–25
Philippi G (1977) On the depth, time and mechanism of origin of the heavy to medium-gravity naphthenic crude oils. Geochim Cosmochim Acta 41:33–52
Pley U, Schipka J, Gambacorta A et al (1991) Pyrodictium abyssi sp. nov. represents a novel heterotrophic marine archaeal hyperthermophile growing at 110oC. Syst Appl Microbiol 14:245–253
Plugge CM, Zhang W, Scholten J, Stams AJ (2011) Metabolic flexibility of sulfate-reducing bacteria. Front Microbiol 2:81
Putra W, Hakiki F (2019) Microbial enhanced oil recovery: interfacial tension and biosurfactant-bacteria growth. J Pet Explor Prod Technol 9:2353–2374
Qian Y, Xu M, Deng T et al (2021) Synergistic interactions of Desulfovibrio and Petrimonas for sulfate-reduction coupling polycyclic aromatic hydrocarbon degradation. J Hazard Mater 407:124385
Rajbongshi A, Gogoi SB (2020) A Review of Microbial Degradation of Petroleum Hydrocarbons. Adv Pet Technol 309–318
Ravenschlag K, Sahm K, Knoblauch C et al (2000) Community structure, cellular rRNA content, and activity of sulfate-reducing bacteria in marine Arctic sediments. Appl Environ Microbiol 66:3592–3602
Ravot G, Magot M, Fardeau M-L et al (1995) Thermotoga elfii sp. nov., a novel thermophilic bacterium from an African oil-producing well. Int J Syst Evol Microbiol 45:308–314
Rees GN, Grassia GS, Sheehy AJ et al (1995) Desulfacinum infernum gen. nov., sp. nov., a thermophilic sulfate-reducing bacterium from a petroleum reservoir. Int J Syst Evol Microbiol 45:85–89
Rees GN, Patel BK, Grassia GS, Sheehy AJ (1997) Anaerobaculum thermoterrenum gen. nov., sp. nov., a novel, thermophilic bacterium which ferments citrate. Int J Syst Evol Microbiol 47:150–154
Risatti J, Capman W, Stahl D (1994) Community structure of a microbial mat: the phylogenetic dimension. Proc Natl Acad Sci 91:10173–10177
Rocha CG, Zaia DAM, da Silva Alfaya RV, da Silva Alfaya AA (2009) Use of rice straw as biosorbent for removal of Cu (II), Zn (II), Cd (II) and Hg (II) ions in industrial effluents. J Hazard Mater 166:383–388
Ron EZ, Rosenberg E (2002) Biosurfactants and oil bioremediation. Curr Opin Biotechnol 13:249–252
Rooney-Varga JN, Anderson RT, Fraga JL et al (1999) Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl Environ Microbiol 65:3056–3063
Rosnes JT, Torsvik T, Lien T (1991) Spore-forming thermophilic sulfate-reducing bacteria isolated from North Sea oil field waters. Appl Environ Microbiol 57:2302–2307
Rozanova E, Nazina T (1979) Occurrence of thermophilic sulfate-reducing bacteria in oil-bearing strata of Apsheron and Western Siberia. Microbiology 48:907–911
Rozanova E, Tourova T, Kolganova T et al (2001) Desulfacinum subterraneumsp. Nov., a new thermophilic sulfate-reducing bacterium isolated from a high-temperature oil field. Microbiology 70:466–471
Sarkar A, Goursaud J, Sharma M, Georgiou G (1989) A critical evaluation of MEOR processes. situ 13:207–238
Sass H, Wieringa E, Cypionka H et al (1998) High genetic and physiological diversity of sulfate-reducing bacteria isolated from an oligotrophic lake sediment. Arch Microbiol 170:243–251
Sen R (2008) Biotechnology in petroleum recovery: the microbial EOR. Prog Energy Combust Sci 34:714–724
Shelton JL, Akob DM, McIntosh JC et al (2016) Environmental drivers of differences in microbial community structure in crude oil reservoirs across a methanogenic gradient. Front Microbiol 7:1535
Sierra-Garcia IN, de Oliveira VM (2013) Microbial hydrocarbon degradation: efforts to understand biodegradation in petroleum reservoirs. Biodegrad-Eng Technol 10:55920
Silva T, Verde L, Neto ES, Oliveira V (2013) Diversity analyses of microbial communities in petroleum samples from Brazilian oil fields. Int Biodeterior Biodegrad 81:57–70
Singer M, Finnerty W (1988) Construction of an Escherichia coli-Rhodococcus shuttle vector and plasmid transformation in Rhodococcus spp. J Bacteriol 170:638–645
Singh A, Van Hamme JD, Ward OP (2007) Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol Adv 25:99–121
So CM, Young LY (2001) Anaerobic biodegradation of alkanes by enriched consortia under four different reducing conditions. Environ Toxicol Chem Int J 20:473–478
Song H, Luo J, Wang Y et al (2010) Application of microbial oil recovery technology in Zichang Oilfield . J Xian Shiyou Univ Nat Sci Ed 4
Song W, Ma D, Zhu Y et al (2014) The role of sulphate-reducing bacteria in oil recovery. Int J Curr Microbiol Appl Sci 7:385–398
Stadnitskaia A, Muyzer G, Abbas B et al (2005) Biomarker and 16S rDNA evidence for anaerobic oxidation of methane and related carbonate precipitation in deep-sea mud volcanoes of the Sorokin Trough, Black Sea. Mar Geol 217:67–96
Stetter KO, Huber R, Blöchl E et al (1993) Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 365:743–745
Stetter KO, König H, Stackebrandt E (1983) Pyrodictium gen. nov., a new genus of submarine disc-shaped sulphur reducing archaebacteria growing optimally at 105oC. Syst Appl Microbiol 4:535–551
Sublette K, McInerney MJ, Montgomery AD, Bhupathiraju V (1994) Microbial oxidation of sulfides by Thiobacillus denitrificans for treatment of sour water and sour gases. Environmental Geochemistry of Sulfide Oxidation, ACS, pp 68–78
Suthar H, Hingurao K, Desai A, Nerurkar A (2008) Evaluation of bioemulsifier mediated microbial enhanced oil recovery using sand pack column. J Microbiol Methods 75:225–230
Svetlichnyj V, Slesarev A, Svetlichnaya T, Zavarzin G (1987) Caldococcus litoralis gen. nov. sp. nov., une nouvelle bactérie marine extrêmement thermophile réduisant le soufre élémentaire. Mikrobiol Mosk 56:831–838
Takahata Y, Hoaki T, Maruyama T (2001) Starvation survivability of Thermococcus strains isolated from Japanese oil reservoirs. Arch Microbiol 176:264–270
Tardy-Jacquenod C, Caumette P, Matheron R et al (1996a) Characterization of sulfate-reducing bacteria isolated from oil-field waters. Can J Microbiol 42:259–266
Tardy-Jacquenod C, Magot M, Laigret F et al (1996b) Desulfovibrio gabonensis sp. nov., a new moderately halophilic sulfate-reducing bacterium isolated from an oil pipeline. Int J Syst Evol Microbiol 46:710–715
Tardy-Jacquenod C, Magot M, Patel B et al (1998) Desulfotomaculum halophilum sp. nov., a halophilic sulfate-reducing bacterium isolated from oil production facilities. Int J Syst Evol Microbiol 48:333–338
Telang AJ, Jenneman GE, Voordouw G (1999) Sulfur cycling in mixed cultures of sulfide-oxidizing and sulfate-or sulfur-reducing oil field bacteria. Can J Microbiol 45:905–913
Tobias C, Neubauer SC (2019) Salt marsh biogeochemistry—an overview. Coast Wetl 539–596
Townsend GT, Prince RC, Suflita JM (2003) Anaerobic oxidation of crude oil hydrocarbons by the resident microorganisms of a contaminated anoxic aquifer. Environ Sci Technol 37:5213–5218
Uchiyama T, Ito K, Mori K et al (2010) Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76:1783–1788
Ulrich AC, Edwards EA (2003) Physiological and molecular characterization of anaerobic benzene-degrading mixed cultures. Environ Microbiol 5:92–102
Van Hamme JD, Singh A, Ward OP (2003) Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67:503–549
Wang LY, Duan RY, Liu JF et al (2012) Molecular analysis of the microbial community structures in water-flooding petroleum reservoirs with different temperatures. Biogeosciences 9:4645–4659
Wang Q, Jiang Y, Wang H et al (2020) Isolation and characterization of a marine bacterium Vibrio diabolicus strain L2-2 capable of biotransforming sulfonamides. Environ Res 188:109718
Ward DM, Brock T (1978) Hydrocarbon biodegradation in hypersaline environments. Appl Environ Microbiol 35:353–359
Watanabe K, Kodama Y, Kaku N (2002) Diversity and abundance of bacteria in an underground oil-storage cavity. Bmc Microbiol 2:1–10
Webster G, Watt LC, Rinna J et al (2006) A comparison of stable-isotope probing of DNA and phospholipid fatty acids to study prokaryotic functional diversity in sulfate‐reducing marine sediment enrichment slurries. Environ Microbiol 8:1575–1589
Widdel F, Knittel K, Galushko A (2010) Anaerobic hydrocarbon-degrading microorganisms: an overview. Handb Hydrocarb Lipid Microbiol, pp 1998–2022
Wilkinson T (1983) Offshore monitoring. Microb Corros 117–122
Windberger E, Huber R, Trincone A et al (1989) Thermotoga thermarum sp. nov. and Thermotoga neapolitana occurring in African continental solfataric springs. Arch Microbiol 151:506–512
Xiu J, Yu L, Zheng C (2010) Application of microbial community structure analysis in microbial enhanced oil recovery. Oil-Gas Field Surf Eng 29:48–50
Yen T (1986) State of the Art Review on Microbial Enhanced Oil Recovery. Univ South Calif NSF OIR–8405134 Los Angel Calif
Yin K, Wang Q, Lv M, Chen L (2019) Microorganism remediation strategies towards heavy metals. Chem Eng J 360:1553–1563
Youssef N, Elshahed MS, McInerney MJ (2009) Microbial processes in oil fields: culprits, problems, and opportunities. Adv Appl Microbiol 66:141–251
Zajic JE, Cooper D, Kosaric N (1983) Microbial enhanced oil recovery. United States
Zengler K, Richnow HH, Rosselló-Mora R et al (1999) Methane formation from long-chain alkanes by anaerobic microorganisms. Nature 401:266–269
Zhang D, Cui L, Madani R et al (2019) Effect of nitrite and nitrate on sulfate reducing ammonium oxidation. Water Sci Technol 80:634–643
Zhang S, Wang Q, Xie S (2012) Stable isotope probing identifies anthracene degraders under methanogenic conditions. Biodegradation 23:221–230
Zhang X, Young L (1997) Carboxylation as an initial reaction in the anaerobic metabolism of naphthalene and phenanthrene by sulfidogenic consortia. Appl Environ Microbiol 63:4759–4764
Zhao G, Sheng Y, Wang C et al (2018) In situ microbial remediation of crude oil-soaked marine sediments using zeolite carrier with a polymer coating. Mar Pollut Bull 129:172–178
Zhao H, Wood AG, Widdel F, Bryant MP (1988) An extremely thermophilic Methanococcus from a deep sea hydrothermal vent and its plasmid. Arch Microbiol 150:178–183
Zhao Y, Zhang H, Boone DR, Mah RA (1986) Isolation and characterization of a fast-growing, thermophilic Methanobacterium species. Appl Environ Microbiol 52:1227–1229
Zhou W, Wu B, Dong X, He S (2015) Effect of Nutrient Supply on the Production of Soluble Microbial Products (SMP) in Anaerobic Reactors. Fermentol Techno 1:2
Zillig W, Holz I, Janekovic D et al (1983) The archaebacterium Thermococcus celer represents, a novel genus within the thermophilic branch of the archaebacteria. Syst Appl Microbiol 4:88–94
Zillig W, Holz I, Klenk H-P et al (1987) Pyrococcus woesei, sp. nov., an ultra-thermophilic marine archaebacterium, representing a novel order, Thermococcales. Syst Appl Microbiol 9:62–70
Zobell CE (1946) Bacteriological process for treatment of fluid-bearing earth formations. United States Patent Office, No. 2,413,278
Funding
This work was funded by the Department of Biotechnology, Government of India in the form of Joint R&D activities under the twinning programme for scientists working in Dibrugarh University and IIT-Kharagpur in the form of a Twinning project no. DBT ENV/2013/222 (Twin 2013).
Author information
Authors and Affiliations
Contributions
Conceptualization: AR, SBG; Methodology: SBG, AR; Formal analysis and investigation: SBG, AR; Writing - original draft preparation: AR; Writing - review and editing: AR, SBG; Funding acquisition: SBG; Resources: AR; Supervision: SBG
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajbongshi, A., Gogoi, S.B. A review on anaerobic microorganisms isolated from oil reservoirs. World J Microbiol Biotechnol 37, 111 (2021). https://doi.org/10.1007/s11274-021-03080-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03080-9