Abstract
As a significant constituent in biosphere, bacteria have a great influence on human activity. The detection of pathogen bacteria is closely related to the human health. However, the traditional methods for detection of pathogenic bacteria are time-consuming and difficult for quantification, although they are practical and reliable. Therefore, novel strategies for rapid, sensitive, and cost-effective detection are in great demand. Aptamer is a kind of oligonucleotide that selected by repeated screening in vitro or systematic evolution of ligands by exponential enrichment (SELEX) technology. Over the past years, owing to high affinity and specificity of aptamers, a variety of aptamer-based biosensors have been designed and applied for pathogen detection. In this review, we have discussed the recent advances on the applications of aptamer-based biosensors in detection of pathogenic bacteria. In addition, we also point out some problems in current methods and look forward to the further development of aptamer-based biosensors for pathogen detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria are single-celled microscopic organisms that are widely spread in nature. They have significant effects on human life—they are used in industrial and medicinal processes, participate in natural material cycles, and can be applied in bioremediation of polluted environments. However, some of them are pathogenic, causing infectious diseases transmitted through food, water, aerosols, and in other ways (Newell et al. 2010; Scallan et al. 2011).
At present, the diagnosis and prevention of bacterial infectious diseases mainly relies on laboratory detection. Common methods for detection of pathogenic bacteria include bacterial culture, immunological methods, and molecular biological methods (Labib and Berezovski 2014; Torres-Chavolla and Alocilja 2009). Traditional culture is the gold standard for bacterial identification. However, culture techniques require specific equipment and professional operators and are time-consuming, making widespread and non-specialist applications difficult (Rajapaksha et al. 2019). Compared with bacterial culture, immunological methods, for example, enzyme-linked immunosorbent assay, are faster, but the complicated operations and the quality of antibodies can influence the experimental results. Molecular biological methods such as polymerase chain reaction (PCR) are highly-sensitive, but may generate a false-positive signal due to many PCR inhibitors in complex samples (e.g., food samples) and any pollution of samples (Levi et al. 2003). Moreover, PCR and PCR-based methods are incapable of discriminating live bacteria from dead ones, which may be a potential shortcoming to limit the future development of PCR (Wu et al. 2020b). Therefore, it is important to develop rapid, accurate, and portable/on-site testing methods for detection of pathogenic bacteria, which is of great significance for public health and clinical diagnosis.
Aptamer is a bioaffinity ligands that can specifically bind to the target, which can be divided into peptide aptamer and oligonucleotide aptamer. The peptide aptamer is a combinatorial protein reagent and its target so far has been focused on protein only. The peptide aptamer of the target protein was commonly selected with traditional yeast two-hybrid system. The peptide aptamer can bind its targets in living cells, resulting a profound influences in functional study within cells (Acquah et al. 2020; Li et al. 2011). Oligonucleotide aptamer is a single-stranded DNA or RNA sequence selected by systematic evolution of ligands by exponential enrichment (SELEX) techniques, also called “chemical antibodies” because of their high affinity to its targets. Generally, aptamer always refer to oligonucleotide one. Compared with natural antibodies, aptamers have some obvious advantages (Table 1). First, aptamers have higher surface density and cause less steric blockage, which helps to improve the efficiency of combination with targets (Lee et al. 2010). Second, their structure allows aptamers to maintain stability at room temperature and in ordinary storage conditions, while natural antibodies need strict storage conditions and are sensitive to environmental change. Third, aptamers can be chemically synthesized, produced, and purified according to strict protocols, which can save money or time in production and decrease the differences between each batch (Ray and White 2010). The obtained aptamers even can be further modified and optimized as needed. Fourth, aptamers can bind a wide range of targets, while natural antibodies can only bind with typical immunogenic macromolecules (Zhuo et al. 2017). Aptamer can specifically bind with analyte such as metal ions (Li et al. 2019b; Liu et al. 2018a), amino acids (Idili et al. 2019; Yuan et al. 2018), nucleotides (Ji et al. 2017), small organic molecules (Liu et al. 2019a; Qiu et al. 2018), peptides (Wu et al. 2019), toxins (Frohnmeyer et al. 2019; Sun and Zhao 2018), enzymes (Chen et al. 2016), other proteins (Lee and Zeng 2017), and even cells and bacteria (Sun et al. 2019a; Wu et al. 2014) with high affinity (Hermann and Patel 2000). Therefore, aptamers have been widely used as excellent molecular recognizers, and aptamer-based biosensors and bioassay methods for bacterial detection are expected to replace traditional methods, making the detection more rapid, sensitive, specific, and reliable.
Biosensors are devices that detect an analyte in biological or chemical reactions by generating signals proportional to the analyte concentrations. It can detect analyte at the concentration of ng/ml or even fg/ml level. Biosensors can be divided into different types on the basis of the signal that is generated, such as electricity, heat, or light. Typical biosensors consist of the analyte, bioreceptor, transducer, electronics, and display (Bhalla et al. 2016) (Fig. 1). In this review, we briefly introduce some typical aptamer-based biosensors for detection of bacteria, most of which have adopted optical, electrochemical, or surface-enhanced Raman scattering (SERS) signal (Fig. 2).
Schematic representation of different type aptamer-based biosensors. When the target bacteria specifically bind to aptamer, the detection system can output different signals such as colorimetric, fluorescent, electrochemical and SERS. And bacteria can be qualitatively or quantitatively detected by the corresponding aptamer-based biosensors
SELEX techniques
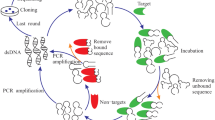
The SELEX technique is a generic means for aptamer screening. In 1990, Ellington and Tuerk used SELEX technology to screen out random oligonucleotides that could specifically bind to organic dyes and DNA polymerase, respectively, and named them “aptamers” (Ellington and Szostak 1990; Tuerk and Gold 1990). Commonly used SELEX technique mainly include conventional SELEX, cell-SELEX, and genomic SELEX. The conventional SELEX technique comprises multiple cycles, and each cycle has three stages: (i) DNA or RNA libraries synthesized in vitro are mixed with the targets to form target–aptamer complexes; (ii) the complexes are separated from the free nucleic acids to remove oligonucleotides that are not bound to targets; (iii) the remaining bound sequences are used as templates for PCR amplification for the next round of screening. After about 20 rounds of selection, aptamers with high affinity and specificity to the target can be obtained (Teng et al. 2016). The cell-SELEX technique uses the whole-cell as target, which does not require protein purification or prior knowledge of the target on cells before selection, and the aptamers selected by it can be used to discover unknown surface proteins or new biomarkers on cells in their native conformation, which is the main merit of cell-SELEX (Marton et al. 2016; Zhuo et al. 2017). In contrast to conventional SELEX, genomic SELEX uses a genomic DNA library, the diversity of which is significantly reduced (Lorenz et al. 2006, 2010). In addition to the SELEX techniques above, some other SELEX technologies have emerged including negative SELEX, capillary electrophoresis-SELEX, magnetic bead-bead SELEX, microfluidic SELEX and in vivo SELEX (Ali et al. 2019; Yan et al. 2019).
Aptamer-based optical biosensors
Optical biosensors realize the detection through the optical signal changes caused by the interaction of the analyte with bioreceptors. Because of its safety and high resolution, optical biosensors have a wide range of applications in the detection of pathogenic bacteria via combination with aptamers. Optical biosensors can be divided into two general modes: label-free and label-based. In label-free sensors, the optical signals are directly generated by interaction between the analyte and the transducer. Label-based sensor uses a label to generates optical signals, which may be colorimetric, fluorescent, or luminescent (Damborský et al. 2016). According to the recent reports for bacteria detection by optical biosensors, here we mainly introduced colorimetric biosensors and fluorescent biosensors.
Aptamer-based colorimetric biosensors
Aptamer-based colorimetric biosensors detect analyte by color change depending on structural colors (Liu et al. 2009; Sivakumar et al. 2005), cationic polymers (Bala et al. 2016), oxidation of substrate by enzyme (Wu et al. 2015), catalyzed oxidation of nanomaterials (Hu et al. 2015), or the aggregation or disaggregation of nanoparticles (Mondal et al. 2018; Yousefi and Saraji 2019). This method does not need complicated analytical instruments, making the aptamer-based colorimetric biosensor simpler than most others.
Aptamer-based colorimetric biosensors usually use a sandwich structure to capture and identify bacteria. Wu et al. designed a gold nanoparticle (AuNP)-based enzyme-linked antibody-aptamer sandwich (nano-ELAAS) method to detect Salmonella typhimurium. Magnetic microparticles (MMP) were modified with S. typhimurium-specific aptamers (MMP-aptamers) to act as capture probes, pre-enriching S. typhimurium from samples; then S. typhimurium were subsequently sandwiched by MMP-aptamers and detection antibodies. Then the sandwich complex (MMP-aptamer/S. typhimurium/detector antibody) reacted with nanoprobes with a reporter antibody and horseradish peroxidase (HRP), which caused a color change that could be seen with the naked eye. Its sensitivity was much higher than that of colorimetric ELAAS and chemiluminescent ELAAS (Wu et al. 2014). Liu et al. adopted a similar sandwich structure to detect Listeria monocytogenes ranging from 10 to 106 colony-forming units (CFU)/mL. The L. monocytogenes could be captured by aptamer-modified magnetic nanoparticles (Apt-MNPs) and then recognized by immunoglobulin Y-coated silver nanoclusters (AgNCs). The AgNCs can oxidize o-phenylenediamine, making the color of reporting system change from blue to red (Liu et al. 2018b).
Aptamer-based colorimetric biosensors coupled with PCR can also achieve bacterial detection. Liu and his group combined PCR and G-quadruplex DNAzyme catalytic reaction to realize sensitive, low-cost, convenient detection of L. monocytogenes and Helicobacter pylori. They constructed a unique modified reverse primer, which bound to an anti-DNAzyme sequence at its 5′-end via a poly-A linker. This strategy can be used to synthesize DNAzyme aptamers at the 3′-end of PCR products. After hemin was added, the aptamer section of the double-stranded products formed a G-quadruplex DNAzyme. Oxidation of 2,2′-azino-bis(3-ethylbenzthiazoline)-6-sulfonic acid was catalyzed by the G-quadruplex DNAzyme, causing the system to change from colorless to green (Fig. 3). This strategy avoids a series of complicating preparations before detection, and can be used in practical samples, so it is more serviceable than other methods (Liu et al. 2018c, d).
The H. pylori detection via aptamer-based colorimetric biosensor coupled with PCR by Liu and his group. (Liu et al. 2018c). (a) The genomic DNA from H. pylori was amplified through PCR with specific forward primer and anti-DNAzyme reverse primer (ADRP). After thermal denatured, a single chain with DNAzyme aptamer was obtained. (b) The DNAzyme aptamer on H. pylori DNA chain combined with hemin to form complete G-quadruplex DNAzyme, which can catalyze the oxidation of ABTS and output the colorimetric signal
To improve the efficiency of aptamers in colorimetric biosensors, researchers have optimized aptamers for bacteria detection. Sun et al. obtained an optimal truncated aptamer with higher affinity after optimizing the original aptamer sequence for Vibrio parahaemolyticus by truncation and site-directed mutagenesis. Then the truncated aptamer was conjugated to magnetic nanoparticles (MNPs) to construct capture probes, and G-quadruplex DNAzyme was used to catalyze chromogenic reaction of 3,3′,5,5′-tetramethylbenzidine (TMB). This method could detect V. parahaemolyticus in the range of 102 ~ 107 CFU/mL (Sun et al. 2019b). Kim et al. also used truncated aptamers for better adsorption onto AuNPs to detect Campylobacter in chicken carcass samples by the aggregation of AuNPs which could causes color change (Kim et al. 2018).
With the successive study of aptamer-based colorimetric biosensors, techniques such as multicolor detection and colorimetric analysis associated with other methods have also been developed. Liu et al. innovatively applied gold nanorods (AuNRs) for detection of L. monocytogenes (Fig. 4). In the presence of L. monocytogenes, a sandwich-type complex formed by Apt-MNPs and nanoenzyme that could oxidize TMB to produce TMB2+ captured the target and quantitatively etched AuNRs with various aspect ratios so that the system output multicolor signals for detection (Liu et al. 2019b). Wu et al. (2018) and Das et al. (2019) associated colorimetric signals with SERS signals and electrochemical signals, making the detection of pathogenic bacteria more sensitive and accurate.
The aptamer-based colorimetric biosensor for L. monocytogenes detection. (Liu et al. 2019b). The Apt-MNP is used as capture probe for L. monocytogenes. The Apt-MNP (black), L. monocytogenes (blue) and oxidase-like nano-artificial enzyme(green) form a sandwich-type immunocomplex that can catalyze TMB to TMB2+. With the concentration of L. monocytogenes increased, more TMB2+ is generated and then etched the AuNRs with various aspect ratios, resulting vivid color change in the solution
Aptamer-based fluorescent biosensors
Aptamer-based fluorescent biosensors achieve quantitative analyte detection through fluorescence change via the combination of aptamers and fluorophores or fluorescent nanomaterials. Compared with colorimetric biosensors, it is easier to realize sensitive and selective detection using fluorescent biosensors.
The sandwich structure applied in aptamer-based colorimetric biosensors can also be employed in aptamer-based fluorescent biosensors. Cheng et al. prepared vancomycin-stabilized fluorescent gold nanoclusters (AuNCs@Van) and aptamer-coated magnetic beads for identifying and capturing Staphylococcus aureus. This dual recognition strategy of vancomycin and aptamer greatly improved the specificity and sensitivity of the detection, and it could be used to detect about 70 CFU/mL S. aureus in complex samples (containing 3 × 108 CFU/mL other bacteria) (Cheng et al. 2016). Yu et al. designed a similar dual recognition strategy with AuNCs@Van and aptamer-modified AuNPs (Apt-AuNPs), with the AuNCs@Van as an energy donor and the Apt-AuNPs as an energy acceptor. Via a fluorescence resonance energy transfer (FRET) mechanism, S. aureus could be detected sensitively and selectively in 30 min. The FRET signal was linear with the concentration of S. aureus (Yu et al. 2017).
Aptamer-based fluorescent biosensors have also been developed to detect different pathogenic bacteria simultaneously. Li et al. reported a method with excellent recovery efficiency and rapid identification capability for simultaneous detection of multiple pathogenic bacteria. The aptamer-modified fluorescent-magnetic multifunctional nanoprobes captured Escherichia coli or S. typhimurium and effectively separated them on the basis of different magnetic responses to the external magnetic field (Li et al. 2018). Duan et al. attached aptamers labeled with fluorescent dyes to carbon nanoparticles, achieving sensitive and stable simultaneous detection of V. parahaemolyticus, S. aureus, and S. typhimurium through a multiple FRET system (Duan et al. 2016b).
Current aptamer-based fluorescence detection technology is developing in the direction of rapidity, simplicity, and convenience to meet the needs of clinical diagnosis, environmental monitoring, and food safety. Qiao et al. established a FRET assay that is expected to be applicable to clinical specimens such as nasal swabs and plasma for detection of methicillin-resistant S. aureus (MRSA) based on a PBP2a protein aptamer. Compared with traditional antimicrobial susceptibility tests, this strategy greatly decreases the time required for detection, and is not necessary to isolate and purify bacteria from clinical samples (Qiao et al. 2018). Shrivastava et al. detected pathogenic bacteria sensitively by quantitative imaging using smartphones, in which they used aptamer-conjugated fluorescent MNPs to capture target bacteria and the smartphone had a light-emitting diode that acted as an excitation source for fluorescence imaging (Fig. 5). This method can realize rapid on-site detection of S. aureus and be applied in remote regions and resource-limited settings (Shrivastava et al. 2018).
Quantitative detection of S. aureus by smartphone with aptamer-based fluorescent. (Shrivastava et al. 2018). Aptamer-conjugated fluorescent MNPs was used to capture target bacteria and the smartphone had a light-emitting diode that acted as an excitation source for fluorescence imaging
Aptamer-based electrochemical biosensors
Aptamer-based electrochemical biosensors can be traced back to 2004. Ikebukuro et al. constructed a sandwich-type biosensor that could be used to quantitatively analyze thrombin with current as the signal, using aptamers labeled with glucose dehydrogenase (Ikebukuro et al. 2004). Since then, aptamer-based electrochemical biosensors have been extensively used in monitoring health, environmental pollution, and food safety (Li et al. 2019c; Mishra et al. 2018).
Roushani et al. constructed a method to detect Pseudomonas aeruginosa in serum by aptamer-based electrochemical biosensors. They deposited silver nanoparticles (AgNPs) on a glassy carbon electrode (GCE) to increase surface area, resulting in significant acceleration of electron transfer and enhancing electrochemical signal efficiently. P. aeruginosa could be captured by NH2-aptamer covalently attached to the AgNP/GCE surface and detected according to the charge transfer resistance in the absence or presence of the target, and the concentration of P. aeruginosa was closely related to the increased degree of impedance. This method could detect P. aeruginosa in the range of 102 to 107 CFU/mL (Roushani et al. 2019). Similarly, Muniandy et al. coated reduced graphene oxide titanium dioxide (rGO-TiO2) nanocomposite on a GCE surface to construct an rGO-TiO2/GCE platform, and combined aptamers with the rGO-TiO2 to capture S. typhimurium. The aptamer–bacteria complex (S. typhimurium/Apt/rGO-TiO2/GCE) caused a physical barrier that could restrain the electronic dynamics at the GCE surface. This strategy needs only small volumes of samples, and is able to achieve quick determination by using a portable instrument. Thus, this approach possesses a huge potential in on-site detection (Muniandy et al. 2019).
Aptamer-based electrochemical biosensors can also be used to monitor bacterial proliferation. Jo et al. developed an aptamer-functionalized capacitance sensor array to monitor bacterial growth and death and to assess bacterial tolerance to antibiotics. The aptamer was immobilized on the sensor surface, and the number of viable bacteria adhering to the sensor surface was closely related to the capacitance. Therefore, the growth of bacteria could be monitored in real-time by measuring the change of capacitance (Jo et al. 2018). Zhang et al. used aptamers linked to magnetic beads to capture target pathogenic bacteria, then the trapped bacteria were cultivated in multichannel conductometric sensors after magnetic separation. The growth kinetics of bacteria could be measured through time-dependent conductivity changes of the culture media. Consequently, a curve of normalized apparent conductivity values against incubation time could be obtained, which reflected the growth of bacteria (Zhang et al. 2019) (Fig. 6).
The procedure of aptamer-based multichannel conductometric sensor for the determination of viable bacteria. (Zhang et al. 2019). The bacteria were captured by the aptamer-functionalized magnetic beads (I, II) and separated through the magnetic separation (III). Then, the growth kinetics of bacteria are obtained by measuring the time-dependent conductivity changes with a conductometric sensor (IV)
Aptamer-based SERS methods
SERS is an emission technique that allows analyte molecules to be absorbed onto the SERS substrate, thus the analyte is exposed to the hot spots created by the substrates, remarkably enhancing the Raman signal (Wu et al. 2020a). Roughened silver, copper or gold is usually used as the SERS substrates (Mosier-Boss 2017). SERS technology can acquire information about the chemical structure of a substance at the molecular level and provide fingerprint identification spectra. It possesses high sensitivity and selectivity, fast detection speed, and very low background interference, and is suitable for multicomponent analysis. Combining SERS detection technology and aptamer recognition ability can improve the detection accuracy and the sensitivity of the analysis (Wang et al. 2019). When analytes combine with the aptamers, the SERS signal can be increased 104 to 106 fold (Chen et al. 2008).
Díaz-Amaya et al. covalently conjugated aptamers to 4-aminothiophenol (4-ATP)-modified AuNPs (4-ATP-AuNPs) to construct nanoprobes for detection of E. coli. In the presence of target bacteria, the E. coli-aptamer-4-ATP-AuNP composite was formed and gradually precipitated, and the concentration of E. coli could be determined based on the supernatant SERS signal intensity due to their negative linear correlation, and E. coli O157:H7 could be detected in the range of 102–106 CFU/mL (Díaz-Amaya et al. 2019).
Gao and He developed a nitrocellulose membrane platform for rapid detection of pathogenic bacteria by using SERS patterns. By incorporating a vacuum filtration system, the bacteria trapped by aptamers were concentrated onto the nitrocellulose membrane, then the AuNPs deposited on the membrane combined with aptamer–bacteria complexes, dramatically enhancing the Raman signal (Gao and He 2019). Pang et al. synthesized aptamer–Fe3O4@gold magnetic nanoparticles for bacteria enrichment and vancomycin and 4-mercaptobenzoic acid (4-MBA)-modified AuNPs (Au@MBA@Van) as Van-SERS tags for sensitive quantification of S. aureus. Owing to the golden surface, aptamer-Fe3O4@gold magnetic nanoparticles also have the ability to be as SERS substrates. Therefore, this strategy provided more hot spots for the amplification of Raman signal to improve the sensitivity of bacteria detection significantly (Pang et al. 2019).
Gold nanoparticle dimers (AuNDs), whether symmetrical or asymmetrical, show stable SERS activity in solution and are excellent SERS substrates because of their uniform and dispersed structure and the strong hot spots between the AuNPs. Wu et al. constructed asymmetric AuNDs with two different sizes of AuNPs, which were modified with HRP, aptamer and Raman reporters, complementary series respectively, to realize dual signal detection of bacteria. In the presence of target bacteria, the HRP-aptamer-AuNPs captured the bacteria and deposited, the asymmetric AuNDs disassembly, the SERS signal intensity of the supernatant ascribing to the single AuNPs declined, while the resuspending solution showed a significant color change on addition of TMB and H2O2 (Wu et al. 2018). Wu et al. constructed symmetrical AuNDs as probes for Shigella sonnei detection. 4-MBA, europium chloride hexahydrate and 1,10-phenanthroline formed a compound structure named as Eu complex. Then, symmetrical AuNDs was constructed by two citrate-stabilized gold nanoparticles (cit-AuNPs) with Eu complex as the linker, and modified with S. sonnei specific aptamers subsequently. When the symmetrical AuNDs specifically captured target bacteria and nonbound symmetrical AuNDs were removed through centrifuging, the symmetrical AuNDs bound with target bacterium were determinated by SERS detector. The intensity of the SERS signal of 4-MBA was proportional to the concentration of target bacteria. The successful synthesis of AuNDs simplified the preparation of SERS substrates and Raman reporters and made the detection procedure much more convenient. This dual-function nanomaterial combined with SERS technology improved the detection performance of this biosensor (Wu et al. 2020a).
AgNPs have also been developed as SERS substrates. For example, Gao et al. took the aptamer interact with S. aureus forming a bind configuration. Then, they used the formation as the template for AgNPs synthesis in situ, synthesizing S. aureus-aptamer AgNPs as the SERS substrates to monitor the concentration of S. aureus (Fig. 7). The method can directly identify a single bacterium through the SERS mapping technique which is particularly useful in the detection and analysis of bacteria because of its capability to scan a certain area to produce heat maps (Gao et al. 2017; Gao and He 2019). Duan et al. synthesized Ag@Au core/shell nanoparticles as SERS substrates and modified them with aptamers to form nanoparticle–bacteria–aptamer sandwich complexes, and used to detect S. typhimurium in real food samples based on the SERS intensity changed with changes of the S. typhimurium concentration (Duan et al. 2016a).
The aptamer-based SERS detection for S. aureus via aptamer dependent AgNPs synthesized in situ. (Gao et al. 2017). The aptamaer were used as capture probe for S. aureus and the template for AgNP synthesized in situ. And the bacteria-aptamer@AgNP generated obvious SERS signals which was linear with the concentration of S. aureus
Other aptamer-based methods
In addition to the above common detection methods, the association of aptamers with other sensitive techniques is also used in the detection of pathogenic bacteria.
The quartz crystal microbalance (QCM) biosensor is a mass-sensitive biosensor, which works based on the change of resonance frequency (Wang et al. 2018). On the basis of this principle, Bayramoglu et al. detected Brucella melitensis in milk and milk products. They first attached aptamers to magnetic nanoparticles and QCM chips, respectively. Then the target bacteria were pre-enriched and eluted by binding to aptamers on magnetic nanoparticles, and were quantitatively detected by the final resonance frequency signals produced after binding to aptamers attached to the QCM chip (Bayramoglu et al. 2019).
Detection of pathogenic bacteria via gas pressure change in a closed vessel is an extremely sensitive method. As illustrated in Fig. 8, Li et al. used aptamer-coated magnetic nanoprobes and vancomycin-functionalized platinum nanoparticles (PtNPs@Van) to capture S. aureus, and the PtNPs@Van catalyzed oxidation of H2O2 to O2, resulting in a marked pressure increase, realizing environmentally friendly detection of S. aureus (Li et al. 2019a).
Schematic illustration for aptamer-based detection of S. aureus via gas pressure. (Li et al. 2019a). The PtNPs@Van catalyze the decomposition of H2O2 in the presence of S. aureus resulting in the pressure increased, which was positively correlated with the concentration of S. aureus
Polyethylene glycol silane (silane–PEG–COOH) is a splendid material for bacterial detection because of its bacteria-repelling surface, which can prevent the adsorption of nonspecific bacteria on the sensor surface. Maldonado et al. used an ultrasensitive photonic biosensor based on a bimodal waveguide interferometer and aptamer for the direct detection of MRSA. With the help of silane–PEG–COOH, the specificity of the detection was greatly improved (Maldonado et al. 2020).
Microchip capillary electrophoresis (MCE) is a technique that possesses a rapid response rate, high separation efficiency, short analysis time, and low sample and reagent consumption (Castro and Manz 2015). MCE is often coupled with laser-induced fluorescence (LIF) to detect bacteria. Zhang et al. (2018) first separated the bacteria–aptamer complex and free aptamer based on the differences in the charge-to-mass ratio by MCE and then detected target bacteria by LIF, and successfully detected S. typhimurium in milk samples. Luo et al. (2020) detected S. typhimurium and P. aeruginosa via the association of aptamer-based strategy, a novel universal primer-duplex PCR process (UP-DPCR), and MCE-LIF technology, achieving high sensitivity of different bacterium detection.
The photo electrochemical (PEC) electrode is an emerging and fast-developing sensing strategy. Compared with traditional electrochemical detection, PEC biosensors have very low background noise and high sensitivity. A high-performance PEC electrode combined with CdS quantum dots modified by an aptamer can detect E. coli with high specificity and stability (Dong et al. 2020).
Besides the aptamer-based biosensors, lateral chromatography test strips is also a simple biosensor for rapid detection of pathogenic bacteria. Liu et al. proposed a novel strategy to visually and sensitively detect viable pathogenic bacteria based on an isothermal RNA amplification reaction-based bioactive paper-based platform (Fig. 9). They devised a low-cost paper-based platform to visually detect L. monocytogenes. RNA isothermal amplification products of L. monocytogenes hybridized with AuNP-thiolated DNA on the paper-based platform. When migrating along the strip, the hybridized products combined with the capture probe embedded in test area, making AuNPs accumulated and characterized a red band. And the two-dimensional bar code of the result could be built by software which could be shared with distant investigators for faster analysis. This portable, integrated test model holds a great promise for rapid on-site analysis of pathogenic bacteria (Liu et al. 2014).
The isothermal RNA amplification and the bioactive paper-based platform devised by Liu et al. (2014). The specific RNA was isothermally amplified from infected food samples and loaded in the sample pad. As migrating to the conjugate pad, it would hybridize with AuNP-thiolated DNA, and next combined with the capture probe embedded in test area, making AuNPs accumulated and characterized a red band. The excess AuNP-thiolated DNA would continually migrate to control area, forming the second red band by hybridized with capture probe here. And the two-dimensional bar code of the result could be built by software which could be shared with distant investigators for faster analysis
The limitations of aptamer-based methods
Aptamers have received extensive attention in the detection of pathogenic bacteria. They simplify the enrichment processes and reduce the detection time owing to their high affinity and specificity. Table 2 shows the comparison between the different type of aptamer-based biosensors. Nevertheless, aptamer-based methods for pathogenic bacteria detection still have some drawbacks, which restrict the development of their applications. Aptamers obtained by SELEX generally have 60–100 nucleotides. Too long aptamers may lead to self-folding, resulting the binding between aptamer and its target reduced (Sun et al. 2019b). And the targets of bacteria-specific aptamers are ordinarily some molecules on the bacteria surface, such as proteins, polysaccharides and so on. Due to this fact, the difficulty of screening would be increased if the bacterial surface is in negative charge when the phosphate backbone of aptamer is also in negative charge (Bayat et al. 2018; Cai et al. 2018). Moreover, although the stability of aptamers in vitro is better than that of natural antibodies, aptamers are easily degraded by nuclease in vivo (Yan et al. 2019; Zhou and Rossi 2017). The high affinity between aptamer and its target only is shown under suitable conditions, which may be difficult to apply in vivo (Keefe et al. 2010). Furthermore, there is no systematic methodology for the development of aptamer, which may hinder the application of aptamer. Therefore, it is necessary to optimize SELEX techniques or develop novel SELEX techniques, which are more suitable for the conditions in complex biologic samples or in vivo.
Conclusion and Future prospects
In summary, we reviewed the recent advances on applications of different type aptamer–based biosensors for pathogenic bacteria detection. The emergence of aptamer and their combination with other technologies has supplied many new sensitive, rapid, and specific methods for bacteria detection. Aptamer as a biomolecule can be modified on various nanomaterials through different chemical reactions. Combining with nanomaterials is able to make aptamer more stable against enzyme degradation (Liang et al. 2014). It can be estimated that, with the rapid development of novel nanomaterials, more drawbacks of aptamer can be overcame. Recent years, there are some researchers report the special nucleic acid molecules constituted by artificial bases, which are designed for great molecular recognition, high stability or other useful features (Bai et al. 2020; Jin et al. 2017; Liang et al. 2016; Wang et al. 2017). As a fast-developing technique, aptamer is expected to be a useful tool for bacteria detection in environment monitoring, food safety inspection, and diagnosis of infectious diseases.
References
Acquah C, Agyei D, Obeng EM, Pan S, Tan KX, Danquah MK (2020) Aptamers: an emerging class of bioaffinity ligands in bioactive peptide applications. Crit Rev Food Sci Nutr 60:1195–1206. https://doi.org/10.1080/10408398.2018.1564234
Ali MH, Elsherbiny ME, Emara M (2019) Updates on aptamer research. Int J Mol Sci 20. https://doi.org/10.3390/ijms20102511
Bai H, Jin C, Zou J, Wang R, Fu T, Tan W (2020) Conformational conversion enhances cellular uptake of F base double-Strand-conjugated oligonucleotides. Anal Chem 92:10375–10380. https://doi.org/10.1021/acs.analchem.0c00614
Bala R, Kumar M, Bansal K, Sharma RK, Wangoo N (2016) Ultrasensitive aptamer biosensor for malathion detection based on cationic polymer and gold nanoparticles. Biosens Bioelectron 85:445–449. https://doi.org/10.1016/j.bios.2016.05.042
Bayat P, Nosrati R, Alibolandi M, Rafatpanah H, Abnous K, Khedri M, Ramezani M (2018) SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 154:132–155. https://doi.org/10.1016/j.biochi.2018.09.001
Bayramoglu G, Ozalp VC, Oztekin M, Arica MY (2019) Rapid and label-free detection of Brucella melitensis in milk and milk products using an aptasensor. Talanta 200:263–271. https://doi.org/10.1016/j.talanta.2019.03.048
Bhalla N, Jolly P, Formisano N, Estrela P (2016) Introduction to biosensors. Essays Biochem 60:1–8. https://doi.org/10.1042/ebc20150001
Cai S, Yan J, Xiong H, Liu Y, Peng D, Liu Z (2018) Investigations on the interface of nucleic acid aptamers and binding targets. Analyst 143:5317–5338. https://doi.org/10.1039/c8an01467a
Castro ER, Manz A (2015) Present state of microchip electrophoresis: state of the art and routine applications. J Chromatogr A 1382:66–85. https://doi.org/10.1016/j.chroma.2014.11.034
Chen JW, Liu XP, Feng KJ, Liang Y, Jiang JH, Shen GL, Yu RQ (2008) Detection of adenosine using surface-enhanced Raman scattering based on structure-switching signaling aptamer. Biosens Bioelectron 24:66–71. https://doi.org/10.1016/j.bios.2008.03.013
Chen Y et al (2016) Aptamer functionalized hydrophilic polymer monolith with gold nanoparticles modification for the sensitive detection of human α-thrombin. Talanta 154:555–559. https://doi.org/10.1016/j.talanta.2016.02.054
Cheng D et al (2016) Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Anal Chem 88:820–825. https://doi.org/10.1021/acs.analchem.5b03320
Damborský P, Švitel J, Katrlík J (2016) Optical biosensors. Essays Biochem 60:91–100. https://doi.org/10.1042/ebc20150010
Das R, Dhiman A, Kapil A, Bansal V, Sharma TK (2019) Aptamer-mediated colorimetric and electrochemical detection of Pseudomonas aeruginosa utilizing peroxidase-mimic activity of gold NanoZyme. Anal Bioanal Chem 411:1229–1238. https://doi.org/10.1007/s00216-018-1555-z
Díaz-Amaya S, Lin LK, Deering AJ, Stanciu LA (2019) Aptamer-based SERS biosensor for whole cell analytical detection of E. coli O157:H7. Anal Chim Acta 1081:146–156. https://doi.org/10.1016/j.aca.2019.07.028
Dong X et al (2020) CdS quantum dots/Au nanoparticles/ZnO nanowire array for self-powered photoelectrochemical detection of Escherichia coli O157:H7. Biosens Bioelectron 149:111843. https://doi.org/10.1016/j.bios.2019.111843
Duan N, Chang B, Zhang H, Wang Z, Wu S (2016a) Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int J Food Microbiol 218:38–43. https://doi.org/10.1016/j.ijfoodmicro.2015.11.006
Duan N, Gong WH, Wang ZP, Wu SJ (2016b) An aptasensor based on fluorescence resonance energy transfer for multiplexed pathogenic bacteria determination. Anal Methods 8:1390–1395. https://doi.org/10.1039/c5ay02608c
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822. https://doi.org/10.1038/346818a0
Frohnmeyer E et al (2019) Aptamer lateral flow assays for rapid and sensitive detection of cholera toxin. Analyst 144:1840–1849. https://doi.org/10.1039/c8an01616j
Gao S, He L (2019) Development of a filtration-based SERS mapping platform for specific screening of Salmonella enterica serovar Enteritidis. Anal Bioanal Chem 411:7899–7906. https://doi.org/10.1007/s00216-019-02204-3
Gao W et al (2017) Intuitive label-free SERS detection of bacteria using aptamer-based in Situ silver nanoparticles synthesis. Anal Chem 89:9836–9842. https://doi.org/10.1021/acs.analchem.7b01813
Hermann T, Patel DJ (2000) Adaptive recognition by nucleic acid aptamers. Science (New York, NY) 287:820–825. https://doi.org/10.1126/science.287.5454.820
Hu J, Ni P, Dai H, Sun Y, Wang Y, Jiang S, Li Z (2015) Aptamer-based colorimetric biosensing of abrin using catalytic gold nanoparticles. Analyst 140:3581–3586. https://doi.org/10.1039/c5an00107b
Idili A, Gerson J, Parolo C, Kippin T, Plaxco KW (2019) An electrochemical aptamer-based sensor for the rapid and convenient measurement of l-tryptophan. Anal Bioanal Chem 411:4629–4635. https://doi.org/10.1007/s00216-019-01645-0
Ikebukuro K, Kiyohara C, Sode K (2004) Electrochemical detection of protein using a double aptamer sandwich. Anal Lett 37:2901–2909. https://doi.org/10.1081/AL-200035778
Ji D et al (2017) Label-free and rapid detection of ATP based on structure switching of aptamers. Anal Biochem 526:22–28. https://doi.org/10.1016/j.ab.2017.03.011
Jin C et al (2017) Fluorinated molecular beacons as functional DNA nanomolecules for cellular imaging. Chem Sci 8:7082–7086. https://doi.org/10.1039/c7sc02819a
Jo N et al (2018) Aptamer-functionalized capacitance sensors for real-time monitoring of bacterial growth and antibiotic susceptibility. Biosens Bioelectron 102:164–170. https://doi.org/10.1016/j.bios.2017.11.010
Keefe AD, Pai S, Ellington A (2010) Aptamers as therapeutics. Nat Rev Drug Discov 9:537–550. https://doi.org/10.1038/nrd3141
Kim YJ, Kim HS, Chon JW, Kim DH, Hyeon JY, Seo KH (2018) New colorimetric aptasensor for rapid on-site detection of Campylobacter jejuni and Campylobacter coli in chicken carcass samples. Anal Chim Acta 1029:78–85. https://doi.org/10.1016/j.aca.2018.04.059
Labib M, Berezovski MV (2014) Electrochemical aptasensors for microbial and viral pathogens. Adv Biochem Eng Biotechnol 140:155–181. https://doi.org/10.1007/10_2013_229
Lee KH, Zeng H (2017) Aptamer-based ELISA assay for highly specific and sensitive detection of Zika NS1 protein. Anal Chem 89:12743–12748. https://doi.org/10.1021/acs.analchem.7b02862
Lee JH, Yigit MV, Mazumdar D, Lu Y (2010) Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv Drug Deliv Rev 62:592–605. https://doi.org/10.1016/j.addr.2010.03.003
Levi K, Smedley and J, Towner KJ (2003) Evaluation of a real-time PCR hybridization assay for rapid detection of Legionella pneumophila in hospital and environmental water samples. Clin Microbiol Infect 9:754–758. https://doi.org/10.1046/j.1469-0691.2003.00666.x
Li J, Tan S, Chen X, Zhang CY, Zhang Y (2011) Peptide aptamers with biological and therapeutic applications. Curr Med Chem 18:4215–4222. https://doi.org/10.2174/092986711797189583
Li L et al (2018) Magnetism-resolved separation and fluorescence quantification for near-simultaneous detection of multiple pathogens. Anal Chem 90:9621–9628. https://doi.org/10.1021/acs.analchem.8b02572
Li J, Jiang H, Rao X, Liu Z, Zhu H, Xu Y (2019a) Point-of-care testing of pathogenic bacteria at the single-colony level via gas pressure readout using aptamer-coated magnetic CuFe2O4 and vancomycin-capped platinum nanoparticles. Anal Chem 91:1494–1500. https://doi.org/10.1021/acs.analchem.8b04584
Li YK, Li WT, Liu X, Yang T, Chen ML, Wang JH (2019b) Functionalized magnetic composites based on the aptamer serve as novel bio-adsorbent for the separation and preconcentration of trace lead. Talanta 203:210–219. https://doi.org/10.1016/j.talanta.2019.05.075
Li Z, Mohamed MA, Vinu Mohan AM, Zhu Z, Sharma V, Mishra GK, Mishra RK (2019c) Application of electrochemical aptasensors toward clinical diagnostics, food, and environmental monitoring: review. Sensors (Basel, Switzerland) 19:5435. https://doi.org/10.3390/s19245435
Liang H et al (2014) Functional DNA-containing nanomaterials: cellular applications in biosensing, imaging, and targeted therapy. Acc Chem Res 47:1891–1901. https://doi.org/10.1021/ar500078f
Liang H, Xie S, Cui L, Wu C, Zhang X (2016) Designing a biostable L-DNAzyme for lead(II) ion detection in practical samples analytical methods: advancing methods and applications. Anal Method 8:7260–7264. https://doi.org/10.1039/c6ay01791f
Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109:1948–1998. https://doi.org/10.1021/cr030183i
Liu H, Zhan F, Liu F, Zhu M, Zhou X, Xing D (2014) Visual and sensitive detection of viable pathogenic bacteria by sensing of RNA markers in gold nanoparticles based paper platform. Biosens Bioelectron 62:38–46. https://doi.org/10.1016/j.bios.2014.06.020
Liu Y, Deng Y, Li T, Chen Z, Chen H, Li S, Liu H (2018a) Aptamer-based electrochemical biosensor for mercury ions detection using AuNPs-modified glass carbon electrode. J Biomed Nanotechnol 14:2156–2161. https://doi.org/10.1166/jbn.2018.2655
Liu Y, Wang J, Song X, Xu K, Chen H, Zhao C, Li J (2018b) Colorimetric immunoassay for Listeria monocytogenes by using core gold nanoparticles, silver nanoclusters as oxidase mimetics, and aptamer-conjugated magnetic nanoparticles. Mikrochim Acta 185:360. https://doi.org/10.1007/s00604-018-2896-1
Liu Z, Yao C, Wang Y, Zheng W (2018c) Visual diagnostic of Helicobacter pylori based on a cascade amplification of PCR and G-quadruplex DNAzyme as a color label. J Microbiol Methods 146:46–50. https://doi.org/10.1016/j.mimet.2018.01.014
Liu Z, Yao C, Yang C, Wang Y, Wan S, Huang J (2018d) Development of DNAzyme-based PCR signal cascade amplification for visual detection of Listeria monocytogenes in food. Anal Biochem 553:7–11. https://doi.org/10.1016/j.ab.2018.05.015
Liu X, Deng K, Wang H, Li C, Zhang S, Huang H (2019a) Aptamer based ratiometric electrochemical sensing of 17β-estradiol using an electrode modified with gold nanoparticles, thionine, and multiwalled carbon nanotubes. Mikrochim Acta 186:347. https://doi.org/10.1007/s00604-019-3465-y
Liu Y et al (2019b) A multicolorimetric assay for rapid detection of Listeria monocytogenes based on the etching of gold nanorods. Anal Chim Acta 1048:154–160. https://doi.org/10.1016/j.aca.2018.10.020
Lorenz C, von Pelchrzim F, Schroeder R (2006) Genomic systematic evolution of ligands by exponential enrichment (Genomic SELEX) for the identification of protein-binding RNAs independent of their expression levels. Nat Protoc 1:2204–2212. https://doi.org/10.1038/nprot.2006.372
Lorenz C et al (2010) Genomic SELEX for Hfq-binding RNAs identifies genomic aptamers predominantly in antisense transcripts. Nucleic Acids Res 38:3794–3808. https://doi.org/10.1093/nar/gkq032
Luo F, Li Z, Dai G, Lu Y, He P, Wang Q (2020) Simultaneous detection of different bacteria by microchip electrophoresis combined with universal primer-duplex polymerase chain reaction. J Chromatogr A 1615:460734. https://doi.org/10.1016/j.chroma.2019.460734
Maldonado J, Estévez MC, Fernández-Gavela A, González-López JJ, González-Guerrero AB, Lechuga LM (2020) Label-free detection of nosocomial bacteria using a nanophotonic interferometric biosensor. Analyst 145:497–506. https://doi.org/10.1039/c9an01485c
Marton S, Cleto F, Krieger MA, Cardoso J (2016) Isolation of an aptamer that binds specifically to E. coli. PLoS One 11:e0153637. https://doi.org/10.1371/journal.pone.0153637
Mazzaracchio V et al (2019) A label-free impedimetric aptasensor for the detection of Bacillus anthracis spore simulant. Biosens Bioelectron 126:640–646. https://doi.org/10.1016/j.bios.2018.11.017
Mishra GK, Sharma V, Mishra RK (2018) Electrochemical aptasensors for food and environmental safeguarding: a review. Biosensors 8. https://doi.org/10.3390/bios8020028
Mondal B, Ramlal S, Lavu PS, N B, Kingston J (2018) Highly sensitive colorimetric biosensor for staphylococcal enterotoxin B by a label-free aptamer and gold nanoparticles. Front Microbiol 9:179. https://doi.org/10.3389/fmicb.2018.00179
Mosier-Boss PA (2017) Review of SERS substrates for chemical sensing. Nanomaterials (Basel, Switzerland) 7. https://doi.org/10.3390/nano7060142
Muniandy S, Teh SJ, Appaturi JN, Thong KL, Lai CW, Ibrahim F, Leo BF (2019) A reduced graphene oxide-titanium dioxide nanocomposite based electrochemical aptasensor for rapid and sensitive detection of Salmonella enterica. Bioelectrochemistry (Amsterdam, Netherlands) 127:136–144. https://doi.org/10.1016/j.bioelechem.2019.02.005
Newell DG et al (2010) Food-borne diseases – the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol 139(Suppl 1):S3–S15. https://doi.org/10.1016/j.ijfoodmicro.2010.01.021
Pang Y, Wan N, Shi L, Wang C, Sun Z, Xiao R, Wang S (2019) Dual-recognition surface-enhanced Raman scattering(SERS)biosensor for pathogenic bacteria detection by using vancomycin-SERS tags and aptamer-Fe3O4@au. Anal Chim Acta 1077:288–296. https://doi.org/10.1016/j.aca.2019.05.059
Qiao J et al (2018) Aptamer-based fluorometric assay for direct identification of methicillin-resistant Staphylococcus aureus from clinical samples. J Microbiol Methods 153:92–98. https://doi.org/10.1016/j.mimet.2018.09.011
Qiu Y, Tang Y, Li B, He M (2018) Rapid detection of cocaine using aptamer-based biosensor on an evanescent wave fibre platform. R Soc Open Sci 5:180821. https://doi.org/10.1098/rsos.180821
Rajapaksha P, Elbourne A, Gangadoo S, Brown R, Cozzolino D, Chapman J (2019) A review of methods for the detection of pathogenic microorganisms. Analyst 144:396–411. https://doi.org/10.1039/c8an01488d
Ray P, White RR (2010) Aptamers for targeted drug delivery. Pharmaceuticals (Basel, Switzerland) 3:1761–1778. https://doi.org/10.3390/ph3061761
Roushani M, Sarabaegi M, Pourahmad F (2019) Impedimetric aptasensor for Pseudomonas aeruginosa by using a glassy carbon electrode modified with silver nanoparticles. Mikrochim Acta 186:725. https://doi.org/10.1007/s00604-019-3858-y
Scallan E et al (2011) Foodborne illness acquired in the United States – major pathogens. Emerg Infect Dis 17:7–15. https://doi.org/10.3201/eid1701.p11101
Shrivastava S, Lee WI, Lee NE (2018) Culture-free, highly sensitive, quantitative detection of bacteria from minimally processed samples using fluorescence imaging by smartphone. Biosens Bioelectron 109:90–97. https://doi.org/10.1016/j.bios.2018.03.006
Sivakumar M, Tominaga R, Koga T, Kinoshita T, Sugiyama M, Yamaguchi K (2005) Studies on visual sensor from self-assembled polypeptides. Sci Technol Adv Mater 6:91–96. https://doi.org/10.1016/j.stam.2004.06.006
Sun L, Zhao Q (2018) Competitive horseradish peroxidase-linked aptamer assay for sensitive detection of Aflatoxin B1. Talanta 179:344–349. https://doi.org/10.1016/j.talanta.2017.11.048
Sun D, Lu J, Zhang L, Chen Z (2019a) Aptamer-based electrochemical cytosensors for tumor cell detection in cancer diagnosis: a review. Anal Chim Acta 1082:1–17. https://doi.org/10.1016/j.aca.2019.07.054
Sun Y, Duan N, Ma P, Liang Y, Zhu X, Wang Z (2019b) Colorimetric aptasensor based on truncated aptamer and trivalent DNAzyme for Vibrio parahemolyticus determination. J Agric Food Chem 67:2313–2320. https://doi.org/10.1021/acs.jafc.8b06893
Teng J et al (2016) Aptamer-based technologies in foodborne pathogen detection. Front Microbiol 7:1426. https://doi.org/10.3389/fmicb.2016.01426
Torres-Chavolla E, Alocilja EC (2009) Aptasensors for detection of microbial and viral pathogens. Biosens Bioelectron 24:3175–3182. https://doi.org/10.1016/j.bios.2008.11.010
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science (New York, NY) 249:505–510. https://doi.org/10.1126/science.2200121
Wang RJ, Xu Y, Zhang T, Jiang Y (2015) Rapid and sensitive detection of Salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe. Anal Methods 7:1701–1706. https://doi.org/10.1039/c4ay02880e
Wang R et al (2017) Artificial base zT as functional “Element” for constructing photoresponsive DNA nanomolecules. J Am Chem Soc 139:9104–9107. https://doi.org/10.1021/jacs.7b02865
Wang L, Wang R, Wei H, Li Y (2018) Selection of aptamers against pathogenic bacteria and their diagnostics application. World J Microbiol Biotechnol 34:149. https://doi.org/10.1007/s11274-018-2528-2
Wang HX, Zhao YW, Li Z, Liu BS, Zhang D (2019) Development and application of aptamer-based surface-enhanced Raman spectroscopy sensors in quantitative analysis and biotherapy. Sensors (Basel, Switzerland) 19. https://doi.org/10.3390/s19173806
Wu W et al (2014) Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of Salmonella Typhimurium. ACS Appl Mater Interfaces 6:16974–16981. https://doi.org/10.1021/am5045828
Wu S, Wang Y, Duan N, Ma H, Wang Z (2015) Colorimetric aptasensor based on enzyme for the detection of Vibrio parahemolyticus. J Agric Food Chem 63:7849–7854. https://doi.org/10.1021/acs.jafc.5b03224
Wu Z, He D, Cui B, Jin Z (2018) A bimodal (SERS and colorimetric) aptasensor for the detection of Pseudomonas aeruginosa. Mikrochim Acta 185:528. https://doi.org/10.1007/s00604-018-3073-2
Wu J, He T, Guo P, Cai F, Zhao C (2019) An electrochemical sense array based on aptamer and biotin-avidin system for the selective detection of glucagon-like peptide-1. Clin Lab 65. https://doi.org/10.7754/Clin.Lab.2018.181208
Wu S, Duan N, He C, Yu Q, Dai S, Wang Z (2020a) Surface-enhanced Raman spectroscopic-based aptasensor for Shigella sonnei using a dual-functional metal complex-ligated gold nanoparticles dimer. Colloids Surf B Biointerfaces 190:110940. https://doi.org/10.1016/j.colsurfb.2020.110940
Wu W, Yu CD, Wang Q et al (2020b) Research advances of DNA aptasensors for foodborne pathogen detection. Crit Rev Food Sci Nut 60(14):2353–2368. https://doi.org/10.1080/10408398.2019.1636763
Yan J et al (2019) Advances in aptamer screening technologies. Talanta 200:124–144. https://doi.org/10.1016/j.talanta.2019.03.015
Yousefi S, Saraji M (2019) Optical aptasensor based on silver nanoparticles for the colorimetric detection of adenosine. Spectrochim Acta A Mol Biomol Spectrosc 213:1–5. https://doi.org/10.1016/j.saa.2019.01.036
Yu M et al (2017) Dual-recognition förster resonance energy transfer based platform for one-step sensitive detection of pathogenic bacteria using fluorescent vancomycin-gold nanoclusters and aptamer-gold nanoparticles. Anal Chem 89:4085–4090. https://doi.org/10.1021/acs.analchem.6b04958
Yuan H et al (2018) An aptamer-based fluorescence bio-sensor for chiral recognition of arginine enantiomers. Spectrochim Acta A Mol Biomol Spectrosc 200:330–338. https://doi.org/10.1016/j.saa.2018.04.038
Zarei SS, Soleimanian-Zad S, Ensafi AA (2018) An impedimetric aptasensor for Shigella dysenteriae using a gold nanoparticle-modified glassy carbon electrode. Mikrochim Acta 185:538. https://doi.org/10.1007/s00604-018-3075-0
Zhang Y et al (2018) A sensitive assay based on specific aptamer binding for the detection of Salmonella enterica serovar Typhimurium in milk samples by microchip capillary electrophoresis. J Chromatogr A 1534:188–194. https://doi.org/10.1016/j.chroma.2017.12.054
Zhang X, Wang X, Yang Q, Jiang X, Li Y, Zhao J, Qu K (2019) Conductometric sensor for viable Escherichia coli and Staphylococcus aureus based on magnetic analyte separation via aptamer. Mikrochim Acta 187:43. https://doi.org/10.1007/s00604-019-3880-0
Zhong Z, Gao X, Gao R, Jia L (2018) Selective capture and sensitive fluorometric determination of Pseudomonas aeruginosa by using aptamer modified magnetic nanoparticles. Mikrochim Acta 185:377. https://doi.org/10.1007/s00604-018-2914-3
Zhong Z, Gao R, Chen Q, Jia L (2020) Dual-aptamers labeled polydopamine-polyethyleneimine copolymer dots assisted engineering a fluorescence biosensor for sensitive detection of Pseudomonas aeruginosa in food samples. Spectrochim Acta A Mol Biomol Spectrosc 224:117417. https://doi.org/10.1016/j.saa.2019.117417
Zhou J, Rossi J (2017) Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov 16:181–202. https://doi.org/10.1038/nrd.2016.199
Zhuo Z et al (2017) Recent advances in SELEX technology and aptamer applications in biomedicine. Int J Mol Sci 18. https://doi.org/10.3390/ijms18102142
Acknowledgements
This work was supported by the National Natural Science Fund of China (81903369), Natural Science Foundation of Hunan Province (2019JJ50491, 2020JJ4527), and the Fund of Hengyang Key Laboratory (2018KJ110).
Funding
Dr. Hao Liang was funded by Natural Science Foundation of Hunan Province (2019JJ50491) and National Natural Science Foundation of China (81903369). Dr. Lili Chen was funded by Natural Science Foundation of Hunan Province (2020JJ4527) and Fund of Hengyang Key Laboratory (2018KJ110).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not necessary; this is a review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, D., Liu, L., Huang, Q. et al. Recent advances on aptamer-based biosensors for detection of pathogenic bacteria. World J Microbiol Biotechnol 37, 45 (2021). https://doi.org/10.1007/s11274-021-03002-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03002-9