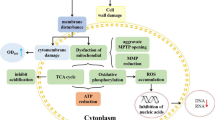
Abstract
Flavonoids from Sedum aizoon L. (FSAL) possess prominent antibacterial activity against Shewanella putrefaciens isolated from sea food. In the current study, the involved molecular mechanisms were investigated using transcriptome analyses combined with bioinformatics analysis in vitro for the first time. Results showed that treatment of FSAL (1.0 MIC) damaged the permeability and integrity of cell membrane and induced 721 differentially expressed genes (DEGs) in tested bacteria at transcriptional levels, including 107 DEGs were up-regulated and 614 DEGs were down-regulated. In addition, the RNA-Seq analysis revealed that the majority of DEGs mainly involved in pathways of lipopolysaccharide biosynthesis, glycerophospholipid metabolism, biosynthesis of amino acids, purine metabolism, ABC transporters and response to stimulus. In summary, the integrated results indicated that the intervention of FSAL induced destruction of cell wall and membrane, disorder of the metabolic process and redox balance, and damage of nucleic acids in S. putrefaciens, at last resulted in the death of cells. This study provided new insights into the anti- S. putrefaciens molecular mechanism underlying the treatment of FSAL, which may be served as the basis guide for identifying potential antimicrobial targets and application of FSAL in food safety.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shewanella putrefaciens, a cold-adapted representative of Shewanella genus (Fonnesbech et al. 2005) diffusely existed in marine environments (Yang et al. 2017), can induce spoilage presented in chilled seafood products especially fresh shrimp even under modified atmosphere packaging (Mace et al. 2014). Result from its higher microorganism metabolic activity, its biochemical actions can generate hypoxanthine from inosine monophosphate or inosine, produce hydrogen sulfide from cysteine, reduce trimethylamine-N-oxide to trimethylamine (Lyu et al. 2018), and contribute to the proteolytic and lipolytic degradations (Ye et al. 2019), thus the formation of volatile compounds produce unpleasant off-odors (Sae-leaw and Benjakul 2019; Yang et al. 2017). Besides, some strains of S. putrefaciens are also assumed to produce tetrodotoxin, one of the neurotoxins as we know (Auawithoothij and Noomhorm 2012). That all leads to decrease the quality of food products. Some recent studies show that S. putrefaciens is also relevant to adhesive biofilm formation on abiotic surfaces and seafood products, resulting in persistent contamination (Liu et al. 2017b; Zhang et al. 2016). Hence, it is vital to control safely the growth of S. putrefaciens on fresh seafood and other products using innovative antimicrobial techniques.
Flavonoids, as a specific genus of secondary plant metabolites, possesses a wide range of bioactive functions such as anti-oxidation, immune regulation, anticancer and antiviral activities (Georgiev et al. 2014). Sedum aizoon L. is regarded as traditional herb widely distributed in China and rich in multiple bioactive constituents including flavonoid, phenolic acid compounds, polysaccharose and alkaloids (Cao et al. 2007; Li et al. 2011; Xu et al. 2015). As a unique natural product, FSAL appears enormous potential in bacteriostatic agent and the antibacterial activities of that against Staphylococcus aureus, Bacillus subtilis and Escherichia coli have demonstrated the study of (Xu et al. 2015) recently. Likewise, our previous research proved the significant effect of FSAL on inhibiting the growth ability of lactic acid bacteria and extending refrigerated storage of fresh pork(Xu et al. 2018). However, up to date, no studies have been carried out to investigate the antibacterial activity of FSAL against S. putrefaciens, and the antibacterial mechanism of FSAL still need to be further elucidated.
With the rapid development of genome sequencing and genomic technologies, transcriptome enables us to globally investigate the complete responses at a transcriptional level (Chen et al. 2019). As yet, transcription analysis has been applied to explore the antibacterial mechanism of numerous bacteriostats, including natural plant ingredients, antibiotics and nano materials (Petek et al. 2010; Sun et al. 2017). Therefore, in this study, transcriptomic analyses combined with bioinformatics analysis were conducted for the first time to explore the molecular mechanism of FSAL against S. putrefaciens in vitro. Differentially expressed genes were identified and then analyzed using Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genome (KEGG). Further investigations including the changes in characteristics of membrane and production of intracellular ROS were performed to support the sequencing data. These results above provided antibacterial mechanism of FSAL in S. putrefaciens, which may be served as the basis for the subsequent study of identifying potential antimicrobial targets to obtain more effective control of this spoilage organism.
Materials and methods
Chemicals and materials
The fresh leaves of Sedum aizoon L. were harvested from the experimental field of Ningbo University (Zhejiang Province, China). Nutrient broth (NB) medium was from Guangdong Huankai Microbial Science & Technology Co., Ltd (Guangzhou, Guangdong, China). Quercetin and gallic acid (HPLC grade) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). AB-8 resin and 2,7′-dichlorofluorescein diacetate (DCFH-DA) were purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). Distilled water was purified using an ultra-pure water system from Heal Force Instruments (Shanghai, China). All other chemicals and reagents were analytical grade.
Preparation of FSAL
The dried leaves of Sedum aizoon L. were prepared and ground into powder using a milling machine, and then passed through a 60-mesh sieve. Briefly, based on our previous experiment(Xu et al. 2018), about 100 g of the powder was extracted with 4 L 70% ethanol at 60 °C for 3 h, homogenized by ultrasonication with a power of 200 W and a frequency of 40 kHz (1 h, 60 °C), then incubated in a 60 °C water bath for 2 h. The extract was concentrated in a vacuum rotary evaporator (Heidolph, Schwabach, Germany). The concentration and double volume of absolute ethanol were placed at 4 °C for 24 h to remove impurities such as protein and polysaccharides. Then the extract was purified using an AB-8 resin. After reaching absorptive saturation, the column was washed by distilled water and then eluted by 80% ethanol. As results, the fractions containing FSAL were concentrated and lyophilized by a freeze-dry system and stored at − 80 °C till further use.
Analysis and characterization of FSAL
The chemical contents of FSAL were determined according to the reported method (Mittal et al. 2013; Zhang et al. 2014) with slight change using high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometer (HPLC-TOFMS) system (Synapt G2, Waters, USA). Briefly, samples were extracted with methanol: the FSAL was dissolved in HPLC grade methanol and cleared by centrifugation (4500×g, 15 min, 4 °C) for preparing sample solution. The pooled supernatant phases were filtered through 0.22 µm filters for analysis. HPLC analysis was performed on Chromatographic separation was conducted on an Agilent Zorbax XDB-C18 column (4.6 × 150 mm, 5 µm), samples were injected (10 µL) into the column and eluted at flow rate of 0.8 mL/min. The mobile phase consisted of formic acid solution (0.1%, A) and methanol (B), and elution was performed with a linear gradient as follows: 0–5 min, A from 70 to 30%; 6–18 min, A from 30 to 10%. The temperature of the column oven was set at 25 °C and detection wavelength was 254 nm. ESI–MS analysis was performed in negative ion mode and the operation parameters were as follows: capillary voltage 2.50 kV, skimmer voltage 50.00 V, the temperature of drying gas 250 °C, flow rate of drying gas 6.66 L/min.
Bacterial strains and culture conditions
The bacteria, S. putrefaciens, obtained from the BeNa Culture Collection (BNCC, 337021), was kept in food biotechnology laboratory of Ningbo University and maintained in slants of nutrient agar (NA) at 4 °C until required. Nutrient broth (NB) was used for pre-cultivation at 30 °C with aeration and agitation (120 rpm). To obtain a fresh experimental culture, the activated S. putrefaciens cells were inoculated in 100 mL of fresh NB medium and incubated at 30 °C in an orbital shaker with 120 rpm till the mid-exponential phase. Then the experimental culture treated with flavonoids from Sedum aizoon L. with a final concentration of 2.0 mg/mL was incubated at 30 °C, and bacterial culture with PBS as control. The minimum inhibitory concentration (MIC, 2.0 mg/mL) was determined by standard broth microdilution assay recommended by the Clinical and Laboratory Standards Institute (Diao et al. 2018). Briefly, two-fold serial dilutions of FSAL were prepared in sterile nutrient broth (NB) to a final volume of 100 µL in sterile 96-well microplates. Then, each well was inoculated with 100 µL of cell culture in the logarithmic phase and the final concentration of FSAL was from 0.00 to 16.00 (0.00, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00) mg/mL. The plate was incubated at 30 °C for 24 h. MIC was defined as the lowest concentration of FSAL at which the growth of the indicator strain was inhibited completely. MIC determination was conducted in triplicate.
Effect of FSAL on S. putrefaciens cell membrane permeability
The effects of FSAL on S. putrefaciens cell membrane were investigated by measuring conductivity and the leakage of protein, reducing sugars and nucleic acids. The bacterial culture treated with FSAL (1.0 MIC) was incubated at 30 °C, meanwhile, only the same volume of PBS was added as the control group. The incubated bacteria were taken at various time intervals (0, 1, 2, 3, 4, 5, 6 h) and centrifugated at 2000×g for 10 min. The supernatant was removed and filtered through 0.22 µm membrane. The release of nucleic acids was determined according to the method described by (Cui et al. 2016) with the OD260 of each supernatant measured. The concentration of protein and total sugars leakage were obtained based on the method of (Das et al. 2013), with minor modifications. Electrical conductivity was measured as described (Cui et al. 2019) with DDS-307 type conductivity meter.
Transmission electron microscopy (TEM) analysis
The effect of FSAL on microscopic morphologies changes of S. putrefaciens was observed using TEM (Liu et al. 2017a). The bacterial culture including the control and FSAL treated were harvested by centrifugation, washed with PBS buffer, subsequently fixed with 2.5% (v/v) glutaraldehyde for 12 h at 4 °C. After being fixed, the samples were rinsed three times in PBS for 15 min at a time and dehydrated with sequential graded cold ethanol (30, 50, 70, and 90%) for 15 min, and ethanol was replaced with anhydrous acetone for 15 min, repeated three times. The specimens were embedded in hard resin after dehydrated and cut into ultra-thin slices (70 nm) using ultramicrotome. The intracellular organization was observed by TEM (H-7650, Hitachi, Japan).
Measurement of ROS production
The production of intracellular ROS detection was analyzed using the fluorescent probe 2,7′-dichlorofluorescein diacetate (DCFH-DA) by confocal laser scanning microscope (CLSM), according to previous reports with some modifications (Ren et al. 2019). The DCFH-DA is non-fluorescent in reduced form and can freely permeates through the cell membrane. Once in the cell, the probe could be hydrolyzed by intracellular esterase to generate DCFH, they are membrane impermeable and trapped inside the cell. When oxidized by ROS, DCFH is converted to the green fluorescing form DCF. Overall, ROS were visualized by fluorescent microplate reader and laser scanning confocal microscope. The bacterial cells were collected till the mid-exponential phase by centrifugation, washed with PBS, re-suspended and mixed thoroughly in presence of 10 µmol/L DCFH-DA and incubated for 20 min at 37 °C in darkness. To fully remove extracellular DCFH-DA, the cells were washed with PBS for 3 times. Then the bacteria were treated with FSAL (1.0 MIC) and the same volume of PBS for 1 h and 3 h at 30 °C. The intracellular ROS values were captured using CLSM (LSM880, Zeiss, Germany) and reported fluorescence intensity.
RNA preparation, library construction and sequencing
The bacterial cells (108 CFU/mL) for RNA-seq analysis were collected 3 h after the addition of FSAL (1.0 MIC), at the same time, only the same volume of PBS was added as the control group. After centrifugation at 14,000×g at 25 °C for 1 min, the supernatant was discarded, then the bacterial pellet was frozen in liquid nitrogen for 4 h and stored at − 80 °C. All treatments were performed in triplicate.
Total RNA was isolated from each sample using the TRIZOL, after extraction, mRNAs were enriched by oligo magnetic beads. Then, for purpose of maximizing the diversity of transcriptional units, the high-quality RNA of 5.0 µg from each sample were mixed into a single large pool. The library construction and the RNA-seq were performed by LC-Bio Technology Co., Ltd., Hang Zhou, Zhejiang Province, China. In general, mRNA was broken into small pieces in fragmentation buffer. RNA fragments were reverse-transcribed to create the final cDNA library using the Truseq™ Stranded RNA sample prep Kit (Illumina, San Diego, USA). The fragments were copied into first strand cDNA using random primers subsequently the second strand cDNA synthesis was performed. The dUTP was used to instead of dTTP for the second strand synthesis, and the index adapters were connected by the Truseq™ Stranded RNA sample prep Kit. Then agarose gel electrophoresis was used to separate and selected fragments and the final sequencing library was acquired by means of PCR amplification. Finally, the paired-end (2 × 150 bp) sequencing was performed on the Illumina HiSeq™ 4000 sequencing platform.
Reads mapping, annotation and analysis
The transcriptome was sequenced by the approach of Illumina paired-end RNA-seq, and a total of million paired-end reads of bp length were generated. To get high-quality data and ensure the accuracy of subsequent biological information analysis, the trimming adapters and removing low-quality sequencing were used to filtered the raw reads. Preparation before assembly, the low-quality reads including reads contained sequencing adaptors, reads contained sequencing primer and nucleotides with q quality values < 20 were discarded. Genes were determined to have different gene expression significantly with ratio changed > twofold with q < 0.05. Besides, in order to eliminate the effect of sequencing artifacts, 75 bp was the minimum acceptance length. The ratios were reported as the up- or down-fold change.
With the analysis of pathway tools, annotation of genes was used to predict biochemical pathways. GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways were retrieved respectively from GO (https://geneontology.org) and KEGG web server (https://www.kegg.jp/kegg).
Defining and analysis of differentially expressed genes
We evaluated the repeated correlation of samples in order to compare gene expression levels in different libraries. To eliminate the effect of differences in gene length and sequencing quantity on calculating gene expression, Reads per Kilobase of Transcript per Million Reads Mapped (RPKM) was used to calculate and normalize the transcript level of each expressed gene. Differentially expressed genes (DEGs) under FSAL treatment were identified as genes with the combined selection standard of fold change (FC) ≥ 2 and a Chi square test (FDR < 0.05).
Quantitative real-time PCR validation
In order to evaluate the validity of Illumina analysis, real-time PCR assay was conducted on S. putrefaciens treated with the same method used in RNA-Seq. Ten DEGs were selected from different pathways to confirm the RNA-Seq results and the relative expression level of the genes was calculated with the 2−ΔΔCT method after normalization, 16S rRNA was used as the internal reference (Liu et al. 2020).
Statistical analysis
All experiments were conducted in triplicates and statistical analysis was performed using the SPSS package program version 19.0 (SPSS Inc., Chicago, IL, USA). Obtained results of comparison between groups were analyzed using one-way analysis of variance (ANOVA). Duncan’s multiple range tests were applied to determine the significant differences with the level of P < 0.05.
Results
Chemical compositions of FSAL
Composition analysis of FSAL was shown in Table 1, as can be seen from the study, quercetin was the major components of FSAL we prepared, followed by gallic acid. The relative contents, retention time and other characterizations of the individual constituent was presented in the results.
Effect of FSAL treatment on S. putrefaciens cell membrane permeability
To analyze cell membrane permeability, the electrical conductivity, protein concentration, sugar concentration and nucleic acids content of the bacterial culture were determined. As shown in Fig. 1a, the constant increase in electrical conductivity was seen in FSAL-treated samples with passage of time, which had significant differences in comparison with the control. The data indicated that some molecules such as Na+, K+ in the cell were leaked due to the addition of FSAL. The protein concentration in suspension of FSAL-treated bacterial culture had a violent rise in the first three hours, and still increased afterwards (Fig. 1b). However, in contrast, that in the control solution did have no significant differences observed as time went by. During the culture period, the sugars concentration in both FSAL-treated and the control samples increased over 6 h time course (Fig. 1c). Meanwhile, after treatment with FSAL, the content of sugars released from cells was markedly higher than the control. Compared with untreated tests, the absorbance value of extracellular nucleic acids of cells had pronounced growth in the first five hours then decreased thereafter (Fig. 1d). However, only little change was observed in control samples. The above results demonstrate that FSAL may profoundly enhance the cells membrane permeability of S. putrefaciens and destroy the membrane structure, causing leakage of intracellular components, thereby affecting the normal physiological metabolism of the cells.
The effects of FSAL (1.0 MIC) on S. putrefaciens cell membrane permeability, and the control group was treated with PBS. The electrical conductivity (a), protein concentration (b), sugar concentration (c) and nucleic acids content (d) of the bacterial culture. Each data in the figures represents the mean ± standard error of three replicate samples. Vertical bars represent standard errors of the means. Asterisks indicate significant differences between FSAL-treated and control samples. The data were analyzed by one-way analysis of variance (ANOVA), *P < 0.05
TEM analysis of S. putrefaciens treated with FSAL
The influence of FSAL on the intracellular organization of S. putrefaciens was observed with TEM. According to Fig. 2a, the intracellular organization of cells from control samples exhibited clear and normal morphology, plump and uniform distribution of cytoplasm and distinct integrity of membranes. In contrast, following treatment with FSAL for 3 h, the ultrastructure of cells was irregularly shaped and disorganized. As shown in Fig. 2b, intracellular structural damage, loss of cytoplasm, discontinuity of membranes and ruptured cell surface were detected. In addition, the destruction of cytoplasmic organization increased with longer processing time. After 6 h of exposure to FSAL, cell structure was further disrupted (Fig. 2c). Due to loss of cytoplasmic components, the lower density of cells was presented, furthermore, vacuolization and cell debris were also emerged under FSAL-treated. Based on ultrastructure analysis, the results of TEM demonstrate that the mechanism of FSAL against S. putrefaciens involves the destruction of cell membrane integrity lead to release of cellular contents and cell death.
Effect of FSAL on the ultrastructure of S. putrefaciens. a TEM images of cells untreated (control); b TEM images of cells treated with FSAL (1.0 MIC) for 3 h; c TEM images of cells treated with FSAL (1.0 MIC) for 6 h. Intracellular structural damage and loss of cytoplasm (arrows #1), discontinuity and ruptured cell surface (arrows #2), cytoplasm material leaked around cell surrounding (arrows #3) as well as vacuolization (arrows #4) were visible
Effect of FSAL treatment on intracellular ROS level in S. putrefaciens cells
The fluorescent probe DCFH-DA was used to detect intracellular ROS, a key factor associated with apoptosis, in S. putrefaciens cells treated with FSAL at 1.0 MIC. DCFH-DA is oxidized to DCF by intracellular ROS, leading to the increase of fluorescence intensity. As shown in Fig. 3, in our study, the intracellular ROS level in S. putrefaciens cells from the control samples had not changed much. On the contrary, after exposing to FSAL, the green fluorescent signal was enhanced in cells. Meanwhile, the accumulation of intracellular ROS dramatically increased as time went on (Fig. 3b, d, f). The result indicates that ROS are produced in S. putrefaciens strains treated with FSAL. Also, ROS enhancement provides oxidative stress sharply and promotes cell damage of S. putrefaciens.
Library construction and RNA-sequencing data
The libraries representing transcriptome of FSAL-treated samples and control samples were constructed for RNA-seq respectively. Absolutely, after getting rid of reads with adaptor contamination, insufficient length, or unacceptable sequencing of low-quality base scores, the clean data of two groups were obtained with average quality values ≥ 30 for more than 94.83% of the reads (Table S1). The results of quality screened reads aligned and mapped to S. putrefaciens reference genome using Rockhopper shown that more than 80.72% of reads in FSAL-treated libraries and 79.16% in control were matched to S. putrefaciens genes, suggesting successful library construction and RNA sequencing.
Identification of DEGs
According to comparative transcriptomic results, FSAL treatment induced significant changes in S. putrefaciens at the mRNA level, indicating a molecular mechanism. Gene expression levels were calculated using FPKM values. As shown in Fig. 4, totally, 721 DEGs were identified in samples with FSAL stress, of which, 107 DEGs were up-regulated and 614 DEGs were down-regulated in comparison with the control group. Table S2 showed description detailed of DEGs between the FSAL-treatment and the control groups.
Distribution of DEGs in FSAL-treated and control S. putrefaciens. a Visualization volcano-plots of DEGs. Each dot represents a gene, red dots (significantly up-regulated genes), blue dots (significantly down-regulated genes) and black dots (non-significantly different genes) for each group indicated on the diagram. b Visualization scatter-plots of DEGs. Colors are as in (a). c Heatmap showing all DEGs. Colors demonstrate expression level patterns in FSAL-treated and control groups. Red cluster represents up-regulated expression and blue cluster represents down-regulated expression
GO classification analysis
Based on the data, in order to gain insight into the functional categories of the DEGs after FSAL intervention, as presented in Fig. 5, DEGs annotated by GO enrichment analysis were classified into three categories of biological process (BP), cellular component (CC) and molecular function (MF), which were composed of 22, 13 and 10 subcategories, respectively. Within the category of BP, the DEGs were mainly distributed in cellular process, metabolic process, response to stimulus and biological regulation. While for the GO functions of CC category, cell, cell part, membrane and membrane part constituted the most abundant components. With regard to MF category, the main groups that DEGs mostly located in were catalytic activity, binding and transporter activity. Table S3 showed the specific description and characteristic of DEGs respectively of BP, CC and MF. In conformity to GO enrichment analysis, the results indicated that the majority of DEGs were found in cell periphery (174 DEGs), plasma membrane (151 DEGs) and membrane (172 DEGs) locations. (Table S3) The results of GO classification between the control and FSAL-treated group showed that FSAL stress obviously affected several membrane functions and major biological processes for S. putrefaciens.
KEGG pathway analysis
To obtain further systematical analysis of the DEGs functions, KEGG pathways significant enrichment analysis, which were tested using KOBAS, was performed to categorize DEGs into different pathways based on bioinformatic databases. As shown in Fig. 6 and Table S4, the results indicated that DEGs were significantly involved in pathway of ABC transporters, flagellar assembly, glycerophospholipid metabolism and two-component system. Moreover, based on the analysis of the data shown in Table S5, DEGs up-regulated by exposure to FSAL were mostly located in pathways of glyoxylate and dicarboxylate metabolism, microbial metabolism in diverse environments, flagellar assembly and carbon metabolism. While DEGs down-regulated were related to biosynthesis of amino acids, lipopolysaccharide biosynthesis, homologous recombination, purine metabolism, biosynthesis of secondary metabolites, metabolic pathways, two-component system and ABC transporters.
RNA-Seq expression validation by quantitative real-time PCR
In order to evaluate the validity of the RNA-Seq data, RT-qPCR assay was used to determine the expression of DEGs with the same RNA samples. The results of DEGs tested were generally in conformity to RNA-Seq results, suggesting that the RNA-Seq results were valid.
Discussion
Cell wall and membrane
As protective barrier to maintain bacterial cell integrity and shape, the cell wall of S. putrefaciens are composed of peptidoglycan, lipopolysaccharide, lipid bilayer and lipoprotein (Brown et al. 2015). Some reports have revealed that the disruption of the cell wall structure has a great impact on the survival of bacterial cells, causing the plasma membrane rupture and cell lysis (Gomes et al. 2020). Composed of phospholipid bilayer and protein, bacterial cell membranes are rich in enzymes that perform many important metabolic functions, including nutrients uptake, homeostasis and the enzyme system contained electron transfer and oxidative phosphorylation, which has some functions of mitochondria (Luo et al. 2014). Thus, even relatively slight changes to the structural integrity of cell membranes can hurtfully influence on cell functions and result in cell death (Yi et al. 2018). In order to elucidate the antibacterial mechanisms of flavonoids, numerous investigators have begun to study the influence of cell wall and membranes under flavonoids treatment (Xu et al. 2018). Hence, as a general phenomenon results in cell death, leakage of intracellular material (Fig. 1) and microscopic morphologies changes (Fig. 2) were investigated in the first of present study to determine effect of FSAL on cell wall structure and membrane permeability. The bacterial plasma membrane supplied a permeability barrier to the passage of small ions such as Na+, K+, which is maintained and regulated by the chemical compositions and structural of the membrane itself (Xiang et al. 2018). The stabilization of necessary electrolytes could maintain proper enzyme activity and promote cell membrane functions, including regulation of metabolism, solute transport, control of turgor pressure and motility (Li et al. 2014). However, remarkable increases in the leakage of ions in FSAL-treated group indicated a disruption of this permeability barrier. Furthermore, compared with the control group, we also found that FSAL caused markedly rapid efflux of cytoplasm material from S. putrefaciens, including protein, nucleic acid and total sugars, which play an important role in many metabolic processes. And they were used as indicators of rough and irreversible damage to the cytoplasmic membrane and inhibition of cell survival (Shen et al. 2015). Consistent with these results, Our TEM observations of S. putrefaciens treated with FSAL decidedly confirmed severe morphological changes even breakdown in structure of the cell wall and membrane. Similar effects were found in phenols and terpenes (Churklam et al. 2020), which exerted their antimicrobial action closely associated with increasing membrane permeability and eventually induced the death of bacterial cell.
Transcriptome analysis was used in the rest of present study to explore the molecular mechanism of FSAL against S. putrefaciens in cell structure. Indeed, based on the results, cell wall and membrane of S. putrefaciens were pretty affected by FSAL treatment. Firstly, according to bioinformatics analysis, the most DEGs were associated with cell wall and membrane. Secondly, the result showed that the genes involved in cell wall structure were significantly differentially expressed in response to FSAL treatment. Compared with the control group, significantly down-regulated genes in the FSAL-treat group related to cell wall component included RS02590, RS04160, RS04545 and RS05150 for lipopolysaccharide biosynthesis. In addition, as well as DEGs (RS13125 and RS16010) for peptidoglycan biosynthesis. As essential ingredient of Gram-negative bacterial cell wall structure, lipopolysaccharide hedges the penetration of antimicrobial components and stimulates the immune function of cell. And the previous study has reported that peptidoglycan biosynthesis was required for cell division and regarded as the targets of antibacterial drug (Brown et al. 2015). Furthermore, in comparison with the control, present study showed that membrane related DEGs RS03115, RS06440 and RS14755 for glycerolipid metabolism, RS03115, RS14755, RS13665, RS03685, RS06440 and RS05190 for glycerophospholipid metabolism, RS19365 for biosynthesis of unsaturated fatty acids, RS04740 for saturated fatty acid biosynthesis, RS16965 for inositol phosphate metabolism and others for encoding membrane protein (15 DEGs for ABC transporters) were significantly down-regulated. At the same time, we also found that RS04980, RS16585 and RS19435 for butanoate metabolism related to maintain the integrity of membrane and RS11765 for RND transporter regarded as major drug efflux pumps in many Gram-negative bacteria were up-regulated expressively. It suggested that cells have begun to adjust their physiology in response to adverse changes around environment. Membrane proteins are responsible for various functions, including nutrient transport, response to environmental stress, biofilm formation and antibiotic resistance (Li et al. 2015; Nakayama et al. 2013). In addition, as one part of them, known for their capability of translocating diverse molecules across cellular membranes in an ATP-dependent manner, ABC transporters are a ubiquitous class of membrane integral efflux transporters. It has also been extensively studied in the past few years about ABC transporters from bacteria (Rees et al. 2009), and numerous DEGs for ATPase activity and transmembrane movement of substances were identified. Therefore, that would be connected to the disruption to transmembrane transport and capacity of using energy of hydrolyzed ATP of cells following FSAL treatment. As we known, phospholipids are importantly principal component of cell membranes, and phosphatidic acid is the key precursor of phospholipid biosynthesis (Lin et al. 2018). Interestingly, it has been studied that, after intervention with morusin (Pang et al. 2019), expression of phosphatidic acid biosynthesis-associated genes was down-regulated significantly. Indeed, fatty acids are the materials for synthesis of phosphatidic acids (Luo et al. 2014) and our data has shown that genes associated with both unsaturated fatty acids biosynthesis and saturated fatty acids biosynthesis pathway were affected after exposing to FSAL. These results indicate that FSAL has potent antibacterial influence on regulation of phospholipids and fatty acid biosynthesis pathway to inhibit the membrane-repair system. Overall, combined with the above analysis, the possible antibacterial mechanisms describing the effect of FSAL on S. putrefaciens involves affecting the protein composition, decreasing synthesis of essential compound required and disrupting integrity and structure of cell wall and membrane.
Amino acid metabolism
Beyond the damage of cell wall and membrane, according to various DEGs related to metabolism process in the present study, S. putrefaciens also underwent metabolism process limitation in the presence of FSAL. Amino acids are the major metabolites involved in the biosynthesis and metabolism of S. putrefaciens. As shown in Table S5, the expressions of many membrane proteins associated with the influx of essential amino acids for bacteria were decreased, which lead to reduce uptake of extracellular nutrients like amino acids. Meanwhile, with uptake limitation of amino acids, previous reports stated that bacteria in turn regulated genes related to synthesis and metabolism of amino acids (Horinouchi et al. 2010; Liu et al. 2020). Under the stimulus of FSAL, RS00975 and RS16585 for tyrosine metabolism, RS04980 and RS10320 for tryptophan metabolism, RS00975 and RS19475 for cysteine and methionine metabolism, RS03990, RS10335 and RS16585 for alanine, aspartate and glutamate metabolism were over-expressed. On the contrary, RS01620, RS06125, RS09215, RS13160 and RS16965 for biosynthesis of amino acids, RS03630, and RS15865 for arginine and proline metabolism RS01620, RS06125 and RS13160 for phenylalanine, tyrosine and tryptophan biosynthesis were significantly down-regulated. Interestingly, it has also shown that bacterial cells commonly activated biosynthetic pathways of some amino acids for survival after several stress treatments (Liu et al. 2020). Natheless, the activation of these biosynthetic pathways also resulted in excessive consumption of several specific amino acids. That indicates that an unbalanced state and metabolism disturbance of amino acids in the cell was produced when treated with FSAL. Therefore, combined with the above considerations, the present study implies that FSAL treatment reduced extracellular amino acid uptake, and then compelled S. putrefaciens cells to acquire several amino acids from intracellular biochemical processes, thus leading to metabolism disturbance and consequently inhibit survival of S. putrefaciens cells.
Oxidative phosphorylation and ROS
In this study, three significantly down-regulated genes (RS11015, RS11050 and RS11225) involved in oxidative phosphorylation were observed in the FSAL-treated group. Oxidative phosphorylation, an intracellular biochemical process, enables the build-up of a proton gradient and ultimately leads to the conversion of ADP to ATP when substance is oxidized. Due to its important part in meeting the energy needs of bacterial survival, oxidative phosphorylation has emerged as major target pathway of new drugs within the last decade. Interestingly, in the case of nutrient deficiency or external stimulation, bacteria have to reduce their metabolic level of oxidative phosphorylation to adapt to the stressful environment they are in (Antonenko et al. 2017). It suggested that FSAL treatment induced energy metabolism disorder and was regarded as a threat against survival of S. putrefaciens cells. In addition, reactive oxygen species (ROS) are an evitable side product of oxidative phosphorylation and considered as intracellular apoptotic factors in bacteria. It was previously described that external stimulus could induce intracellular ROS production and the primary targets are thought to promote the oxidation of nicotinamide adenine dinucleotide (NADH) through the electron transport chain (Huang et al. 2018). In the present study, the accumulation of intracellular ROS dramatically produced in S. putrefaciens strains treated with FSAL (Fig. 3), and transcriptome analysis showed that 6 DEGs associated with detoxification, 6 DEGs related to antioxidant activity and 23 DEGs involved in response to stimulus were up-regulated. Particularly, antioxidant defense systems (RS14135 for thioredoxin TrxA, RS10320 for peroxidase, RS13500 for superoxide dismutase, RS00400 for cytochrome-c peroxidase, RS07550 for peroxiredoxin and RS05455 for glutathione peroxidase were over expressed) was stimulated under oxidative stress produced by FSAL treatment. Meanwhile, previous study has reported that the production of intracellular ROS caused serious cellular damage, lipid peroxidation lead to degradation of the membrane structures of cells, protein peroxidation resulted in metabolism disorder, and DNA breaks (Ren et al. 2019). Thus, it can be inferred that FSAL treatment can give rise to disorder of energy metabolism and imbalance of redox state in S. putrefaciens strains.
DNA replication and repair system
Another possible antimicrobial mode of FSAL was associated with 260-nm absorbing materials included DNA and RNA (nucleic acids), which are the key structural components in the cytoplasm (Ning et al. 2017). As we known, folate regarded as methyl donors plays an important part in the synthesis of DNA nucleotides such as thymine. In this study, DEGs (RS02440, RS02810, RS06430, RS06435 and RS15655) involved in folate biosynthesis were down-regulated in the FSAL-treated group. Therefore, it suggests that FSAL treatment can bring folate deficiency to S. putrefaciens cells and then lead to damage of nucleic acids. In addition, under FSAL stress, the DNA replication and repair system is more important to the survival of S. putrefaciens cells. According to the results of transcriptome analysis, there are many down-regulated DEGs encoding proteins involved that system, mainly containing 5 DEGs for homologous recombination, 3 DEGs for pyrimidine metabolism, 2 DEGs for DNA replication and 8 DEGs for purine metabolism. Particularly, the two down-regulation of DEGs (RS00770 and RS16795) participating in DNA polymerase subunits and the over expression of RS13860 encoding nucleoside phosphorylase were also observed with FSAL intervention. Thus, it implies that FSAL may destroy the processes of DNA replication, repair, and recombination in S. putrefaciens. Similar to our results, a previous study expounded profoundly that action of antibacterial agent might result in generating double-stranded DNA breaks trapped by covalently-linked topoisomerases and then destroy its function (Kang et al. 2020). In summary, as a result of exposure to FSAL, replication machinery and synthesis of DNA was restrained, which leads to inhibit protein biosynthesis and metabolism, eventually cell death.
Conclusions
The study makes use of transcriptome combined with bioinformatics analysis for the first time to obtain an insight into the antibacterial mechanism of FSAL against S. putrefaciens. The results showed that FSAL intervention caused damage permeability and integrity of cell membrane, increased dramatically concentration of intracellular ROS and induced significant changes in numerous genes. For GO enrichment analysis, the DEGs were mainly distributed in membrane functions, metabolic process and response to stimulus. Meanwhile, KEGG pathways analysis indicated that DEGs were mostly involved in pathway of ABC transporters, glycerophospholipid metabolism, biosynthesis of secondary metabolites and two-component system. Thus, the results revealed that FSAL has obvious antibacterial effects that disrupt the structural integrity of cell wall and membranes and detrimentally influence the metabolism process to disturb cellular functions, and result in cell death. The above results provided theory basis for the antibacterial mechanisms of FSAL, which may promote the guide for exploitation and applications of FSAL and other plant-derived bacteriostats in food-related preservation technology.
References
Antonenko YN, Denisov SS, Khailova LS, Nazarov PA, Rokitskaya T, Tashlitsky VN, Firsov AM, Korshunova GA, Kotova EA (2017) Alkyl-substituted phenylamino derivatives of 7-nitrobenz-2-oxa-1,3-diazole as uncouplers of oxidative phosphorylation and antibacterial agents: involvement of membrane proteins in the uncoupling action. Biochim Biophys Acta Biomembr 1859:377–387
Auawithoothij W, Noomhorm A (2012) Shewanella putrefaciens, a major microbial species related to tetrodotoxin (TTX)-accumulation of puffer fish Lagocephalus lunaris. J Appl Microbiol 113:459–465
Brown L, Wolf JM, Prados-Rosales R, Casadevall A (2015) Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol 13:620–630
Cao X, Li S, Wang C, Lu M (2007) Potential use of the herbicide quizalofop-p-ethyl for eicosapentaenoic acid overproduction by the diatom Nitzschia laevis. Chin J Biotechnol 23:885–890
Chen Y, Cheng L, Zhang X, Cao J, Wu Z, Zheng X (2019) Transcriptomic and proteomic effects of (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3"Me) treatment on ethanol-stressed Saccharomyces cerevisiae cells. Food Res Int 119:67–75
Churklam W, Chaturongakul S, Ngamwongsatit B, Aunpad R (2020) The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 108:106864
Cui H, Ma C, Li C, Lin L (2016) Enhancing the antibacterial activity of thyme oil against Salmonella on eggshell by plasma-assisted process. Food Control 70:183–190
Cui H, Zhang C, Li C, Lin L (2019) Antibacterial mechanism of oregano essential oil. Ind Crop Prod 139:111498
Das MR, Sarma RK, Borah S, Kumari R, Saikia R, Deshmukh AB, Shelke MV, Sengupta P, Szunerits S, Boukherroub R (2013) The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloid Surf B Biointerfaces 105:128–136
Diao M, Qi D, Xu M, Lu Z, Lv F, Bie X, Zhang C, Zhao H (2018) Antibacterial activity and mechanism of monolauroyl-galactosylglycerol against Bacillus cereus. Food Control 85:339–344
Fonnesbech VB, Venkateswaran K, Satomi M, Gram L (2005) Identification of Shewanella baltica as the most important H2S-producing species during iced storage of Danish marine fish. Appl Environ Microbiol 71:6689–6697
Georgiev V, Ananga A, Tsolova V (2014) Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients 6:391–415
Gomes TA, Zanette CM, Spier MR (2020) An overview of cell disruption methods for intracellular biomolecules recovery. Prep Biochem Biotechnol 19:1–20
Horinouchi T, Tamaoka K, Furusawa C, Ono N, Suzuki S, Hirasawa T, Yomo T, Shimizu H (2010) Transcriptome analysis of parallel-evolved Escherichia coli strains under ethanol stress. BMC Genomics 11:579
Huang Z, He K, Song Z, Zeng G, Chen A, Yuan L, Li H, Hu L, Guo Z, Chen G (2018) Antioxidative response of Phanerochaete chrysosporium against silver nanoparticle-induced toxicity and its potential mechanism. Chemosphere 211:573–583
Kang S, Kong F, Shi X, Han H, Li M, Guan B, Yang M, Cao X, Tao D, Zheng Y, Yue X (2020) Antibacterial activity and mechanism of lactobionic acid against Pseudomonas fluorescens and methicillin-resistant Staphylococcus aureus and its application on whole milk. Food Control 108:106876
Li W, Luo Q, Wu L (2011) Two new prenylated isoflavones from Sedum aizoon L. Fitoterapia 82:405–407
Li Y, Han Q, Feng J, Tian W, Mo H (2014) Antibacterial characteristics and mechanisms of ɛ-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control 43:22–27
Li YQ, Sun XX, Feng JL, Mo HZ (2015) Antibacterial activities and membrane permeability actions of glycinin basic peptide against Escherichia coli. Innov Food Sci Emerg Technol 31:170–176
Lin Y, Bogdanov M, Lu S, Guan Z, Margolin W, Weiss J, Zheng L (2018) The phospholipid-repair system LplT/Aas in Gram-negative bacteria protects the bacterial membrane envelope from host phospholipase A2 attack. J Biol Chem 293:3386–3398
Liu G, Ren G, Zhao L, Cheng L, Wang C, Sun B (2017a) Antibacterial activity and mechanism of bifidocin A against Listeria monocytogenes. Food Control 73:854–861
Liu J, Yu S, Han B, Chen J (2017b) Effects of benzalkonium chloride and ethanol on dual-species biofilms of Serratia liquefaciens S1 and Shewanella putrefaciens S4. Food Control 78:196–202
Liu M, Feng M, Yang K, Cao Y, Zhang J, Xu J, Hernandez SH, Wei X, Fan M (2020) Transcriptomic and metabolomic analyses reveal antibacterial mechanism of astringent persimmon tannin against Methicillin-resistant Staphylococcus aureus isolated from pork. Food Chem 309:125692
Luo Q, Shi M, Ren Y, Gao H (2014) Transcription factors FabR and FadR regulate both aerobic and anaerobic pathways for unsaturated fatty acid biosynthesis in Shewanella oneidensis. Front Microbiol 5:736
Lyu F, Hong YL, Cai JH, Wei QQ, Zhou X, Ding YT, Liu ZF, Liu L (2018) Antimicrobial effect and mechanism of cinnamon oil and gamma radiation on Shewanella putrefaciens. J Food Sci Technol 55:3353–3361
Mace S, Cardinal M, Jaffres E, Cornet J, Lalanne V, Chevalier F, Serot T, Pilet MF, Dousset X, Joffraud JJ (2014) Evaluation of the spoilage potential of bacteria isolated from spoiled cooked whole tropical shrimp (Penaeus vannamei) stored under modified atmosphere packaging. Food Microbiol 40:9–17
Mittal A, Kadyan P, Gahlaut A, Dabur R (2013) Nontargeted identification of the phenolic and other compounds of Saraca asoca by high performance liquid chromatography-positive electrospray ionization and quadrupole time-of-flight mass spectrometry. ISRN Pharm 2013:293935
Nakayama M, Shimatani K, Ozawa T, Shigemune N, Tsugukuni T, Tomiyama D, Kurahachi M, Nonaka A, Miyamoto T (2013) A study of the antibacterial mechanism of catechins: Isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Control 33:433–439
Ning Y, Yan A, Yang K, Wang Z, Li X, Jia Y (2017) Antibacterial activity of phenyllactic acid against Listeria monocytogenes and Escherichia coli by dual mechanisms. Food Chem 228:533–540
Pang D, Liao S, Wang W, Mu L, Li E, Shen W, Liu F, Zou Y (2019) Destruction of the cell membrane and inhibition of cell phosphatidic acid biosynthesis in Staphylococcus aureus: an explanation for the antibacterial mechanism of morusin. Food Funct 10:6438–6446
Petek M, Baebler S, Kuzman D, Rotter A, Podlesek Z, Gruden K, Ravnikar M, Urleb U (2010) Revealing fosfomycin primary effect on Staphylococcus aureus transcriptome: modulation of cell envelope biosynthesis and phosphoenolpyruvate induced starvation. BMC Microbiol 10:159
Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Biol 10:218–227
Ren X, An P, Zhai X, Wang S, Kong Q (2019) The antibacterial mechanism of pterostilbene derived from xinjiang wine grape: A novel apoptosis inducer in Staphyloccocus aureus and Escherichia coli. LWT-Food Sci Technol 101:100–106
Sae-leaw T, Benjakul S (2019) Prevention of quality loss and melanosis of pacific white shrimp by cashew leaf extracts. Food Control 95:257–266
Shen S, Zhang T, Yuan Y, Lin S, Xu J, Ye H (2015) Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 47:196–202
Sun D, Zhang W, Mou Z, Chen Y, Guo F, Yang E, Wang W (2017) Transcriptome analysis reveals silver nanoparticle-decorated quercetin antibacterial molecular mechanism. ACS Appl Mater Interfaces 9:10047–10060
Xiang Q, Kang C, Niu L, Zhao D, Li K, Bai Y (2018) Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT-Food Sci Technol 96:395–401
Xu F, Wang C, Wang H, Xiong Q, Wei Y, Shao X (2018) Antimicrobial action of flavonoids from Sedum aizoon L. against lactic acid bacteria in vitro and in refrigerated fresh pork meat. J Funct Food 40:744–750
Xu T, Wang Z, Lei T, Lv C, Wang J, Lu J (2015) New flavonoid glycosides from Sedum aizoon L. Fitoterapia 101:125–132
Yang SP, Xie J, Qian YF (2017) Determination of spoilage microbiota of pacific white shrimp during ambient and cold storage using next-generation sequencing and culture-dependent method. J Food Sci 82:1178–1183
Ye J, Yang S, Qian Y, Xie J (2019) Effect of cell-free supernatant from Aeromonas sobria on the spoilage of Shewanella putrefaciens in pacific white shrimp (Litopenaeus vannamei) with the influence of temperature fluctuation. Appl Sci 9:587
Yi L, Li X, Luo L, Lu Y, Yan H, Qiao Z, Lü X (2018) A novel bacteriocin BMP11 and its antibacterial mechanism on cell envelope of Listeria monocytogenes and Cronobacter sakazakii. Food Control 91:160–169
Zhang F, Zhu J, Wang H (2016) Inhibitory activity of tea polyphenols on biofilm development of Shewanella putrefaciens. J Food Process Preserv 40:910–917
Zhang X, Wu Z, Weng P (2014) Antioxidant and hepatoprotective effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3''Me) from Chinese oolong tea. J Agric Food Chem 62:10046–10054
Acknowledgements
This study was supported by Public Welfare Project of Zhejiang Province (LGN19C200012), Natural Science Foundation of Zhejiang Province (LY16C200003), Natural Science Foundation of Ningbo City (2015A610273) and Key Research and Development Projects of Zhejiang Province (2017C02021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Chi, Z., Zhao, K. et al. A transcriptome analysis of the antibacterial mechanism of flavonoids from Sedum aizoon L. against Shewanella putrefaciens. World J Microbiol Biotechnol 36, 94 (2020). https://doi.org/10.1007/s11274-020-02871-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02871-w