Abstract
Probiotics are known to modulate gut microbiota, intestinal barrier function and host immune response, but due to the species and strain specific response their mechanisms are not clearly understood. Thus, the present study was designed to isolate, assess the anti-inflammatory potential and underlying modulatory mechanisms of indigenous probiotics in murine macrophage cell line, RAW 264.7. Forty lactic acid bacteria (LAB) were isolated from different sources and monitored for their anti-inflammatory potential against lipopolysaccharide (LPS) induced inflammatory stress employing RAW 264.7 cells. Among these isolates, only four LAB isolates exhibited more than 90% nitric oxide inhibition and possessed the probiotic attributes. Further, these selected LAB isolates reduced the level of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, inhibited the phosphorylation of Mitogen Activated Protein Kinases (MAPKs) i.e. p38 MAPK, ERK1/2 and SAPK/JNK and expression of cyclooxygenase-2 (COX-2) in LPS stimulated RAW 264.7 cells. The in vitro analysis suggested that the selected probiotic isolates attenuated the LPS-induced inflammation by downregulating MAPK pathway vis-a-vis inhibiting COX-2 and can be employed as anti-inflammatory agents in various inflammatory diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gut microbiota plays an important role in human health and alteration of the normal gut microbiome may lead to various diseases. Presently, functional foods employing natural biointerventions have gained significant importance in various gastrointestinal diseases. Functional foods are dietary components or foods that may provide health benefits beyond basic nutrition such as prebiotics, probiotics and synbiotics (Baboota et al. 2013; Cencic and Chingwaru 2010). Probiotics are live microorganisms which when administered in adequate amount confer health benefits to the host and are referred as the gastrointestinal interference therapy due to their GRAS (Generally recognized as safe) status (Hill et al. 2014). Probiotics impart an array of health benefits in digestive and respiratory functions, suppression of mutagenesis, tumorigenesis, prevention of diarrhea, colitis and constipation which may be attributed to their immunomodulatory response and alleviation of gut inflammation (Kerry et al. 2018; Rajoka et al. 2019; Verma and Shukla 2013; Wilkins and Sequoia 2017).
Inflammation is considered as one of the major causes of many diseases such as inflammatory bowel disease, cancers, cardiovascular diseases, diabetes, obesity, and metabolic syndrome (Laveti et al. 2013). Substantial evidences have demonstrated that gut dysbiosis leads to enhanced gut-derived lipopolysaccharides (LPS), which interacts with macrophages and intestinal epithelial cells, responsible for the development and progression of chronic low-grade inflammation and metabolic disorders via activation of various signaling pathways i.e. nuclear factor-kB (NF-kB), MAPK etc. (Guha and Mackman 2001; Belizário et al. 2018; Singh et al. 2018).
Various in-vitro and in-vivo studies have observed that probiotics modulate the inflammatory response either with its cell-wall associated components, secretory products or modifying the composition of gut microbiota (Llewellyn and Foey 2017; Plaza-Diaz et al. 2017; Li et al. 2019). Since it is known that probiotics are multifactorial and have species and strain specific response, therefore the present study was designed to isolate indigenous probiotics from different sources having anti-inflammatory potential and to investigate their molecular anti-inflammatory mechanisms in murine macrophage RAW 264.7 cell line.
Material and methods
Isolation, maintenance and culture conditions of lactic acid bacteria
Lactic acid bacteria (LAB) were isolated from fermented foods like dosa batter, fresh fruits example orange, apple and infant (1–6 months old) feces collected in sterile containers. Fresh fecal sample from healthy breastfed infants were collected only after obtaining informed consent from their parents. Briefly, 1 g of each sample was serially diluted and plated on De Man Rogosa and Sharpe (MRS) (HiMedia Laboratories, Mumbai, India) agar plate, incubated at 37 °C for 48 h. Suspected colonies were picked individually and further purified by repeated subculturing on MRS agar plates to obtain pure cultures. Preliminary phenotypic characterization of isolated LAB was carried out by performing gram staining, catalase and oxidase test. Purified LAB cultures were preserved in 50% (v/v) sterile glycerol (HiMedia Laboratories) for long term storage at − 80 °C. For experimental use, the isolated LAB were inoculated in MRS broth and incubated at 37 °C overnight, cold centrifuged at 4000×g, washed twice with PBS (pH 7.4) and resuspended at a concentration of 109 colony forming units/ml (CFU/ml).
Cell culture
RAW 264.7 cell lines procured from National Centre for Cell Science, Pune, India were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco, Thermo Fisher Scientific, NY, USA) with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific) and 1% penicillin- streptomycin solution (Gibco, Thermo Fisher Scientific) at 37 °C in 5% CO2 humidified incubator and were regularly subcultured after every 2 days.
Nitric oxide assay
In order to assess the anti-inflammatory activity of isolated LAB, they were screened for their potential to mitigate LPS induced nitric oxide (NO) production in murine macrophage RAW 264.7 cell line as per Singh et al. 2018. Briefly, RAW 264.7 cells (1.0 × 105 cells/ml) were seeded in a 24-well micro plate and treated with LAB isolates (109 CFU/ml) with or without LPS from E. coli 055:B5 (1 µg/ml) (Sigma-Aldrich, St. Louis, USA). However, RAW cells treated with LPS alone (1 µg/ml) served as the positive control. After 16 h of incubation at 37 °C in 5% CO2 humidified incubator, the amount of nitrite was determined by treating the supernatant with equal volume of Griess reagent (2% Sulfanilamide in 5% phosphoric acid and 0.2% N-1-naphthyl ethylenediamine dihydrochloride, 1:1). The optical density (OD) was measured at 540 nm using a microplate reader (Tecan Infinite M200). Results were expressed in terms of percent nitric oxide production and were calculated as \(\left( {{\text{OD of test}}/{\text{OD of positive control}}} \right) \, \times {1}00\). The tests were performed in triplicate and repeated thrice.
Cell viability assay
MTT assay was performed to assess the effect of LAB isolates on the viability of RAW 264.7 cells (Chiang et al. 2012). Briefly, RAW 264.7 cells (1 × 105 cells/ml) were seeded in 96 well culture plates, treated with LAB isolates (109 CFU/ml) and incubated at 37 °C for 16 h. Thereafter, supernatants were discarded and 20 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml) (HiMedia Laboratories) was added followed by incubation in dark at 37 °C for 4 h. Finally, the supernatant was removed and the formazan crystals were dissolved by adding 150 µl of dimethylsulfoxide. OD was measured at 570 nm using microplate reader. The percent cell viability was calculated as \(({\text{OD of test}}/{\text{OD of untreated control}}) \times 100\).
Probiotic characterization of LAB isolates
Isolated LAB possessing anti-inflammatory potential were further monitored for probiotic characters as per the DBT-ICMR guidelines (Ganguly et al. 2011).
Acid and bile tolerance
MRS broth (pH 2.5) was inoculated with overnight grown cultures of selected LAB isolates and incubated at 37 °C for 3 h. To determine the effect of acidic pH on viable LAB counts, samples were collected at different time intervals (0, 1 h, 2 h and 3 h) using the standard spread plate method and expressed as CFU/ml. Further, the bile tolerance of selected LAB isolates was performed using modified MRS broth [0.2% and 0.4% (w/v) of bile salt mixture (HiMedia Laboratories) that was inoculated with different LAB isolates and incubated at 37 °C for 4 h (Zhang et al. 2016). Samples were collected at 0 and 4 h respectively, serially diluted, spread plated onto MRS agar and viable counts were determined by incubating at 37 °C for 24 h whereas standard MRS broth served as the control.
Adhesion assays
Adhesion to hydrocarbons
The cell surface hydrophobicity of isolated and selected LAB was evaluated as per Rosenberg et al. 1980. Briefly, to 3 ml of isolated LAB cell suspension, 1 ml of hydrocarbon (xylene, hexane, chloroform or hexadecane) (Merck, USA) was added, vortexed and incubated at 37 °C for 1 h whereas control comprised of only isolated LAB. Thereafter, optical density of aqueous phase was measured at 600 nm. Cell surface hydrophobicity was calculated in terms of percent hydrophobicity (H%) \({\text{H}}\% = \left( {{1} - {\text{A1}}/{\text{A}}0} \right) \times {1}00;\) where A0 = absorbance of the control; A1 = absorbance of aqueous phase.
Adhesion to Caco-2 cell
Caco-2 cells cultured in DMEM containing 10% fetal bovine serum and 1% penicillin–streptomycin solution were allowed to reach confluency for 14 days by incubating at 37 °C in 5% humidified CO2 incubator. LAB isolates (109 CFU/ml) prepared in DMEM and FBS without antibiotic was added to the confluent monolayer and allowed to adhere in 5% CO2 humidified incubator at 37 °C for 2 h. Culture media was removed and the cell monolayer were washed twice with sterile phosphate-buffered saline (PBS, pH 7.4) to remove non-adherent bacterial cells. Caco-2 monolayers were then treated with 1% Triton X100. The resulting lysates were centrifuged and the pellet was resuspended in 1 ml PBS. Appropriate dilutions were spread on MRS agar plate to calculate viable bacterial counts (CFU/ml) for bound bacterial cells and incubated at 37 °C for 48–72 h. Total bacterial count (CFU/ml) was calculated by spread plating the bacterial suspensions of selected LAB isolates (109 CFU/ml) on MRS agar plates (Singh et al. 2018). Percent binding was calculated as
Adhesion to mucin
In order to assess the mucin binding ability of selected isolates, 96 well black polystyrene plate were coated with mucin (0.5 mg/ml in PBS, pH 7.4) (Sigma-Aldrich, St. Louis, USA) followed by blocking with 1% PBST (PBS with 0.05% tween 20). LAB isolates were labeled with 100 mM CFDA (Carboxyfluorescein diacetate) (Sigma-Aldrich Co.) by incubating at 37 °C for 40 min. CFDA labelled bacteria (200 µl/well) were added to the plate and incubated at 37 °C for 4 h. Unbound cells were removed by washing the wells thrice with 0.05% PBST (pH 7.4). Cells were lysed for 1 h by adding 200 µl lysis solution (1% sodium dodecyl sulfate (w/v) in 0.1 mol/l NaOH) to release the dye from bound cells. Fluorescence was measured in SPECTRA MAX 5Me fluorimeter (Molecular devices LLC, USA) at 485 nm and 520 nm as excitation and emission wavelengths respectively. Adhesion to mucin was expressed as the percentage of fluorescence recovered after binding relative to the fluorescence of CFDA labelled bacterial suspension added to the wells (Singh et al. 2018). Assay was performed in triplicate, and experiment was conducted thrice.
Prebiotic profiling of isolates
Selected LAB isolates were screened for their ability to utilize different prebiotics like inulin, isomaltooligosaccharide (IMOs), resistant starch, fructooligosaccharide (FOS) (Sigma-Aldrich Co.) by agar plate screening. Briefly, MRS agar plates with respective prebiotics at different concentrations (0.5%, 1% and 2% w/v) and 0.035% phenol red indicator were streaked with LAB isolate and incubated at 37 °C for 72 h. Utilization of prebiotic was indicated as change in color from red to yellow (Singh et al. 2018).
Identification of effective LAB isolates
The LAB isolates were further characterized phylogenetically using 16 s rRNA sequencing. Genomic DNA was extracted from the isolates using standard method for bacterial DNA isolation. Quality of DNA was checked using agarose gel electrophoresis and 16S rRNA gene was amplified using universal primers (UNI 8F- 5′ AGAGTTTGATCCTGGCTGAG 3′, UNI 1492R- 5′ GGTTACCTTGTTACGACTT 3′). The conditions for PCR was 94 °C for 4 min, 94 °C for 30 s, 49 °C for 40 s, 72 °C for 100 s and 72 °C for 5 min. Amplicon obtained was purified and sequenced. The sequence obtained was subjected to nucleotide Blast at NCBI database for species level identification of bacteria.
Analysis of molecular markers for anti-inflammatory potential of selected isolates
Quantification of proinflammatory cytokines
RAW 264.7 cells (1.0 × 105 cells/ml) were seeded in a 24-well plate and treated with selected LAB isolates (109 CFU/ml) with or without LPS (1 µg/ml). After 16 h of incubation at 37 °C in 5% CO2 humidified incubator, supernatants were collected. Quantification of proinflammatory cytokines interleukin-6 (IL-6), interleukin-1β (IL-1β), tumor necrosis factor- α (TNF-α) in the supernatants was determined using commercially procured ELISA kits (Elabscience, Texas, USA) as per manufacturer’s instructions.
Western blot analysis
To elucidate the mechanism by which selected LAB isolates inhibit the LPS induced inflammatory stress in macrophages, their effect on the expression of COX-2 and MAPK pathway proteins i.e. p38 MAPK, extracellular signal-regulated kinase (ERK1/2) and stress-activated protein kinase/Jun-amino-terminal kinase (SAPK/JNK) was analysed. The RAW 264.7 cells were cultured in 6-well plates at a density of 1.0 × 105 cells/ml and treated with selected LAB isolates (109 CFU/ml) with or without LPS (1 µg/ml). After 16 h of incubation, the supernatant was pipetted out carefully and the cells were lysed in Radio-Immunoprecipitation Assay buffer (RIPA, Sigma-Aldrich Co.) containing 1% protease phosphatase inhibitor cocktail, centrifugation at 8000×g for 20 min at 4 °C and protein concentration in the cell lysate was determined by Bradford assay (Bradford 1976). Equal amounts (15 µg) of protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated protein was transferred onto PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA) and subsequently blocked with Tris-buffered saline (10 mM Tris–Cl, pH 7.4) containing 0.5% Tween-20 and 5% non-fat dry milk for 1 h at room temperature. The blots were probed with corresponding primary antibodies (phosphorylated ERK1/2, ERK1/2, phosphorylated p38, p38, phosphorylated SAPK/JNK, SAPK/JNK, COX-2, GAPDH, all at 1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA) at 4 °C overnight. After probing with the primary antibodies, the membranes were incubated with horseradish peroxidase conjugated anti-rabbit immunoglobulin G (Ig G, 1:2000 dilution) as secondary antibody. Immunoreactive bands were detected using the enhanced chemiluminescence (ECL) detection system (Amersham Imager 600). The relative densities of protein bands were determined by using Image J software (NIH, Bethesda, Maryland, USA).
LPS binding assay
In order to study the LPS binding potential of LAB isolates, selected LAB (109 CFU/ml) were incubated with LPS (1 µg/ml) for 2 h in DMEM (with 10% FBS and without antibiotic) at 37 °C, cold centrifuged at 4000 × g for 10 min and the supernatant was used to treat the RAW 264.7 (1.0 × 105 cells/ml) cells seeded in a 24-well plate followed by 16 h incubation. The optical density (OD) was measured at 540 nm using a microplate reader (Tecan Infinite M200) and results were expressed in terms of percent nitric oxide production. However, the positive control comprised of RAW cells treated only with LPS (1 µg/ml).
Statistical analysis
Results were analysed statistically and represented as mean ± SD. Statistical significance was calculated using One-way ANOVA followed by Tukey’s post-hoc test. P ≤ 0.05 was considered as significant. Graph pad PRISM-5.0 software was used for all analysis.
Results
Isolation of potential lactic acid bacteria
Total forty LAB isolates were isolated from different sources. Among these, sixteen LAB were isolated from infant feces (#1 to 15 and V), fourteen from fruit peels (#16A to 30A) and ten from fermented foods (#SK1-SK10). Phenotypically, all these LAB isolates showed white, circular, slightly elevated mucoid colony with smooth texture on MRS agar and were Gram positive rods or cocci, catalase and oxidase negative (Supplementary Table S1).
Nitric oxide assay
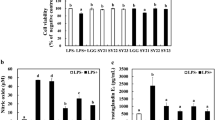
It was observed that among forty LAB isolates, only four isolates, SK2, V, 22A and 26A reduced the NO production to less than 10% (ranging from 5–9%) in LPS-stimulated RAW 264.7 cells compared with untreated LPS stimulated macrophages having 100% NO production. Interestingly, non LPS stimulated RAW 264.7 cells treated with these four selected LAB isolates did not elicit NO production (Fig. 1a, b). Since, isolates SK2, V, 22A and 26A, had maximum nitric oxide inhibition, they were selected and employed to assess their probiotic characteristics and anti-inflammatory mechanism.
a Effect of LAB isolates on nitric oxide production in LPS induced RAW 264.7 cells treated with LPS + LAB isolates; b Effect of LAB isolates on nitric oxide production in RAW cells treated with LAB isolates alone; c Effect of selected LAB isolates on viability of RAW 264.7 cells. Values are mean ± SD. #p < 0.05 v/s untreated control, *p < 0.05 v/s LPS
Cell viability by MTT assay
Cell viability assay was performed to rule out any potential cytotoxicity of selected LAB isolates and it was observed that treatment of RAW 264.7 cells with these four selected LAB isolates did not alter the viability of RAW 264.7 cells and was comparable to untreated control cells that were 100% viable (Fig. 1c).
Probiotic characterization of selected LAB isolates
As it was observed that the four LAB isolates were non toxic to RAW 264.7cells and had maximum nitric oxide inhibition, they were further monitored for probiotic attributes as for human use, the LAB isolates must be able to survive in gastrointestinal tract i.e. tolerate extreme acidic conditions and bile salt concentration.
Acid and bile tolerance
It was interesting to observe that all selected LAB isolates, tolerated the acidic pH (2.5) and bile salt concentration (0.2% and 0.4%) at each point of observation as indicated by the viable cell counts (Table 1).
Adhesion to hydrocarbons
Cell surface hydrophobicity, an important attribute to assess the interaction between bacteria and host epithelial cells was performed with selected LAB isolates using different hydrocarbons (Fig. 2a). It was observed that LAB isolate 26A exhibited maximum hydrophobicity of 41% with hexadecane, followed by SK2 (40%), V (37%) and 22A (29%) respectively. However, with xylene SK2 exhibited maximum hydrophobicity of 20% followed by 26A (16%), V (12%), 22A (11%) and with chloroform also SK2 had maximum hydrophobicity (31%) followed by V (28%), 26A (26%) and 22A (26%) respectively. Further, it was observed that with hexane, LAB isolate 26A showed maximum hydrophobicity of 24% followed by 22A (23%), SK2 (21%) and V (14%) respectively (Fig. 2a). It was interesting to observe that different LAB isolates exhibited different percentage hydrophobicity but isolates SK2 and 26A showed higher percent hydrophobicity to different hydrocarbons compared with other selected LAB isolates.
Adhesion to Caco-2 cells and mucin
For Caco-2 binding assay, LAB isolate 26A was found to exhibit maximum adhesion of 55% followed by SK2 (52%), V (49%) and 22A (30%) whereas SK2 had maximum mucin adherence of 58% followed by 48% (22A), 41% (26A) and 38% of V respectively (Fig. 2b, c).
Prebiotic profiling of isolates
Among four selected LAB isolates, only SK2 and 26A metabolized 1% and 2% IMOs while other prebiotics used were not metabolized by any of the four selected isolates (Table 2; Supplementary Fig. S1).
Identification of effective LAB isolates
The four selected LAB isolates exhibiting maximum nitric oxide inhibition and possessing probiotic characters were identified phylogenitically by 16 s rDNA sequencing. Sequences obtained were submitted to the Gen Bank and LAB isolate SK2 was identified as Lactobacillus pentosus (Gen Bank ID: MK955491), isolate V as Lactobacillus fermentum (Gen Bank ID: KT998657), isolate 22A as Weissella cibaria (Gen Bank ID: MK294344) and isolate 26A as Lactobacillus plantarum (Gen Bank ID: MK955489).
Analysis of molecular markers for anti-inflammatory potential of selected probiotic isolates
Quantification of proinflammatory cytokines
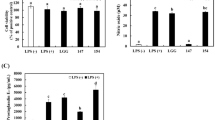
The anti-inflammatory potential of selected probiotics was further confirmed by their inhibitory effect on LPS-induced inflammatory cytokines in RAW 264.7. It was observed that all four selected probiotics significantly (p < 0.05) inhibited the secretion of proinflammatory cytokines (IL-6, IL-1β, TNF-α) in LPS stimulated RAW264.7 cells compared with enhanced secretion of proinflammatory cytokines in untreated LPS stimulated RAW 264.7 cells (Fig. 3). However, unstimulated and probiotic treated RAW 264.7 cells did not significantly (p < 0.05) stimulate the production of proinflammatory cytokines, IL-6 and TNF-α as compared to untreated control for all the selected isolates. Similarly, treatment of unstimulated RAW 264.7 cells with isolate SK2 and 22A did not significantly (p < 0.05) increase the production of IL-1β.
Western blot analysis
It was observed that treatment of LPS stimulated RAW 264.7 cells with selected probiotics significantly (p < 0.05) inhibited the expression of COX-2 (Fig. 4a). Further, it was observed that LAB isolate SK2, 26A and V significantly (p < 0.05) suppressed the phosphorylation of p38 MAPK, ERK1/2 and SAPK/JNK in LPS treated RAW 264.7 cells compared with LPS treated RAW 264.7 cells. However, LAB isolate 22A significantly (p < 0.05) suppressed the phosphorylation of ERK1/2 and SAPK/JNK but did not show significant inhibition of p38 MAPK (Fig. 4 b–d).
Effect of selected LAB isolates on the expression of MAPK pathway in LPS stimulated RAW 264.7 cells: a expression of COX-2 relative to GAPDH, b expression of P-ERK 1/2 relative to ERK 1/2, c expression of P-P38 relative to P-38 and d expression of P-SAPK relative to SAPK. Values are mean ± SD. *p < 0.05 v/s LPS
LPS binding assay
It was observed that NO production was significantly (p < 0.05) less in RAW 264.7 cells treated with supernatant of LAB isolate previously incubated with LPS compared to RAW 264.7 cells treated with LPS alone (Fig. 5).
Discussion
Gut microbiota, a symbiotic partner of good health, is gaining interest for its comprehensive physiological and pathological functions as it plays an important role in gut homeostasis mainly by regulation of intestinal junctions, gut permeability, nutrient absorption, immune-modulation etc. (Plaza-Diaz et al. 2014). Moreover, there is growing evidence indicating that dysbiosis of the gut leads to various gastrointestinal diseases and metabolic disorders such as obesity, diabetes and chronic inflammation. Thus, manipulation of gut microbiota by supplementation of probiotics, is an active area of research and warrants further investigation (Carding et al. 2015). Therefore, the present study was aimed at isolating indigenous probiotics exhibiting anti-inflammatory potential that may be used in attenuating various metabolic disorders.
Recent studies have indicated that dysbiosis disrupts the intestinal barrier function resulting into LPS induced macrophage activation vis-à-vis NO generation and triggering of the pro-inflammatory cascade (Belizário et al. 2018). Interestingly, it was observed that among various isolated LAB, only selected isolates significantly reduced the NO production in LPS activated macrophages indicating their anti-inflammatory potential. The reduced NO production by LAB isolates may be due to downregulation of inducible nitric oxide synthase, the main mediators of various chronic inflammatory diseases (Oh et al. 2012). In earlier studies too, scientists have demonstrated that various probiotics Lactobacillus, Bifidobacteria and Weissella strains inhibited the level of nitric oxide in RAW 264.7 cells and HT 29 cells (Oh et al. 2018; Singh et al. 2018; Li et al. 2019). Additionally, it was observed that supernatants of all selected LAB isolates coincubated with LPS significantly reduced the production of NO in RAW 264.7 cells, indicating the binding of LPS with these LAB isolates thereby inhibiting LPS induced macrophage activation (Thomas and Versalovic 2010). Similarly, Park et al. (2007) have also reported that probiotic Bifidobacteria strains have the LPS binding ability.
It was interesting to observe that all the selected probiotics significantly reduced the level of proinflammatory cytokines (IL-6, IL-1β, TNF-α), the key mediators of inflammation in LPS induced RAW 264.7 cells and corroborates with earlier studies (Khokhlova et al. 2012; Oh et al. 2018). These scientists have also found that Lactobacillus rhamnosus, Lactobacillus gaserri, Lactobacillus acidophilus, Bifidobacteria and Weissella strains inhibited the level of proinflammatory cytokines in RAW 264.7 cells, Caco 2 and HT 29 cells. The reduced level of proinflammatory cytokines may be due to the inhibition of multiple inflammatory pathways like nuclear factor kB (NFkB) and MAPKs by these selected probiotic strains (Striz et al. 2014).
In order to understand the underlying molecular mechanism of anti-inflammatory potential of selected probiotics, the expression of COX-2 was studied. Interestingly, the selected probiotic isolates reduced the expression of COX-2 in LPS-stimulated RAW 264.7 cells and is in concordance with earlier study where scientists have also reported the anti-inflammatory potential of Lactobacillus casei 3260 due to the inhibition of COX-2 via suppression of NFkB in RAW 264.7 cells (Lee et al. 2008). Further, it was found that most of the selected probiotic isolates also reduced the phosphorylation of all the MAPKs in LPS-stimulated macrophages and corroborates with previous study (Li et al. 2019). Li et al. (2019) have observed that combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. Lactis reduced the expression of p38 MAPK in LPS induced HT-29 cells whereas, Griet et al. (2014) reported that anti-inflammatory effect of Lactobacillus reuteri CRL1098 was due to its metabolites and was not induced by MAPK inhibition. In another study, Jeong et al. (2015) have documented that lipoteichoic acids from different lactic acid bacteria activate MAPK signaling pathway in RAW 264.7 cells to different extents thereby highlighting the species and strain specificity of LAB. Additionally, all four selected LAB possessed the probiotic attributes and did not affect the viability of RAW 264.7 cells indicating their GRAS status and suitability for human use (Ganguly et al 2011).
Based on this study, the proposed anti-inflammatory molecular mechanism of isolated and selected probiotics may be attributed to the inhibition of inflammatory mediators such as NO, proinflammatory cytokines and down-regulation of COX-2 due to suppression of MAPK pathway that in turn regulated NFkB signaling vis-à-vis LPS binding ability of the probiotics leading to the inhibition of LPS induced macrophage activation (Fig. 6).
The results of the present study indicate that the selected indigenous probiotics possess anti-inflammatory activity. However, detailed study is underway to validate these in vitro observations in experimental model of metabolic syndrome.
References
Baboota RK, Bishnoi M, Ambalam P et al (2013) Functional food ingredients for the management of obesity and associated co-morbidities—a review. J Funct Foods 5:997–1012
Belizário JE, Faintuch J, Garay-Malpartida M (2018) Gut microbiome dysbiosis and immunometabolism: new frontiers for treatment of metabolic diseases. Mediat Inflamm. https://doi.org/10.1155/2018/2037838
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Carding S, Verbeke K, Vipond DT et al (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. https://doi.org/10.3402/mehd.v26.26191
Cencic A, Chingwaru W (2010) The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2:611–625
Chiang SS, Liu CF, Tseng KC et al (2012) Immunomodulatory effects of dead Lactobacillus on murine splenocytes and macrophages. Food Agric Immunol 23(2):183–202
Ganguly NK, Bhattacharya SK, Sesikeran B et al (2011) ICMR-DBT guidelines for evaluation of probiotics in food. Indian J Med Res 134:22–25
Griet M, Zelaya H, Mateos MV et al (2014) Soluble factors from Lactobacillus reuterii CRL1098 have anti-inflammatory effects in acute lung injury induced by lipopolysaccharide in mice. PLoS ONE 9(10):e110027. https://doi.org/10.1371/journal.pone.0110027
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Sign 13:85–94
Hill C, Guarner F, Reid G et al (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Jeong JH, Jang S, Jung BJ et al (2015) Differential immune-stimulatory effects of LTAs from different lactic acid bacteria via MAPK signaling pathway in RAW 264.7 cells. Immunobiology 220(4):460–466
Kerry RG, Patra JK, Gouda S et al (2018) Benefaction of probiotics for human health: a review. J Food Drug Anal 26(3):927–939. https://doi.org/10.1016/j.jfda.2018.01.002
Khokhlova EV, Smeianov VV, Efimov BA et al (2012) Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol 56:27–39
Laveti D, Kumar M, Hemalatha R et al (2013) Anti-inflammatory treatments for chronic diseases: a review. Inflamm Allergy Drug Targets 12(5):349–361
Lee JM, Hwang KT, Jun WJ et al (2008) Antiinflammatory effect of lactic acid bacteria: inhibition of cyclooxygenase-2 by suppressing nuclear factor-κB in Raw 264.7 macrophage cells. J Microbiol Biotechnol 18(10):1683–1688
Li SC, Hsu WF, Chang JS, Shih CK (2019) Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis shows a stronger anti-inflammatory effect than individual strains in HT-29 cells. Nutrients 11(5):969. https://doi.org/10.3390/nu11050969
Llewellyn A, Foey A (2017) Probiotic modulation of innate cell pathogen sensing and signaling events. Nutrients 9(10):1156. https://doi.org/10.3390/nu9101156
Oh NS, Joung JY, Lee JY, Kim Y (2018) Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 13(2):e0192021. https://doi.org/10.1371/journal.pone.0192021
Oh Y, Cho W, Oh JH et al (2012) Fermentation by Lactobacillus enhances antiinflammatory effect of Oyaksungisan on LPS stimulated RAW 264.7 mouse macrophage cells. BMC Complement Altern Med 12:17. https://www.biomedcentral.com/1472-6882/12/17
Park MS, Kim MJ, Ji JE (2007) Assessment of lipopolysaccharide-binding activity of Bifidobacterium and its relationship with cell surface hydrophobicity, autoaggregation, and inhibition of interleukin-8 production. J Microbiol Biotechnol 17(7):1120–1126
Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A (2014) Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol 20(42):15632–15649
Plaza-Diaz J, Ruiz-Ojeda FJ, Vilchez-Padia LM, Gill A (2017) Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 9(6):555. https://doi.org/10.3390/nu9060555
Rajoka MSR, Zhao H, Mehwish HM et al (2019) Anti-tumor potential of cell free culture supernatant of Lactobacillus rhamnosus strains isolated from human breast milk. Food Res Int 123:286–297. https://doi.org/10.1016/j.foodres.2019.05.002
Rosenberg M, Gutnick D, Rosenberg E (1980) Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett 9(1):29–33
Singh S, Bhatia R, Singh A et al (2018) Probiotic attributes and prevention of LPS-induced pro-inflammatory stress in RAW264.7 macrophages and human intestinal epithelial cell line (Caco2) by newly isolated Weissella cibaria strains. Food Funct 9(2):1254–1264
Striz I, Brabcova E, Kolesar L, Sekerkova A (2014) Cytokine networking of innate immunity cells: a potential target of therapy. Clin Sci 126(9):593–612
Thomas CM, Versalovic J (2010) Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 1:148–163
Verma A, Shukla G (2013) Probiotic Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Sprague Dawley rats. Nutr Cancer 65:84–91
Wilkins T, Sequoia J (2017) Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician 96(3):170–178
Zhang B, Wang Y, Tan Z et al (2016) Screening of probiotic activities of Lactobacilli strains isolated from traditional tibetan Qula, a raw yak milk cheese. Asian-Australas J Anim Sci 29(10):1490–1499
Acknowledgements
The financial support provided by the Indian Council of Medical Research, India (3/1/3 JRF-2016/HRD-51) for carrying the present study; Panjab University, Chandigarh and National Agri-food Biotechnology Institute, Mohali and Department of Biotechnology, Government of India, New Delhi for providing core funds and facilities are highly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict of interests among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khanna, S., Bishnoi, M., Kondepudi, K.K. et al. Isolation, characterization and anti-inflammatory mechanism of probiotics in lipopolysaccharide-stimulated RAW 264.7 macrophages. World J Microbiol Biotechnol 36, 74 (2020). https://doi.org/10.1007/s11274-020-02852-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02852-z