Abstract
Ganoderma boninense, the main causal agent of oil palm (Elaeis guineensis) basal stem rot (BSR), severely reduces oil palm yields around the world. To reduce reliance on fungicide applications to control BSR, we are investigating the efficacy of alternative control methods, such as the application of biological control agents. In this study, we used four Streptomyces-like actinomycetes (isolates AGA43, AGA48, AGA347 and AGA506) that had been isolated from the oil palm rhizosphere and screened for antagonism towards G. boninense in a previous study. The aim of this study was to characterize these four isolates and then to assess their ability to suppress BSR in oil palm seedlings when applied individually to the soil in a vermiculite powder formulation. Analysis of partial 16S rRNA gene sequences (512 bp) revealed that the isolates exhibited a very high level of sequence similarity (> 98%) with GenBank reference sequences. Isolates AGA347 and AGA506 showed 99% similarity with Streptomyces hygroscopicus subsp. hygroscopicus and Streptomyces ahygroscopicus, respectively. Isolates AGA43 and AGA48 also belonged to the Streptomyces genus. The most effective formulation, AGA347, reduced BSR in seedlings by 73.1%. Formulations using the known antifungal producer Streptomyces noursei, AGA043, AGA048 or AGA506 reduced BSR by 47.4, 30.1, 54.8 and 44.1%, respectively. This glasshouse trial indicates that these Streptomyces spp. show promise as potential biological control agents against Ganoderma in oil palm. Further investigations are needed to determine the mechanism of antagonism and to increase the shelf life of Streptomyces formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil palm diseases, such as vascular wilt (Fusarium oxysporum f. sp. elaedis), basal stem rot (Ganoderma sp.), red ring disease (Rhadinaphelenchus cocophilus), sudden wilt (Phytomonas staheli) and bud rot disease (Phytophthora palmivora) (Idris 2011), can have a severe economic impact on palm oil production. In Malaysia, basal stem rot (BSR) disease is the most serious disease of oil palm and a solution to this problem needs to be found urgently. The yields obtained from an infected oil palm are reduced owing to their reduced weight and the production of fewer fruit bunches. The yield of fresh fruit bunches (FFB) from infected palms is only 19–43% of that obtained from uninfected palms (Idris 2012). According to Singh (1991), a disease incidence of 31–67% can lead to a reduction in FFB yield of approximately 26–46%. In severe infection cases, palms are prone to collapse and death. In 2009–2010, the incidence of BSR disease was 3.71% with 59,148 hectares of palm oil affected, which resulted in estimated yield losses of RM 1.5 billion (~ US$480 million) (Arif et al. 2011; Idris et al. 2011). Many different strategies have been recommended and applied to manage the disease and to prolong the economic lifespan of the palm, including cultural practices, sanitation, chemical fungicides and biological control (Idris 2012). Biological control is an attractive and green alternative to chemical fungicides. Introducing an antagonistic control agent to the rhizosphere area should manipulate the soil’s indigenous microorganism communities, leading to the suppression of soil-borne pathogens, and should interfere with the survival or disease-producing activities of the phytopathogen. Although the control of pathogens using a biological control agent is not widely practiced on a commercial scale, a number of experimental approaches have been reported that show the potential of beneficial microorganisms, including actinomycetes, bacteria and fungi, for further development as biological control agents. For example, Azizah (2003) reported that seedlings inoculated with beneficial microbes were protected from Ganoderma. Most of the reports on promising microbial agents for controlling Ganoderma have emphasized the potential of endophytic fungi (Nurrashyeda et al. 2011), Trichoderma spp. (Sariah et al. 2005; Susanto et al. 2005; Sundram et al. 2008), mycorrhiza (Sundram et al. 2015), Pseudomonas sp. and Burkholderia sp. (Maizatul Suriza and Idris 2009; Zaiton et al. 2008; Nurrashyeda et al. 2016), with little attention given to actinomycetes. However, Tan et al. (2002) showed that actinomycetes were able to affect the growth of Ganoderma in vitro. Our initial in vitro study showed that Streptomyces sp. was able to inhibit the growth of G. boninense in dual inoculations on culture plates (Shariffah-Muzaimah et al. 2015).
Actinomycetes are widely distributed and are one of the major groups of the soil population (Kustner 1968), representing a large part of the rhizosphere microbial flora (Sardi et al. 1992). The important roles played by actinomycetes in the rhizosphere in terms of influencing plant growth and protecting plant roots against invasion by root pathogenic fungi have been recognized by studies around the world (Cao et al. 2004; Crawford et al. 1993). Actinomycetes, especially Streptomyces species isolated from the rhizosphere, have been reported to have potential as biological control agents for soil-borne plant diseases (Shimizu et al. 2000), such as Pythium ultimum (Crawford et al. 1993), Colletotrichum spp. (Intra et al. 2011; Taechowisan and Lumyong 2003), Fusarium oxysporum (Alimuddin et al. 2011; Getha and Vikineswary 2002; Taechowisan and Lumyong 2003), Phytophthora sp. (Xiao et al. 2002), Magnaporthe oryzae (Ebrahimi-Zarandi et al. 2009) and Sclerotium rolfsii (Errakhi et al. 2007). These inhibitory effects are probably related to their ability to produce biologically active metabolites such as antibiotics. Over 50% of the 10,000 antimicrobial compounds known to be produced by microorganisms are produced by actinomycetes (Anderson and Wellington 2001). Furthermore, approximately 60% of the biologically active compounds developed for agricultural use originated from Streptomyces species (Ilic et al. 2007). Many studies around the world have shown that species belonging to the Streptomyces genus are able to reduce the growth of fungal plant pathogens, such as Sclerotinia sclerotiorum (Baniasadi et al. 2009), Helminthosporium solani (Elson et al. 1997), damping-off in tomato (Sabaratnam and Traquair 2002), Rhizoctonia solani (Shahrokhi et al. 2005) and Fusarium wilt in tomato (Anitha and Rabeeth 2009). However, there are no published reports on the use of actinomycetes as a biological control against G. boninense in oil palm. This paper describes the characterization of rhizosphere actinomycetes isolated from oil palm plantations and assessed their potential to control Ganoderma disease in oil palm seedlings under glasshouse conditions.
Materials and methods
Culture of Streptomyces-like actinomycete isolates
The four Streptomyces-like actinomycetes (AGA43, AGA48, AGA347 and AGA506) used in this study had been isolated as part of a previous study from the rhizosphere soil of healthy 20-year-old oil palms (Elaeis guineensis Jacq.) growing in oil palm plantations with some history of Ganoderma boninense infection (Shariffah-Muzaimah et al. 2015). Of the 600 actinomycetes that were isolated in our initial investigation, these four isolates showed the strongest in vitro antagonistic activity against G. boninense in dual culture inoculations (Shariffah-Muzaimah et al. 2015). See Shariffah Muzaimah et al. (2015) for the methods used to isolate and culture the actinomycetes. Spore and mycelial suspensions (1 ml) of each isolate were maintained at − 80 °C in 20% (v/v) glycerol for long-term preservation. Working cultures were obtained by streaking one loopful of the stock suspension onto a yeast extract-malt extract isolation medium (International Streptomyces Project Medium No 2, ISP2), and incubated at 28 °C until a well-sporulated mature colony was obtained.
Cultural and morphological characterization
Cultural characterization was performed by referring to the International Streptomyces Project (ISP). All the isolates were streaked on ISP2, oatmeal agar (ISP3), inorganic salts-starch agar (ISP4) and glycerol-asparagine agar (ISP5) (Shirling and Gottlieb 1966). The cultural characteristics of the isolates on various media were recorded after incubation at 28 ± 2 °C for 7 –14 days. The morphological and cultural characteristics of the isolates were assessed by observing the colour of mature sporulating aerial mycelium (the surface colour of the isolates), substrate mycelium (the colour of the isolates on the reverse side of the culture) and soluble pigment production on the culture media, other than brown or black (Lyons and Pridham 1965; Pridham 1965; Shirling and Gottlieb 1966). All the isolates were streaked on ISP2 agar before placing sterile microscope slide covers on the culture streaks. The cover slides were viewed under a light microscope after 7 days of incubation at 28 ± 2 °C. The appearance of hyphae and spore chains was assessed using Shirling and Gottlieb (1966) and Sousa et al. (2008).
Molecular identification
The isolates were grown in ISP2 liquid medium for 4 days at 28 ± 2 °C with continuous shaking at 150 rev min−1. The biomass was harvested by centrifugation at 10,000×g for 15 min and washed thrice with 50 ml of sterile distilled water (Franco-Correa et al. 2010). Between 100 mg and 1 g of biomass was ground in liquid nitrogen using a sterile mortar and pestle. The homogenized tissues were transferred to a clean tube for genomic DNA extraction using a DNeasy Plant Mini Kit (Qiagen, Venlo, The Netherlands) following the manufacturer’s protocol. The lysate (DNA) obtained was then stored at − 20 °C until ready for further analysis.
The PCR amplification reaction was performed in a total volume of 20 µl of PCR mixture (Invitrogen, Waltham, MA, USA) containing 2 µl of DNA template (50–500 ng µl−1), 2 µl of 10 × PCR buffer, 2 µl of 25 mM MgCl2, 1 µl of 10 mM dNTPs (0.25 µl of each of dCTP, dGTP, dATP and dTTP), 0.5 µl of 1U µl−1 Taq polymerase, 10.5 µl of sterile distilled water, 1 µl of 10 nM forward 16S rDNA primer and 1 µl of 10 nM reverse 16S rDNA primer. The primers used in this study were 16SF (5′ ACGTGCCAGCAGCCGCGGTAATAC 3′), 16SR (5′ GGCCCCCGTCAATTCCTTTGAGTTT 3′), 16SAct1F [16SAct1F (5′ CGCGGCCTATCAGCTTGTTG 3′)] and 16S Act1R (5′ CCGTACTCCCCAGGCGGGG 3′). All oligonucleotide primers were synthesized by 1st Base Sdn Bhd, Selangor, Malaysia. The amplification was performed in a Vapoprotect thermal cycler (Eppendorf, Hamburg, Germany). The PCR programme involved 5 min of initial denaturation at 94 °C, followed by 35 cycles of denaturation for 1 min at 95 °C, annealing for 30 s at 58 °C and elongation for 1 min at 72 °C, and a final 10 min extension at 72 °C. The size of the PCR product was estimated to be 512 bp. Amplicons were analysed using electrophoresis on 1% agarose gel containing 5 µl of ethidium bromide (EtBr) and run at 70 volts (V) for 1 h in 1 × TBE (1 M Tris-base, 1 M boric acid, 0.02 M EDTA, 1 l ddH2O, pH 8). The resulting DNA patterns were examined under UV light and photographed by transillumination using Biospectrum Imaging Systems (UVP, USA).
A single band amplicon was subsequently excised and purified using a gel extraction kit (Qiagen) according to manufacturer’s instruction. The purified PCR products were sequenced using an ABI DNA analyser (NHK Bioscience Solutions Sdn Bhd, Kuala Lumpur, Malaysia). The sequences obtained were compared to actinomycete reference sequences in the NCBI GenBank database using the Basic Alignment Search Tool (BLASTn, http://blast.ncbi.nlm.nih.gov/). Multiple sequence alignments were performed using ClustalW in Biology Workbench (http://seqtool.sdsc.edu/) using the sequences obtained from this study and additional sequences obtained from GenBank or published elsewhere. A phylogenetic tree of partial 16S rDNA sequences was constructed using the MEGA6 program (Tamura et al. 2011) using the neighbour-joining method (Saitou and Nei 1987) for grouping and characterization of the submitted sequences. A bootstrap analysis was performed using 1000 resamplings of the data to provide statistical confidence values for the tree branches. The actinomycetes Actinoplanes regularis, Actinomyces urogenitalis, Nocardia asteroides and Micromonospora sp. were included as outgroups.
Extracellular enzymatic activity
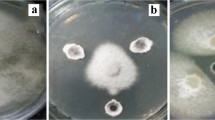
To determine the enzymatic activity of the isolates, agar plugs were excised from well-sporulating cultures and transferred to Tween 80 agar to determine lipase production, carboxymethylcellulose (CM-cellulose) agar to determine cellulase production and colloidal chitin agar to determine chitinase production. After 7 days of incubation at 28 °C, the formation of a clear zone in the growth media around the colonies was noted. CM-cellulose plates were stained with 0.1% Congo red: clear areas around the colonies indicated cellulase activity (Sahilah et al. 2010; Gopalakrishnan et al. 2013). Siderophore production was determined using the double Chrome azurol S (CAS) agar method (Hu and Xu 2011). The CAS agar plates were spot inoculated with the isolates and incubated for 24–48 h at 28 °C. The formation of a distinct orange zone around a colony indicated positive siderophore production.
β-1,3-Glucanase activity was determined using the method described by Singh et al. (1999). All the actinomycetes were cultured in ISP2 broth supplemented with 1% (weight/volume) colloidal chitin and incubated at 28 °C. After 4 days of incubation, the cultures were centrifuged at 10,000×g for 15 min and the supernatants collected. Then, 1 ml of the supernatant was added to 0.1 ml of laminarin solution (2%, w/v) in 0.2 M acetate buffer (pH 5.4) and incubated at 40 °C for 1 h. Next, 3 ml of dinitrosalicylic acid (DNS) were added to the mixture and boiled for 10 min. The development of a dark-red colour indicated the presence of a reducing sugar. The concentration of the reducing sugar was determined by measuring the absorbance at 540 nm using a UV–Vis spectrophotometer (Genesys 10S UV–Vis, Thermo Fisher Scientific, Waltham, MA, USA). A standard curve was prepared using glucose ranging from 0 to 1 mg ml−1 with intervals of 0.2 mg ml−1. One unit of β-1,3-glucanase activity was defined as the amount of enzyme that liberated 1 mol of glucose h−1 under the defined conditions.
Phosphate solubilization activity was determined following the method described by Katznelson and Bose (1959). Agar plugs excised from cultures of the isolates were transferred to phosphate solubilization agar consisting of 10 g of glucose, 20 g of agar, 2 g of asparagine, 0.5 g of MgSO4, 0.1 g of NaCl, 0.1 g of KCl, 0.5 g of yeast extract and 1 l of distilled water. The pH was adjusted to 7.0 with 1 M NaOH before autoclaving. Fifty millilitres of sterile 10% K2HPO4 solution and 100 ml of sterile 10% CaCl2 solution were added aseptically after autoclaving. The formation of a clear area around the colonies indicated phosphate solubilization activity. The production of indole acetic acid (IAA) was determined according to the method described by Bric et al. (1991). A single colony of each of the isolates was streaked onto ISP2 supplemented with 5 mM tryptophan, 0.06% sodium dodecyl sulfate (SDS) and 1% glycerol. The plates were then overlaid with a nitrocellulose membrane and incubated at 28 °C. After 7 days of incubation, the membrane was removed and soaked into Salkowski’s reagent until adequate colour developed. Isolates with positive IAA production were identified by the formation of a red halo surrounding the colony area on the membrane. Four replicates of each assay were performed.
Effect of pH, salinity and temperature on isolate growth
The effect of pH, salinity and temperature on isolate growth on ISP2 agar was investigated. Normal ISP2 agar was used to determine the temperature effect. Isolates were streaked on agar and incubated at 28, 30, 40 or 50 °C for 7 days. ISP2 agar media with pHs ranging from 4.5 to 8.5 were used to determine the effect of pH on growth. The isolates were also streaked on ISP2 media supplemented with sodium chloride (NaCl) at concentrations of 0, 2, 4, 6, 8, 10 or 12% (Gopalakrishnan et al. 2014). The plates used to assess salinity and pH effects were incubated at 28 °C in an inverted position to avoid evaporation. The growth of each isolate was recorded on the seventh day.
Preparation of Streptomycete powder formulations
Inoculum of each of the four Streptomyces-like isolates and of Streptomyces noursei ATCC 11455 (a standard Streptomyces strain that produces the antifungal compound nystatin) was prepared by inoculating a flask of production medium consisting of molasses, yeast extract, starch, pharmamedia and trace elements and a pH of 7 with 10 ml of starter culture of each isolate. The inoculated flask was incubated at 28 °C on a rotary shaker (215 rev min−1) for 7 days. Starter medium was prepared by inoculating individual flasks containing starter medium [glucose 0.5%, soluble starch 1%, NZ-case 0.3%, yeast extract 0.2%, tryptone (Difco Laboratories, Detroit, MI, USA) 0.5%, dipotassium hydrogen phosphate (K2HPO4) 0.1%, magnesium sulfate (MgSO4·7H2O) 0.05% and calcium carbonate (CaCO3) 0.3% (pH 7.0)] with a loopful of mature spores of one of the Streptomyces isolates. All the flasks were incubated on a rotary shaker (215 rev min−1) at 28 °C for 3 days.
Fifty grams of vermiculite were weighed into individual autoclavable plastic bags, autoclaved twice at 121 °C for 30 min on consecutive days and dried in an oven (Memmert, Schwabach, Germany) at 60 °C for 2 days. Forty millilitres of 7-day-old cultures of each Streptomyces isolate (108 c.f.u. g−1) were added to a separate bag and mixed evenly under sterile conditions. Three 1-g sub-samples of the formulation were immediately diluted in 9 ml of sterile distilled water (final culture, 106 c.f.u. g−1) and plated on to ISP2 agar to determine the initial density of the viable cells in the formulation. The formulation was stored at 28 ± 2 °C in a sealed bag. The shelf life of each of the formulated isolates was evaluated in triplicate at monthly intervals over an 8-month period and expressed as the number of c.f.u. g−1 based on the standard dilution plate count method. The efficacy of each powder formulation in vitro was assessed by adding 0.1 g of formulation to the centre of a PDA plate and then inoculating the plate with two agar discs excised from a G. boninense culture. The plates were incubated at 28 ± 2 °C for 7 days. Each formulation was replicated four times.
The Ganoderma inoculum was prepared using fresh rubber wood blocks (RWB, 6 cm × 6 cm × 6 cm). The RWB were autoclaved at 121 °C for 20 min before placing into individual heat-resistant polypropylene bags (15 cm × 33 cm × 0.05 mm) containing 100 ml of malt extract agar (MEA) and then re-autoclaved at 121 °C for 30 min. To ensure a good coverage of nutrients on the RWB, the blocks were rotated in the polypropylene bag after 10 min cooling at room temperature. The RWB were inoculated with five 5-mm plugs excised from a 5-day-old G. boninense PER71 culture and incubated in the dark at 28 ± 2 °C for 3 weeks, or until the RWB were fully colonized by G. boninense mycelium (Idris et al. 2006).
Evaluation of Streptomyces spp. formulations for biological control activity against Ganoderma in oil palm seedlings
Commercial oil palm seedlings of the dura × pisifera (D×P) cultivar were used in this experiment. The seedlings were grown in individual polypropylene bags containing a soil mixture of topsoil and sand (1:3) and kept in the nursery under glasshouse conditions until they reached the four- to five-leaf stage (3 months old). The soil mixture was not sterilized so as to more closely imitate the soil that palm oil seedlings experience under field conditions. Seedlings were pre-treated with 50 g of the respective powder formulation by mixing the formulation with the soil. Fourteen days after the pre-treatment, each seedling was artificially inoculated with G. boninense PER71 using the RWB sitting technique (Idris et al. 2006). A RWB that was completely colonized by Ganoderma was placed in direct contact with at least three seedling roots and then covered with soil mixed with the respective powder formulation. Seedlings treated with a vermiculite-based powder that lacked Streptomyces and seedlings treated with a vermiculite-based powder formulated with S. noursei (ATCC 11455) acted as controls. All seedlings were grown under glasshouse conditions in a shaded nursery (30% light absorbance), supplemented with fertilizer once a month and watered daily (Izzati and Abdullah 2008; Zaiton et al. 2008). The experiment involved six treatments (five Streptomyces formulations and one formulation that lacked Streptomyces) with three replicates of each treatment (i.e. six seedlings/replicate) and was arranged in a completely randomized design (CRD).
The incidence of BSR among the seedlings was assessed on a monthly basis for sixth months following their inoculation with Ganoderma. Qualitative assessments were based on the observation of disease symptoms such as yellowing and wilting of the leaves, and the appearance of white mycelia or a white button at the basal stem area of the seedling that later developed into a fruiting body. These qualitative data assessments were used to generate measurable quantitative assessments: the percentage of disease incidence (DI), the severity of foliar symptoms (SFS) and the number of dead seedlings (DS). DI (%) referred to the proportion of seedlings that were visually assessed as infected (chlorotic and necrotic leaves, with or without the production of a fruiting body):
where a is the number of seedlings infected and b is total number of seedlings assessed in this study.
The SFS (%) was calculated using the formula
where a is the number of desiccated leaves (wilted/desiccated), b is the number of yellowing leaves, c is the total number of leaves, l is the index of desiccated leaves and 0.5 is the index of yellowing leaves (Maizatul Suriza and Idris 2009; Sariah and Zakaria 2000). Disease reduction was expressed as area under the disease progress curve (AUDPC) values and the effectiveness of each treatment in suppressing the disease was measured by comparing the reduction of disease incidence in treated seedlings with the untreated control seedlings (Izzati and Abdullah 2008; Sundram et al. 2008; Zaiton et al. 2008).
All percentage data (DI, SFS and DS) values were arcsine transformed (Gomez and Gomez 1984) and subjected to ANOVA. Means were compared using the least significant different (LSD) at P ≤ 0.05 using SAS® software (SAS Institute Inc., Cary, NC, USA).
Results
Characterization of isolates
The morphological characterization of the Streptomyces-like isolates was performed on four agar media. At 1–3 days after streaking, the surface of all the colonies was smooth. These colonies later developed aerial mycelia with a powdery or velvety appearance and developed sporulating aerial mycelium. Most of the isolates grew well on ISP2 and showed poor to average growth on ISP3, ISP4 and ISP5 with no production of soluble diffusible pigments (except for AGA43 and AGA48) on all the media tested. The colonies of isolates AGA43 and AGA48 were grey to light grey with a yellowish-brown reverse colour on ISP2 media. The agar colour changed to greenish-yellow indicating the production of a soluble diffusible pigment. Aerial mycelia were abundant, light and powdery (Fig. 1, i and ii) with a strong earthy smell. Colonies were visible to the naked eye from 1 day after spreading or streaking, forming colonies with irregular, lobate margins that lacked aerial mycelia. White aerial mycelium developed after 3 days, becoming grey (light-grey for AGA43) when mature. The colonies of isolate AGA347 were white to brownish-grey with a white to light-brown reverse. No pigmentation was observed on plates. The colonies were wrinkled on ISP2 and formed vegetative hyphae with an undulating margin (Fig. 1, iii). Colonies were visible to the naked eye 3 days after inoculation. Isolate AGA506, was brownish to greyish brown with a white to light-brown reverse. No pigmentation was observed on plates. The colonies were abundant, light, smooth and powdery. Colonies were visible to the naked eye 3 days after inoculation. The surface of each colony appeared convex, white and velvety, becoming grey 5 days after inoculation as the aerial spores matured (Fig. 1, iv). Observations under the light microscope at 40× revealed that the four isolates possessed either spiral or straight spore chains. Isolates AGA43 and AGA48 were characterized as open loop, whereas isolate AGA347 was characterized as closed spiral and AGA506 as biverticillus with an open spiral at the tips of non-fragmented hyphae. The characteristics of each isolate are summarized in Table 1.
3–5-day-old actinomycete colonies growing on yeast extract malt extract agar (ISP2) that were isolated from the rhizosphere of oil palm (Elaeis guineensis): a surface, b reverse and c sporulating aerial mycelium of strains at ×40 magnification under a light microscope. i Isolate AGA043, ii isolate AGA048, iii isolate AGA347 and iv isolate AGA506. Scale bar = 10 µm
Molecular identification of the Streptomycete-like isolates
The molecular identity of the isolates was determined by performing 16S rRNA gene sequence analysis. The partial 16S rRNA gene sequence (~ 512 bp) of isolates AGA43, AGA48, AGA347 and AGA506 showed a very high level of similarity (> 98%) with sequences related to different Streptomyces species deposited in the GenBank. Isolates AGA347 and AGA506 showed 99% similarity with known Streptomyces species and were identified as Streptomyces hygroscopicus subsp. hygroscopicus and Streptomyces ahygroscopicus, respectively. Isolates AGA43 and AGA48 were only identified down to genus level. The phylogenetic tree that was constructed using partial 16S rRNA sequence data showed the relationship between isolates AGA43, AGA48, AGA347 and AGA506 and representatives of the genus Streptomyces (Fig. 2). Although isolates AGA43 and AGA48 were both classified as Streptomyces sp., they were clustered into the same group as Streptomyces flavogriseus and Streptomyces sanglieri, with 96% sequence similarity using Blastn analysis. Isolate AGA347 was predicted to be Streptomyces hygroscopicus subsp. hygroscopicus (Accession number: AB184760.1), with a sequence similarity of 99%. Meanwhile, isolate AGA506 was identical to Streptomyces ahygroscopicus (Accession number: GQ162433.1), with a sequence similarity of more than 99%. All four isolates showed divergence from the pathogenic actinomycetes in the outgroups, and, herewith, were defined as putative biological control agents that could potentially to be used to control plant pathogens.
Phylogenetic tree generated using the neighbour-joining method showing the relationships of the partial 16S rRNA gene sequences of the four Streptomyces isolates investigated in this study (highlighted in green) and 18 Streptomyces reference species obtained from GenBank (accession numbers are given parentheses). Single asterisks denote species with known biological control activity. Double asterisks denote known plant pathogenic actinomycetes. Branch lengths represent the number of nucleotide differences and the numbers at the branch nodes indicate the bootstrap percentage frequencies of a given branch (1000 bootstrap replicates; most of bootstrap values indicate more than 50% support). Actinoplanes regularis, Actinomyces urogenitalis, Nocardia asteroides and Micromonospora sp. were included as outgroups. The scale bar indicates 0.02 nucleotide substitutions per nucleotide position
Production of extracellular enzymes and effect of pH, salinity and temperature on isolate growth
All the isolates produced lipase and isolates AGA43 and AGA48 produced siderophores (Fig. 3). Only isolate AGA347 showed positive chitinase and cellulase production. Isolates AGA347 and AGA506 were able to solubilize phosphate. The membranes used in the IAA assay showed no colour changes, indicating that the isolates lacked the ability to produce IAA. In general, the four Streptomyces isolates were able to grow on ISP2 with a pH of 4.5 and 5.5. Isolates AGA347 and AGA506 showed moderate growth compared to isolates AGA43 and AGA48, which exhibited poor growth. The colonies of isolates AGA43 and AGA48 were smaller than those of AGA347 and AGA506, the substrate mycelia adhered to the agar and no observable aerial mycelia developed. By contrast, when inoculated on ISP2 with pH ranges from 6.5 to 8.5, all four isolates developed abundant aerial spores as the mycelia matured. All four isolates were able to grow and produce abundant aerial spores on ISP2 when incubated at temperatures ranging from 20 to 40 °C. No differences in colony size or cultural characteristics were observed within this temperature range. However, none of the isolates was able to grow at 50 °C. The salinity test revealed that all four isolates were able to grow abundantly on ISP2 with NaCl concentrations of 2–4%. The formation of spores was less abundant on ISP2 with 6% NaCl and no growth occurred on plates of ISP2 with NaCl concentrations of more than 6% (Table 2).
Viability of Streptomyces isolates in powder formulation and efficacy against G. boninense in vitro
The viability of each Streptomyces isolate when formulated with vermiculite was monitored for 8 months. The initial density of each of the Streptomyces isolates in the powder formulation was 108 c.f.u. g−1. After 3 months storage, the formulated isolates showed significantly different levels of viability. Isolates AGA48 (105 c.f.u. g−1) and AGA506 (105 c.f.u. g−1) showed significantly higher levels of viability throughout the storage period than AGA43 (104 c.f.u. g−1) and AGA347 (104 c.f.u. g−1), which showed high viability levels during the first few months after inoculation but then started to decrease with time in storage. By contrast, the S. noursei isolate showed a dramatic reduction in viability during storage, with only 103 c.f.u. g−1 of viable cells at the end of the 8-month storage period.
After 7 days of incubation at 28 °C, the G. boninense colonies on the control plates completely covered the plates. The colonies developed white mycelial mats that were compact and cottony. Colonies appeared dark in reverse due to the formation of a brownish pigmentation. The darkened region had an undulating surface that buckled into the agar. By contrast, the growth of the Ganoderma colonies on the assay plates was significantly inhibited by the Streptomyces powder formulations and showed a stunted growth pattern (Table 3; Fig. 4).
Efficacy of Streptomyces powder formulations as a pre-treatment for oil palm seedlings to suppress the development of BSR
The oil palm seedlings showed no visible signs or symptoms of BSR at 1–3 months after inoculation with Ganoderma. However, 3 months after the seedlings were inoculated with G. boninense, external symptoms of BSR started to appear, such as a white mycelial mass at the basal area of the seedling, which later developed into a white button, which was followed by the emergence of fruiting body. At 3 months, the lower leaves also became progressively yellow, which was later followed by desiccation of the oldest to the youngest leaves. Desiccation began at the leaf tip. This was followed by the rapid yellowing and drying of the entire lamina.
Seedlings treated with Streptomyces AGA347 showed lower levels of DI and SFS (33.3 and 35.6%, respectively) than seedlings that received the other treatments. Seedlings treated with Streptomyces AGA048 showed DI and SFS levels of 53.33 and 57.2%, respectively. Seedlings treated with Streptomyces AGA506 showed DI and SFS levels of 66.7 and 67.2%, respectively. Seedlings treated with S. noursei showed DI and SFS levels of 66.7 and 70.8%, respectively. Seedlings treated with AGA043 showed DI and SFS levels of 80 and 70.8%, respectively. Untreated seedlings showed the highest DI and SFS levels (93.3 and 83.8%, respectively). Only 26.7 and 33.3% of the seedlings treated with Streptomyces AGA347 or Streptomyces AGA48 died. By contrast, 60.0% of the seedlings treated with Streptomyces AGA506, S. noursei or Streptomyces AGA43 died and 86.67% of the untreated seedlings died. By the end of the experiment, severe root rot was seen in all seedlings with observable foliar desiccation. Longitudinal sections of the affected seedlings showed extensive rotting that extended into the stem. No root rot or stem decay was observed in healthy seedlings. The disease severity index (DSI), was based on the extent of the root rot and bole decay. Seedlings treated with Streptomyces AGA347 had the lowest DSI value (30.0%) followed by Streptomyces AGA048 (38.7%). By contrast, seedlings treated with Streptomyces AGA043, Streptomyces AGA506 or S. noursei showed high DSI values (66.7, 60.0 and 65.0%, respectively). The untreated seedlings showed the greatest number of necrotic lesions in the bole (DSI, 88.7%) (Fig. 5).
Destructive sampling of oil palm seedlings 6 months after inoculation with G. boninense. a Seedlings treated with the Streptomyces AGA347 formulation with 20% tissue rot (scale 1 on the DSI). b Untreated seedlings showing more than 50% tissue rot (scale 3). c Untreated seedlings showing more than 90% tissue rot (scale 4)
The highest DSI (96.67%) was recorded for untreated control seedlings sixth months after inoculation with G. boninense, indicating the severe impact that a Ganoderma infection can have on seedlings. 6 months after inoculation with G. boninense, seedlings treated with Streptomyces AGA48 and Streptomyces AGA347 recorded the lowest DSI values (31.67 and 16.67%, respectively) and seedlings treated with Streptomyces AGA43, S. noursei or AGA506 (56.7%) showed the highest DSI values (66.7, 60.0 and 56.7%, respectively). Observable symptoms such as dying leaves were in line with the production of G. boninense sporophores at the base of stems on seedlings severely infected with G. boninense. Seedlings treated with Streptomyces AGA347 showed the lowest AUDPC value (83.34 units) followed by Streptomyces AGA48 (139.99 units). Seedlings treated with Streptomyces AGA043, Streptomyces AGA506 and S. noursei showed AUDPC values of 216.66, 173.34 and 163.34 units, respectively. The percentage of disease reduction (% DR), which was calculated using the AUDPC values, indicated the ability of the Streptomyces powder formulations to reduce BSR damage. Seedlings treated with Streptomyces AGA347 showed the greatest % DR, followed by those treated with Streptomyces AGA048, S. noursei, Streptomyces AGA506 and Streptomyces AGA506 (73.11, 54.84, 47.31, 44.08 and 30.11%, respectively) (Table 4).
Discussion
Actinomycetes in the rhizosphere area may be beneficial for plants owing to their production of plant growth hormones such as IAA (Khamna et al. 2009) and siderophores, which improve nutrient uptake (Merckx et al. 1987), and their ability to solubilize nutrients such as phosphate (Katznelson and Bose 1959; Sousa et al. 2008). All the actinomycete isolates investigated in this study demonstrated the potential to produce lipase and siderophores. The production of siderophores gives a microorganism a competitive advantage in soil. Enzymes such as chitinase, cellulase, lipase and protease play important roles in nutrient mineralization and decomposition (Gopalakrishnan et al. 2014; Sousa et al. 2008; Tokala et al. 2002). In addition, the ability to produce enzymes related to the fungal cell-wall degradation enzymes chitinase and glucanase could also be a key factor in biocontrol mechanisms given that polysaccharides such as chitin and glucan are the main components of most fungal cell walls (Gooday et al. 1992; Larralde-Corona et al. 2008; Sousa et al. 2008). Larralde-Corona et al. (2008) demonstrated a positive relationship between the enzymatic activity of Trichoderma strains and the degree of antagonism that the Trichoderma strains showed towards Macrophomina phaseolina in an antagonistic assay. However, not all the isolates investigated in this study showed a significant positive relationship between their enzymatic assay results and their antagonistic assay results. This suggests that the inhibitory activity of some isolates may be due to the production of secondary metabolites given that actinomycetes are known to be prolific producers of metabolites with activity against a broad range of pathogens (Saxena and Pandey 2001). However, we did not investigate the production of secondary metabolites in this study.
All the isolates were able to grow abundantly on ISP2 with a pH range between 6.5 and 8.5. Two of the four isolates showed medium growth with less abundant mycelia at a pH of 4.5 and 5.0, indicating that they were acid-tolerant isolates. The ability of isolates to grow on media with pHs ranging from 4.5 to 5.5 suggests that these isolates are able to compete and survive in acidic soils (Sousa et al. 2008). The ability of the isolates to grow on ISP2 with NaCl concentrations of up to 6% signifies that these Streptomyces isolates have a high salt tolerance. This result agrees with a similar observation reported by Gopalakrishnan et al. (2013), and the ability of Streptomyces strains to tolerate high salt concentrations is well known (Waksman 1959). The ability of isolates AGA43, AGA48, AGA347 and AGA506 to tolerate pH and salinity ranges may assist their competitive ability in the rhizosphere and should enable them to play a role in plant protection under various environmental conditions (Sousa et al. 2008).
All four isolates showed typical morphological characteristics of the genus Streptomyces. In general, Streptomyces isolates show good sporulation on agar and form velvety or powdery dry colonies of different colours (Lo et al. 2002; Oskay 2009). The growth and physiological appearance of a Streptomyces colony depends on the composition of the medium and the culture age (Awad et al. 2009). According to Mutitu et al. (2008), abundant mycelial growth indicates that an adequate level of nutrition and other components required for growth are present in the medium. A variety of pigmentation colours and colony types can be produced on different media by the same isolate. The different colours of the aerial mycelium are produced by pigments such as phenazines, phenoxazinones and prodiginines. However, in this study, culturing the isolates on different media had little effect on the morphological appearance of the isolates, other than showing some variations in pigmentation and colony types.
16S rRNA gene sequence analysis confirmed that all the isolates belonged to the genus Streptomyces and confirmed the identity of isolate AGA347 as Streptomyces hygroscopicus subsp. hygroscopicus and AGA506 as Streptomyces ahygroscopicus. Both S. hygroscopicus and S. ahygroscopicus have been reported to have potential as biocontrol agents against many pathogens, including Colletotrichum gloeosporioides, which is the causal agent of anthracnose disease in orchid (Prapagdee et al. 2008). They are also known to reduce strawberry root rot by Fusarium oxysporum (Shen et al. 2016) and to have an antagonistic effect on Botrytis cinerea (Kim et al. 2015). The phylogenetic analysis grouped isolate AGA347 into the same cluster as S. griseoviridis and isolate AGA506 was found to share a high level of sequence similarity with S. lydicus, which is known to exhibit biological control activity towards many phytopathogens (Tahvonen 1988; Yuan and Crawford 1995). Analyses of the 16S rDNA sequences of AGA043 and AGA048 showed that the isolates clustered together on the same branch as Streptomyces sanglieri strain A14. S. sanglieri has been reported to promote oil palm growth and to have an antagonistic effect on Ganoderma (Nur Azura et al. 2016). Both isolates showed the highest similarity (top BLAST hit and the lowest E-value) with the adjacent Streptomyces sp. Analysis using multiple sequence alignments (http://www.ebi.ac.uk/Tools/msa/) showed gaps within the sequence, indicating that both isolates may belong to a different strain.
To evaluate the ability of these four Streptomyces isolates to control Ganoderma disease in oil palm seedlings, we formulated each isolate in a vermiculite-based powder. Vermiculite was used as a carrier due to its ability to retain water and to hold microbial cells within the porous material, allowing the microbial agent to be slowly released into the soil. In this experiment, the initial inoculum volume of each Streptomyces sp. was 1 × 108 c.f.u. g−1. The viability of each product was maintained for the first 3 months of storage; however, the viability then dropped to 105 c.f.u. g−1 and remained at this level until the sixth month of storage. This phenomenon might reflect the unavailability or depletion of nutrient sources other than the available nutrients left in the fermentation broth. The storage temperature can also affect the growth rate and pH of formulations. In this study, the formulations were stored at room temperature (28 °C) and the effect of temperature on cell viability was not determined. However, previous studies indicate that the shelf life and efficacy of microbial cells in formulations stored at temperatures ranging between 4 and 20 °C was maintained for longer than those in formulations stored at higher temperatures. Higher temperatures promote microbial growth, which consequently cause the microbes to produce more waste. The waste includes lytic enzymes, which are toxic to microbial cells. According to Kolombet et al. (2008), as well as nutrient depletion, the decreasing number of viable cells may also be related to the accumulation of lytic enzymes during storage. This may explain why the viability of the Streptomyces formulations decreased throughout the storage period, which would also affect the percentage inhibition of Ganoderma radial growth.
To determine whether the Streptomyces formulations were able to control Ganoderma disease in oil palm seedlings, we used an artificial inoculation technique. Artificially inoculating oil palm seedlings with G. boninense PER71 is an effective strategy for inducing the infection at the nursery stage (Izzati and Faridah 2008). In this study, infected seedlings that were treated with the Streptomyces formulations showed reduced disease and slower disease development compared with the untreated controls. Lower DI and SFS percentages in this experiment indicate disease suppression by the Streptomyces sp. Among the isolates tested, treating seedlings with a formulation of either the Streptomyces isolate AGA347 or AGA48 was the most effective strategy for reducing Ganoderma disease in oil palm seedlings.
Streptomyces AGA347 and AGA48 may have established in the rhizosphere, thus hindering pathogen proliferation and invasion of the seedling roots. The vermiculite carrier may provide the Streptomyces isolates with some protection from the environment and support the growth and establishment of the Streptomyces isolates in the soil rhizosphere. However, even though all the Streptomyces isolates were formulated in the same carrier, S. noursei, Streptomyces AGA43 and Streptomyces AGA506 were less effective at reducing disease in infected seedlings, despite strong antagonistic effects towards G. boninense in vitro. This phenomenon may perhaps arise because the active compounds involved in the antagonistic mechanism in vitro are reduced or less effective in the soil environment. Similar results were reported by Pattanapipitpaisal and Kamlandharn (2012).
This study indicates that Streptomyces AGA347 and Streptomyces AGA347 show promise as potential biological control agents against G. boninense to suppress the development of BSR disease in oil palm. The Streptomyces formulations will be further developed to prolong their shelf life. Further studies should identify the mechanisms of action of these potential biological control agents, which may lead to the discovery of novel biocontrol activity and growth promotion in oil palm.
References
Alimuddin A, Widada J, Asmara W, Mustofa M (2011) Antifungal production of a strain of Actinomycetes spp. isolated from the rhizosphere of cajuput plant: selection and detection of exhibiting activity against tested fungi. Indones J Biotechnol 16(1):1–10
Anderson AS, Wellington EM (2001) The taxonomy of Streptomyces and related genera. Int J Syst Evol Microbiol 51(3):797–814
Anitha A, Rabeeth M (2009) Control of Fusarium wilt of tomato by bioformulation of Streptomyces griseus in greenhouse condition. Afr J Basic Appl Sci 1:9–14
Arif MA, Roslan A, Idris AS, Ramle M (2011) Economics of oil palm pests and Ganoderma disease and yield loses. In: Proceedings of the Third MPOB-IOPRI International Seminar: Integrated Oil Palm Pests and Disease Management, Kuala Lumpur, Malaysia. MPOB Publishing, pp 83–98
Awad MH, El-Shahed KYI, El-Nakkadi AEM (2009) Isolation, screening and identification of newly isolated soil Streptomyces (Streptomyces sp. NRC-35) for β-lactamase inhibitor production. World Appl Sci J 7(5):637–646
Azizah H (2003) Ganoderma versus mycorrhiza. Oil Palm Bull 47:6–14
Baniasadi F, Shahidi Bonjar GH, Baghizadeh A, Karimi Nik A, Jorjandi M, Aghighi S, Rashid Farokhi P (2009) Biological control of Scleroctinia sclerotiorum, causal agent of sunflower head and stem rot disease, by use of soil borne actinomycetes isolates. Am J Agri BioSci 4(2):146–151
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57(2):535–538
Cao L, Qiu Z, Dai X, Tan H, Lin Y, Zhou S (2004) Isolation of endophytic actinomycetes from roots and leaves of banana (Musa acuminata) plants and their activity against Fusarium oxysporum f. sp. cubense. World. J Microbiol Biotechnol 20:501–504
Crawford LD, Lynch JM, Whipps JM, Ousley MA (1993) Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl Environ Microbiol 59(11):3899–3905
Ebrahimi-Zarandi M, Shahidi Bonjar GH, Padasht Dehkaei F, Ayatollahi Moosavi SA, Rashid Farokhi P, Aghighi S (2009) Biological control of rice blast (Magnaporthe oryzae) by use of Streptomyces sindeneusis isolate 263 in greenhouse. Am J Appl Sci 6:194–199
Elson MK, Schisler DA, Bothast RJ (1997) Selection of microorganisms for biological control of silver scurf (Helminthosporium solani) of potato tubers. Plant Dis 81:647–652
Errakhi R, Bouteau F, Lebrihi A, Barakate M (2007) Evidences of biological control capacities of Streptomyces spp. against Sclerotium rolfsii responsible for damping-off disease in sugar beet (Beta vulgaris L.). World J Microbiol Biotechnol 23(11):1503–1509
Franco-Correa M, Quintana A, Duque C (2010) Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl Soil Ecol 45:209–217
Getha K, Vikineswary S (2002) Antagonistic effects of Streptomyces violaceus niger strain G10 on Fusarium oxysporum f. sp. cubense race 4: indirect evidence for the role of antibiosis in the antagonistic process. J Ind Microbiol Biotechnol 28:303–310
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. Wiley, New York
Gooday GW, Zhu WY, O’Donnell RW (1992) What are the roles of chitinase in the growing fungus? FEMS Microbiol Lett 100(1–3):387–391
Gopalakrishnan S, Vadlamudi S, Apparla S, Bandikinda P, Vijayabharathi R, Bhimineni RK, Rupela O (2013) Evaluation of Streptomyces spp. for their plant-growth-promotion traits in rice. Can J Microbiol 59(8):534–539
Gopalakrishnan S, Vadlamudi S, Bandikinda P, Sathya A, Vijayabharathi R, Rupela O, Kudapa B, Katta K, Varshney RK (2014) Evaluation of Streptomyces strains isolated from herbal vermicompost for their plant growth-promotion traits in rice. Microbiol Res 169:40–48
Hu QP, Xu JG (2011) A simple double-layered chrome azurol S agar (SD-CASA) plate assay to optimize the production of siderophores by a potential biocontrol agent Bacillus. Afr J Microbiol Res 5(25):4321–4327
Idris AS (2011) Biology, detection and control of Ganoderma in oil palm. In: Basri MW, Choo YM, Chan KW (eds) Further advances in oil palm research (2000–2010-Volume 1). MPOB, Malaysia, pp 521–845
Idris AS (2012) Latest research and management of Ganoderma disease in oil palm. In: Proceedings of the Fourth IOPRI-MPOB International Seminar: Existing and Emerging Pests and Diseases of Oil Palm Advances in Research and Management. Bandung, Indonesia, pp. 1–23
Idris AS, Kushairi D, Ariffin D, Basri MW (2006) Technique for inoculation of oil palm germinated seeds with Ganoderma. MPOB Inf Ser 314:1–4
Idris AS, Mior MHAZ., Maizatul SM, Kushairi A (2011) Survey on status of Ganoderma disease of oil palm in Malaysia 2009–2010. In: Proceedings of the PIPOC 2011 International Palm Oil Congress (Agriculture, Biotechnology and Sustainability), Kuala Lumpur, Malaysia. MPOB Publishing, pp. 235–238
Ilic SB, Konstantinovic SS, Todorovic ZB, Lazic ML, Veljkovic VB, Jokovic N, Radovanovic BC (2007) Characterization and antimicrobial activity of the bioactive metabolites in streptomycete isolates. Mikrobiologiia 76(4):478–480
Intra B, Mungsuntisuk I, Nihira T, Igarashi Y, Panbangred W (2011) Identification of actinomycetes from plant rhizospheric soils with antagonist activity against Colletotrichum spp., the causative agent of anthracnose disease. BMC Res Notes 4:98
Izzati NMZ, Abdullah F (2008) Disease suppression in Ganoderma-infected oil palm seedlings treated with Trichoderma harzianum. Plant Prot Sci 44(3):101–107
Katznelson H, Bose B (1959) Metabolic activity and phosphate-dissolving capability of bacterial isolates from wheat roots, rhizosphere, and non-rhizosphere soil. Can J Microbiol 5(1):79–85
Khamna S, Yokata K, Pebery JF, Lumyong S (2009) Antifungal activity of Streptomyces spp. isolated from rhizosphere of Thai medicinal plants. Int J Integr Biol 6:143–147
Kim YS, Lee IK, Yun BS (2015) Antagonistic effect of Streptomyces sp. BS062 against Botrytis diseases. Mycobiology 43(3):39–342
Kolombet LV, Zhigletsova SK, Kosareva NI, Bystrova EV, Derbyshev VV, Krasnova SP, Schisler D (2008) Development of an extended shelf-life, liquid formulation of the biofungicide Trichoderma asperellum. World J Microbiol Biotechnol 24:123–131
Kustner E (1968) The actinomycetes. In: Burges A, Raw F (eds) Soil biology. Academic Press, London, pp 111–124
Larralde-Corona CP, Santiago-Mena MR, Sifuentes-Rincon AM, Rodriguez-Luna IC, Rodriguez-Perez MA, Shirai K, Narvaez-Zapata JA (2008) Biocontrol potential and polyphasic characterization of novel native Trichoderma strains against Macrophomina phaseolina isolated from sorghum and common bean. Appl Microbiol Biotechnol 80:167–177
Lo CW, Lai NS, Cheah HY, Wong NKI, Ho CC (2002) Actinomycetes isolated from soil samples from the Crocker Range Sabah. ASEAN Rev Biodiver Environ Conserv 9:1–7
Lyons AJ, Pridham TG (1965) Colorimetric determination of color of aerial mycelium of Streptomycetes. J Bacteriol 89:159–169
Maizatul Suriza M, Idris AS (2009) Nursery evaluation of Agrobacterium radiobacter, Burkholderia cepacia and Pseudomonas syringae to control Ganoderma boninense infection in oil palm. In Proceedings of Agriculture, Biotechnology and Sustainability Conference Volume 3. PIPOC 2009-International Palm Oil Congress: Kuala Lumpur, Malaysia. MPOB Publishing
Merckx R, Dijkstra A, Den Hartog A, Van Veen JA (1987) Production of root-derived material and associated microbial growth in soil at different nutrient levels. Biol Fertil Soils 5(2):126–132
Mutitu EW, Muiru WM, Mukunya DM (2008) Evaluation of antibiotic metabolites from actinomycete isolates for the control of late blight of tomatoes under greenhouse conditions. Asian J Plant Sci 7:284–290
Nur Azura AB, Yusoff M, Tan GYA, Jegadeesh R, Appleton DR, Vikineswary S (2016) Streptomyces sanglieri which colonised and enhanced the growth of Elaeis guineensis Jacq. seedlings was antagonistic to Ganoderma boninense in in vitro studies. J Ind Microbiol Biotechnol 43(4):485–493
Nurrashyeda R, Idris AS, Madihah AZ, Ramle M, Kushairi A (2011) Hendersonia GanoEF1 granules for the control of Ganoderma boninense in oil palm. MPOB Information Series, No. 556. MPOB TT No. 483
Nurrashyeda R, Maizatul SM, Idris AS, Madihah AZ, Nasyaruddin M (2016) The potential of endophytic bacteria as a biological control agent for Ganoderma disease in oil palm. Sains Malays 45(3):401–409
Oskay M (2009) Antifungal and antibacterial compounds from Streptomyces strains. Afr J Biotechnol 8(13):3007–3017
Pattanapipitpaisal P, Kamlandharn R (2012) Screening of chitinolytic actinomycetes for biological control of Sclerotium rolfsii stem rot disease of chilli. Songklanakarin J Sci Technol 34
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci 4(5):330–337
Pridham TG (1965) Color and Streptomycetes—report of an International Workshop on Determination of Color of Streptomycetes. Appl Environ Microbiol 1:43–61
Sabaratnam S, Traquair JA (2002) Formulation of Streptomyces biological control agent for the suppression of Rhizoctonia damping-off in tomato transplant. Biocontrol 23(3):245–252
Sahilah AM, Tang SY, Zaimawati MN, Rosnah H, Kalsum MU, Son R (2010) Identification and characterization of actinomycetes for biological control of bacterial wilt of Ralstonia solanacearum isolated from tomato. J Trop Agric Food Sci 38(1):103–114
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sardi P, Saracchi M, Quaroni S, Petrolini B, Borgonovi GE, Merli S (1992) Isolation of endophytic Streptomyces strains from surface-sterilized roots. Appl Environ Microbiol 58:2691–2693
Sariah M, Zakaria K (2000) The use of soil amendments for the control of basal stem rot of oil palm seedling. In: Flood J, Bridge PD, Holderness M (eds) Ganoderma diseases in perennial crops. CABI Publishing, Wallingford, pp 88–99
Sariah M, Choo CW, Zakaria H, Norihan MS (2005) Quantification and characterization of Trichoderma spp. from different ecosystems. Mycopathologia 159:113–117
Saxena S, Pandey AK (2001) Microbial metabolites as eco-friendly agrochemicals for the next millennium. Appl Microbiol Biotechnol 55(4):395–403
Shahrokhi S, Shahidi Bonjar GH, Saadoun I (2005) Biological control of potato isolate of Rhizoctonia solani by Streptomyces olivaceus strain 115. Biotechnology 4(2):132–138
Shariffah-Muzaimah SA, Idris AS, Madihah AZ, Dzolkhifli O, Kamaruzzaman S, Cheong PCH (2015) Isolation of actinomycetes from rhizosphere of oil palm (Elaeis guineensis Jacq.) for antagonism against Ganoderma boninense. J Oil Palm Res 27:19–29
Shen T, Wang C, Yang H, Deng Z, Wang S, Shen B, Shen Q (2016) Identification, solid-state fermentation and biocontrol effects of Streptomyces hygroscopicus B04 on strawberry root rot. Appl Soil Ecol 103:36–43
Shimizu M, Nakagawa Y, Sato Y, Furumai T, Igarashi Y, Onaka H, Yoshida R, Kunoh H (2000) Studies on endophytic actinomycetes (I) Streptomyces sp. isolated from Rhododendron and its antifungal activity. J Gen Plant Pathol 66(4):360–366
Shirling EB, Gottlieb D (1966) Methods for characterization of Streptomyces species. Int J Syst Bacteriol 16:313–340
Singh G (1991) Ganoderma—the scourge of oil palm in the coastal areas. The Planter 67:421–444
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological controls of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Sousa CS, Soares ACF, Garrido MS (2008) Characterization of Streptomycetes with potential to promote plant growth and biocontrol. Sci Agric 65:50–55
Sundram S, Abdullah F, Ahmad ZAM, Yusuf UK (2008) Efficacy of single and mixed treatments of Trichoderma harzianum as biocontrol agents of Ganoderma basal stem rot in oil palm. J Oil Palm Res 20:470–483
Sundram S, Meon S, Seman IA, Othman R (2015) Application of arbuscular mycorrhizal fungi with Pseudomonas aeruginosa UPMP3 reduces the development of Ganoderma basal stem rot disease in oil palm seedlings. Mycorrhiza 25(5):387–397
Susanto A, Sudharto PS, Purba RY (2005) Enhancing biological control of basal stem rot disease (Ganoderma boninense) in oil palm plantations. Mycopathologia 159:153–157
Taechowisan T, Lumyong S (2003) Activity of endophytic actinomycetes from roots of Zingiber officinale and Alpinia galangal against phytopathogenic fungi. Ann Microbiol 53(3):291–298
Tahvonen RT (1988) Microbial control of plant diseases with Streptomyces spp. EPPO Bull 18(1):55–59
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28(10):2731–2739
Tan CJ, How KC, Loh-Mia PP, Ismet A, Getha K, Seki T, Vikineswary S (2002) Bioactivity of selected actinomycetes against Ganoderma boninense. Asia Pacific J Mol Biol Biotechnol 10:119–125
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA, Bailey JF, Morra MJ (2002) Novel plant–microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68(5):2161–2171
Waksman SA (1959) The Actinomycetes. vol. I. Nature, occurrence and activities. The Williams & Wilkins Company, Baltimore
Xiao K, Kinkel LL, Samac DA (2002) Biological control of Phytophthora root rots on alfalfa and soybean with Streptomyces. Biol Control 23(3):285–295
Yuan WM, Crawford DL (1995) Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Appl Environ Microbiol 61(8):3119–3128
Zaiton S, Sariah M, Ahmad ZAM (2008) Effect of endophytic bacteria on growth and suppression of Ganoderma boninense infection in oil palm. Int J Agric Biol 10:127–132
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shariffah-Muzaimah, S.A., Idris, A.S., Madihah, A.Z. et al. Characterization of Streptomyces spp. isolated from the rhizosphere of oil palm and evaluation of their ability to suppress basal stem rot disease in oil palm seedlings when applied as powder formulations in a glasshouse trial. World J Microbiol Biotechnol 34, 15 (2018). https://doi.org/10.1007/s11274-017-2396-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-017-2396-1