Abstract
Tuberculosis is the most widespread and deadly airborne disease caused by Mycobacterium tuberculosis. The two-pronged lethal effect on the bacteria using lipids/surfactants and anti-tubercular drugs may render the miniaturization of dose owing to synergistic and tandem effect of both. The current research has been focused on screening and evaluating various lipids/surfactants possessing inherent anti-mycobacterium activity that can ferry the anti-tubercular drugs. In vitro anti-mycobacterium activity was evaluated using agar well diffusion method. Furthermore, time-concentration dependent killing and DNA/RNA content release studies were performed to correlate the findings. The exact mechanism of bacterial killing was further elucidated by electron/atomic force microscopy studies. Finally, to negate any toxicity, in vitro hemolysis and toxicity studies were performed. The study revealed that capmul MCM C-8, labrasol and acconon C-80 possessed highest in vitro anti-mycobacterium activity. Electron/atomic force microscopy results confirmed in vitro studies and verified the killing of Mycobacterium owing to the release of cytoplasmic content after cell wall fragmentation and disruption. Moreover, the least hemolysis and hundred percent survivals rate of mice using the excipients demonstrated the safety aspects of explored excipients that can ferry the anti-tubercular drugs. The present study concluded the safe, efficient and synergistic activity of the explored excipients and anti-tubercular drugs in controlling the menace of tuberculosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There were an estimated 8.7 million cases of Tuberculosis (TB) equivalent to 125 cases per 100,000 populations and 8.6 million incident cases of TB equivalent to 122 cases per 100,000 populations globally in 2011 and 2012, respectively. Of the 8.7 million incident cases, an estimated 0.5 million and 2.5 million were children and women, respectively. Moreover, India and China alone were accounted for 26 and 12 % of 8.7 million population, respectively (Global Tuberculosis Report 2012; Global Tuberculosis Report 2013). In 2013, the figure was 9.0 million cases which is equivalent to 126 cases per 100,000 populations. Of these total estimated cases, 550,000 and 3.3 million were children and women, respectively. Global tuberculosis report published this year indicated that India and China accounted 24 and 11 % of global cases, suggesting the reduction in TB as compared to year 2011 and 2012 respectively (Global Tuberculosis Report 2014). Tuberculosis is a deadly airborne disease caused by Mycobacterium tuberculosis. The bacteria grow slowly in cells and the relapse of the infection depends on the host immune system (Fujiwara et al. 2012).
Amongst various rapidly growing non-pathogenic Mycobacterium species, M. smegmatis has been widely used for molecular analysis (Fujiwara et al. 2012). M. smegmatis (a non-pathogenic Mycobacterium) was originally isolated in the year 1885 by Alvarez and Tavel from human smegma (a natural lubricant of male sex organ). This saprophytic Mycobacterium offers a suitable alternative model for studying the pathogenesis of the tubercle bacilli in normal laboratory conditions. The bacterium neither enters into the epithelial cells nor persists in phagocytic cells. Furthermore, the short generation time (3–4 h) makes the bacteria a good surrogate to study the pathogenesis rapidly (Barry 2001). Cell wall of the Mycobacterium is the first barrier for anti-tubercular drugs and formulations. Therefore, understanding the chemical composition and nature of the cell wall is required for effective delivery of anti-mycobacterial drugs. The distribution of the type of glycolipids and phospholipids in the bacterial cell wall determines the surface properties, permeability and bacterial phenotype (Etienne et al. 2005; Gopalaswamy et al. 2008).
Most of the first-line anti-tubercular drugs act by inhibiting the cell wall synthesis of the Mycobacterium. Free fatty acids (FFAs) have been reported as antimicrobial towards an array of microorganism and most of the research has been focused on medium and long chain FFAs (Immanuel et al. 2011). These compounds are naturally present in high fat foods and the FFAs produced after lipolysis are regarded as potential bactericides and bacteriostatics (Kamdem et al. 2011). Recently, some authors reported that the nanoemulsions prepared from glyceryl monocaprylate and labrasol possessed an inherent antibacterial activity against E. coli and S. aureus (Singh et al. 2013, 2014). In addition, surfactants (Tween® 80, cremophor and labrasol) were reported to enhance oral drug absorption by inhibiting p-glycoprotein efflux pump associated with intestinal brush border (Shen et al. 2011; Zhang et al. 2003; Rahman et al. 2013). It has been accounted that cremophor EL is similar to Tween-80 in transporter inhibition as both contain poly(ethylene oxide) as common group. Both are reported to have equipotent in fluidizing the lipid bilayer below their respective critical micelle concentration (Cremophor EL ~30 mM and Tween-80 ~50 mM). Furthermore, only cremophor-EL has been reported to inhibit monocarboxylic acid transporter (Rege et al. 2002). Cremophor EL and Tween 80 were effective in increasing Rhodamine 123 permeability from apical to basolateral side and decreasing from basolateral to apical side through intestinal P-gp efflux transporter thereby causing conformational changes in membrane bound transporter (Dudeja et al. 1995; Woodcock et al. 1992; Chang et al. 1996).
Till date, no pharmaceutical products containing lipid(s) and surfactant(s) have been approved for therapeutic or prophylactic use against Mycobacterium infection. The present investigation, therefore aimed to select lipids, surfactants and co-surfactants possessing inherent anti-mycobacterium efficacy against non-pathogenic M. smegmatis. The mechanism of anti-mycobacterium activity of lipids and surfactants were evaluated using various microscopic techniques (scanning/transmission electron microscopy and atomic force microscopy). The anti-mycobacterium activity exhibited by selected lipids and surfactants were further corroborated in a pathogenic strain of M. tuberculosis H37 Rv. Furthermore, the toxicity studies of the excipients were carried out in a suitable animal model. The selected lipids and surfactants can be exploited to formulate a suitable self-nanoemulsifying drug delivery system. The novelty embodied in the study are (1) ability to reduce the dose and dosing frequency of anti-tubercular drugs, (2) miniaturization of dose owing to two-pronged synergistic and tandem effect of lipids/surfactants and anti-tubercular drugs, (3) insight into basic mechanism of bacterial killing, (4) evaluation of biosafety aspects of excipients in animal model and (5) establishment of anti-mycobacterium activity of excipients against pathogenic strain (Mycobacterium tuberculosis H37 Rv).
Materials and methods
Materials
Non-pathogenic Mycobacterium smegmatis strains (MTCC-995; MS-995 and MTCC-942; MS-942) were obtained from the Microbial Type Culture Collection IMTECH (Institute of Microbial Technology, Chandigarh, Punjab, India). M. tuberculosis H37 Rv (ATCC 25618) was used in the study as pathogenic strain for susceptibility test (Tubercular centre, Ranchi, India). Labrasol and cremophor® EL were gifted from Gattefosse (St-Priest, France). Lipids, surfactants and co-surfactants along with their trade names, suppliers and compositions used in the present study are listed in supplementary Table T1. Milli-Q water was used in all experiments. All other reagents used were of analytical grade.
Bacterial growth and excipient preparations
The strains (MS-995 and MS-942) were grown to logarithmic phase (OD600–0.6) in nutrient broth media as specified in the procured culture leaflet. Stocks were prepared by harvesting the bacteria and re-suspending in 100 ml sterilized nutrient broth. The cultures were then allowed to grow in an incubated condition at 37 ± 1 °C. The pH of stock culture was found to be 7.2 by using electronic pH meter (Systronics, μ pH system 361, Ahmadabad, India). Lipids explored in the present study were emulsified with cremophor EL (lipid: cremophor EL = 1:1) to get 10 %v/v (or w/v). Cremophor-EL was found to be inactive against mycobacterium strains (within explored the concentration) and was helpful only to facilitate the diffusion of lipids in agar media.
In vitro anti-mycobacterium activities
The preliminary assessment of anti-mycobacterium activities of oils/lipids, surfactants and co-surfactants were performed by the agar well diffusion method as described previously (Hussain et al. 2013). A weighed amount of nutrient agar (28 g/L) was prepared and sterilized by autoclaving at 121 °C for 15 min. After achieving normal temperature (before solidification) the media was then mixed with MS-995 (2.5 × 107 CFU/ml) and MS-942 (1.8 × 107 CFU/ml) separately, in an aseptic condition. The mixed suspensions were then poured into 85 mm diameter petri dishes and left for solidification. The wells were made in the solidified agar media aseptically under laminar flow. In each well, 150 µl of oils, surfactants and co-surfactants were poured. The petri dishes were incubated at 37 ± 1 °C. The zone of inhibition was measured by determining the diameter created around the wells using vernier calipers.
Determination of minimum inhibitory concentration
Minimum inhibitory concentrations (MIC) of selected excipients (capmul MCM C8, labrasol and acconon-C80) were determined against both the strains. Nutrient agar media was prepared and sterilized by autoclaving at 121 °C for 15 min. After cooling, the media was mixed with 2 ml of MS-995 (2.5 × 107 CFU/ml) containing various concentrations of excipients. The mixture was then poured into 85 mm diameter petri dishes (approx. 30 ml each) and kept aside for solidification. All serial dilutions were made using sterilized milli-Q water. Analogous method was adopted for MS-942 (1.8 × 107 CFU/ml). The MIC was determined as lowest concentration causing no visible growth of the bacteria after incubation period.
Concentration and time-dependent killing of M. smegmatis strains
The killing kinetic of screened excipients was evaluated by a method reported earlier with some modifications (Zhang et al. 2010). To investigate the anti-mycobacterium activities of capmul MCM C-8, labrasol and acconon C-80 at various concentrations (2.5, 5.0, 7.5 and 10 % w/v), 2 ml of bacterial cultures (2.5 × 107 CFU/ml for MS-995; 1.8 × 107 CFU/ml for MS-942) were added in respective sterilized tube containing excipients (1.0 ml) and incubating for 24 h at 37 ± 1 °C. Control bacterial sample was prepared using analogous method containing milli-Q water instead of excipients. Treated bacterial suspensions were taken from each labeled tube at 30, 60, 120 and 240 min and added to sterilize molten nutrient agar plate followed by incubation (Malik et al. 2005). After incubation, plates were counted for viable colonies and percent survival was reported at various time intervals. All experiments were done in triplicate under same experimental conditions.
Analysis of released cytoplasmic content
UV-absorbing cellular material released after bacterial damage was performed as reported previously (Su et al. 2012). Two milliliter of bacterial cultures (MS-995 ~2.5 × 107 and MS-942 ~1.8 × 107 CFU/ml) were centrifuged at 3000 rpm for 15 min. The supernatant was removed to get pellets. The pellets were re-suspended with Milli-Q water and mixed with each excipient to get the final concentration of MIC of each selected excipients. The samples were then vortexed and incubated with constant stirring at 37 ± 1 °C. Next, the samples were collected at different time intervals (60, 120 and 240 min) and centrifuged (Micro centrifuge, REMI, Mumbai, India) at 3000 rpm for 10 min to remove the bacterial debris. The supernatants were diluted with milli-Q water and absorbance was measured at 260 and 280 nm using UV–Vis Spectrophotometer (Schneider 1957).
Morphological evaluation
Scanning electron microscopy
Scanning electron microscopy (SEM) was performed as previously described with some modifications (Zhang et al. 2003). Two milliliter of bacterial suspension (MS-995 ~2.5 × 107 and MS-942 ~1.8 × 107 CFU/ml) were harvested separately by centrifugation at 3000 rpm for 5 min. The obtained pellets were washed again with milli-Q water and centrifuged. The procedure was repeated to ensure the absence of the adhered media which may interfere in morphological studies. The pellets were treated with capmul MCM C-8, labrasol and acconon C-80 at their MIC value and incubated for 4 h at 37 ± 1 °C after shaking. After incubation, the treated cultures were centrifuged to obtain pellets and re-dispersed in 2.5 % v/v glutaraldehyde solution. Further, 10 µl from each sample was kept on cover slips previously treated with poly-l-Lysin. Control groups were similarly prepared in milli-Q water under same experimental conditions. All samples were kept overnight at 37 °C temperature. The samples were fixed on brass specimen club using double sided adhesive tape and were made electrically conductive by coating with platinum in vacuum (Beg et al. 2013; Bleck et al. 2010; Dahl 2005). Samples were imaged at different resolutions using SEM (JEOL JSM-6390LV, Tokyo, Japan).
Transmission electron microscopy
Bacterial cultures (2.0 ml) from exponential phase were collected and centrifuged at 3000 rpm for 5 min to get pellets. The obtained pellets were rinsed with milli-Q water thrice and were treated with labrasol, capmul MCM C-8 and acconon C-80 (at MIC value) at two different time-points (15 and 120 min). The treated bacterial samples were spread on copper grid coated with carbon film and excess samples were instantly removed with filter paper followed by negative staining using 2 % (w/v) of phosphotungstic acid solution. The stained samples were air dried at ambient temperature before analysis (Singh et al. 2013; Hanh et al. 2013) using transmission electron microscopy (Tecnail 2, 120 kV, FEI Company, Eindhoven, Netherlands). Control samples were prepared similarly without any treatments.
Atomic force microscopy
Bacterial suspensions (treated as well as control) were prepared as per method reported under scanning electron microscopy. Treated and control samples were placed on a glass cover slip previously treated with poly-l-Lysin and air dried. Poly-l-lysin was used to immobilize the bacterial cells on the glass surface so that they are stable to tip force while AFM imaging. Morphological study was assessed at ambient temperature (25 °C) using AFM Solver Pro 47 (NT MDT, Saint-Petersburg, Russia). Images were obtained with a scan speed of 0.8 Hz in air at room temperature and at semi-contact angle mode (Verma et al. 2014). All the scanning and dimensional parameters were set as previously reported (Singh et al. 2013).
In vitro hemolysis study
To negate any possibilities of hemolysis induced by labrasol, acconon-C-80 and capmul MCM C8, in vitro hemolysis studies were performed as reported previously with slight modifications (Hussain et al. 2013). Different concentrations (MIC and 5 × MIC) of these excipients were prepared to carry out the test. Red blood cells (RBCs) treated with phosphate buffer solution (pH 7.4) and Triton X-100 were used as negative and positive control, respectively (Falamarzian and Lavasanifar 2010). Blood was collected in blood collecting tube containing few drops of EDTA (ethylene diamine tetra acetic acid). The collected blood was centrifuged to separate erythrocyte from the plasma. The RBCs obtained were washed thrice with PBS solution and 4 % v/v RBCs suspension was prepared (Perkins et al. 1997). All the samples were stored at 0–4 °C temperature before incubation. Test samples (0.5 ml of MIC and 5× MIC of excipients were mixed with 0.5 ml of RBCs suspension and final volume was adjusted to 4 ml with PBS. RBCs (treated and control) were incubated at 37 ± 1 °C temperature for 24 h followed by centrifugation to get pellets. The supernatant (0.3 ml) was removed and mixed with milli-Q water (2.7 ml) followed by measuring absorbance at 550 nm (λmax) using UV–Vis Spectrophotometer. The percentage hemolysis was calculated using: (Abs − Abs0/Abs100 − Abs0) × 100; Abs, Abs0 and Abs100 are the absorbance of excipients treated, negative control and positive control, respectively.
Excipients susceptibility test against M. tuberculosis H37 Rv
M. tuberculosis H37 Rv was grown to logarithmic phase (equivalent to OD595 ~ 0.5) in Middlebrook 7H9 broth (Difco Laboratory, USA) supplemented with albumin dextrose complex (Difco Laboratory, USA) for stock preparation. The bacterium was sub-cultured under aerobic condition in the same media from the stock at 37 °C. Tenfold diluted suspension of this standard suspension (1 mg/ml) were streaked on Lowenstein-Johnsen (LJ) media for determination of CFU value.
Excipient susceptibility test was performed by proportion method as reported earlier with slight modification (Hassan et al. 2014; Gupta et al. 2010). This method determines the percent growth (number of colonies) of a defined inoculum on control media (free from drug) versus growth of culture on media containing test sample (in critical amount) supposed to inhibit tuberculosis strain Rv. Inoculated media was prepared on LJ media by inoculating (one loopful equivalent to 6.0 μl, 3 mm diameter) with previously grown culture and incubated at 37 °C for 6 weeks on LJ media. Some colonies were dispersed in water for injection (2 ml) to achieve 1 mg/ml suspension equivalent to McFarland standard 1.0. The suspension was vortexed for 1 min to get a homogeneous and left for some time to reduce aerosol formation in further process. Standard dilution of 10−4 was prepared to inoculate the LJ media contained in McCartney vial. Control vial free from any excipient and vial containing test excipients were inoculated with same dilution (10−4) and incubated overnight in a slant position with loosened cap at same temperature. After 12 h of incubation (overnight), the cap was tightened and incubated at same temperature for period of 6 weeks in upright position. First reading of susceptibility was assessed on 28th day of incubation and again incubated even-after showing susceptibility. Final result was declared after completion of 6th week. The absence of colonies indicates susceptibility of excipient against the strain Rv. The experiment was performed in Class II Biological Safety Cabinet and taking all care of infection.
In vivo biosafety evaluation
Preparation of excipients emulsion or solution
Capmul MCM C8 was prepared in oil-in-water emulsion form using cremophor EL as emulsifier (10 % w/v). Other excipients were water soluble and prepared into solution form in same concentration. To explore the possibility of toxicity of excipient in explored concentration, toxicity study was performed as per the methods reported earlier with slight modification (Pandey et al. 2006; Jain et al. 2010).
Toxicity assessment in mice
Swiss albino mice of either sex, weighing about 30–40 g and age of about 12–16 weeks old were used in the study. Animals were randomly grouped and each group contained three animals. Total twelve mice were grouped into four groups. Group-1 was served as control without any treatment, group-2 received labrasol solution, group-3 received capmul MCM C8 and group-4 received cremophor-EL. These excipients were administered intra-peritoneally as reported earlier (Jain et al. 2003). All animals were monitored for survival after dosing till 14th day. The surviving mice were immediately subjected to histopathological studies.
Histopathological assessment
Treated and untreated animals were ethically sacrificed by cervical dislocation after completing 14 days of toxicity study (Hussain et al. 2013). Major visceral organs like lungs, kidney, heart, brain and liver were taken out and preserved immediately in 10 %v/v formalin solution. These organs were studied for any possible abnormalities after treatment with excipient and compared with control group by histopathological examination. The isolated and excised organs were stained with basic hematoxylin and acidic eosin. The prepared slides were viewed using leical optical microscopy (Leica application suite version 2.4.0R1, Leica microsystems limited, Switzerland) under different magnifications. Anatomical structure of each treated group was compared with control group. In vivo investigation was performed after approval of the Institutional Ethic Committee of the Department of Pharmaceutical Science and Technology, BIT, Mesra Ranchi-835215 (BIT/PH/IAEC/25/2013).
Results and discussion
In vitro anti-mycobacterium assessment
Zone of inhibition (mm) exhibited by different oils, surfactants/co-surfactants against MS-995 and MS-942 are tabulated in the Supplemental Table T2. The in vitro results revealed the maximum ZOI in capmul MCM C-8 (22.05 ± 0.95 mm), labrasol (17.25 ± 0.5 mm) and acconon C-80 (16.25 ± 0.5 mm) against MS-995. Accordingly, capmul MCM C-8 (15.5 ± 0.5 mm), labrasol (18.0 ± 2.0 mm) and acconon C-80 (13.75 ± 1.6 mm) exhibited maximum inhibition against MS-942 (Supplemental Table T2). Furthermore, MS-995 was found more sensitive to capmul MCM C8 and acconon C-80 as compared to MS-942 while labrasol demonstrated similar antibacterial efficiency against both the strains. In all classes of capmul, caprylic acid (C8:0) content is more than 80 % as compared to other fatty acids such as capric acid (C10:0). Similarly, miglyol classes contain more than 50 % caprylic acid (C8:0). Gelucire 44/14 contains lauric cid (C12:0) as major component (44.7 %) and caprylic acid nearly 7.29 %. Labrasol is mixture of mono-, di-and triglycerides that contains mainly caprylocaproyl macrogol-8-glycerides. Labrasol exclusively contains 50–80 % caprylic (C8:0) and 20–30 % capric acid (C10:0) (Rahman et al. 2013). Hence, the inherent anti-mycobacterium activity may be attributed to caprylic acid present lipids and surfactants. A detail composition of fatty acids in Capmul MCM, Miglyol-812, Gelucire 44/14 and Labrafil is reported by Arnold et al. (2012). Furthermore, in the present study, capmul MCM C-8 that contains caprylic acid (>95 %) was emulsified in nanometric dimension using cremophor-EL, which further reinforces the killing propensity of lipid plausibly due to augmented diffusion of the oil into the media. Labrasol and acconon C-80 apart from eliciting anti-mycobacterium activity have also been reported to be a potent P-pg inhibitor (Shen et al. 2011; Lin et al. 2007). This may lead to enhanced oral bioavailability of anti-tubercular drugs and may cause reduced drug resistance. The selected excipients are considered as generally regarded as safe category (GRAS).
Determination of minimum inhibitory concentration
Capmul MCM C8, acconon C-80 and labrasol were subjected for determination of MIC against MS-995 and MS-942 (Supplemental Table T3). Capmul MCM C-8, acconon C-80 and labrasol have MIC value of 12.5 ± 0.5, 12.5 ± 0.5 and 25.0 ± 1.0 mg/ml respectively, against MS-995. Similarly, Capmul MCM C8, acconon C-80 and labrasol showed the MIC of 18.75 ± 0.8, 15.625 ± 2.5 and 25.0 ± 1.0 mg/ml against MS-942 (Supplemental Table T3). The anti-mycobacterium activity of the selected excipients can be attributed to caprylic acid that forms a major constituent.
Concentration and time dependent killing of M. smegmatis
The concentration and time dependent killing of M. smegmatis (MS-995 and MS-942) using capmul MCM C-8, labrasol and acconon C-80 is portrayed in the Fig. 1a–f. A reduction in viable colonies was observed as the concentration of excipients increases. Maximum inhibition was found with Capmul MCM C-8 during initial first and second hours within the explored concentration (2.5–10 %w/v). When MS-942 and MS-995 were treated with 10 %w/v of capmul MCM C-8, labrasol and acconon C-80, a complete loss of viable colonies was apparent after 4 h of treatment (Fig. 1a–f). Furthermore, labrasol was found to be more effective against MS-995 over MS-942 even at lower concentration. The finding is in compliance with ZOI studies which showed better anti-mycobacterium activity against MS-995 compared to MS-942 (Supplemental Table T2). Similar trend was found with both capmul MCM C8 and acconon C-80 (Fig. 1). The killing effect of capmul MCM C-8 (in first hour) was found to be comparable (about 40 % surviving colonies) in both strains at higher concentration (10 % w/v) as shown in the Fig. 1. However, there is complete loss of viable colonies after 4 h at 10 % concentration in both strains. Similarly, there was complete disappearance of viable colonies after 4 h of treatment with co-surfactant acconon C-80 against both strains at higher concentration (10 %). These finding can be explained as following:
The cell wall of M. smegmatis comprises of dense electron rich inner layer with transparent outer layer covalently linked to peptidoglycan. The distribution of glycolipid and phospholipid in the cell envelop determine the cell surface properties, permeability and phenotype (Fujiwara et al. 2012). Intracellular persistence of the M. smegmatis depends upon the permeability of the outer membrane. The outermost layer of Mycobacterium contains mycolic acid which provides a lipophilic layer with negative charge. Killing of these bacteria might be due to lipid extraction from the cell wall (by labrasol and acconon C-80) and lipid–lipid desolvation caused by capmul MCM C-8. Furthermore, the surfactants (labrasol and acconon C-80) may reduce the interfacial tension at the interface between cell wall facilitating the enhanced permeation of the surfactants.
Cytoplasmic content release study
This study was based on the release of cytoplasmic content from the M. smegmatis (MS-995 and MS-942) when treated at the MIC of each excipients. It was hypothesized that time dependent killing of the Mycobacterium can be correlated to cytoplasmic content released. The released UV-absorbing cytoplasmic material from killed bacteria is an indicator of membrane perturbation (Su et al. 2012). Therefore, we measured the supernatant of the treated culture with excipients at different time point (Fig. 2). The high molecular weight compound such as DNA, RNA, protein and complex polysaccharides are released after bacterial cell fragmentation. The purity of the DNA and RNA can be assessed by determining absorbance ratio at two different wavelengths (260 and 280 nm). Low concentration of excipients had minimal effect on the cell fragmentation leading to minimum release of UV-absorbing material (Fig. 2). As depicted in the Fig. 2, it can be observed that the absorption ratio (260/280) achieved a plateau phase after ~2 h with all excipients against both the strains indicating cell fragmentation owing to release of essential cellular contents. Furthermore, the plateau phase achieved the absorption ratio of 1.74 and 2.21 at 120 min in case of capmul MCM C8 against MS-995 and MS-942, respectively. However, the plateau phase was found at 1.28 and 1.16 at 120 min for labrasol and acconon C-80 respectively, against MS-995. Similarly, the plateau phase was found at 1.43 and 1.45 at 120 min for labrasol and acconon C-80 respectively, against MS-942. The finding construes that the capmul MCM C8 has higher piercing potential causing maximum cytoplasmic release as compared to labrasol and acconon C-80. Since, all excipients were explored at their respective MIC the killing mechanism can also be attributed to the extraction of essential lipidic content of the cell wall along with the release of DNA/RNA content.
Morphological evaluation
Scanning electron microscopy
Scanning electron microscopy (SEM) was performed to elicit the morphological changes in cellular architecture of MS-995 (Fig. 3a–d) and MS-942 (Fig. 3e–h) in presence of various excipients (capmul MCM C-8, labrasol and acconon C-80). The photomicrograph of SEM revealed significant surface perturbation after 4 h of treatment in both strains. The control groups (Fig. 3a, e of MS-995 and MS-942, respectively) showed the rod-shaped bacilli with intact and apparently smooth continuous surface membrane. However, after treatment with excipients, profound perturbations and damages were observed on bacterial cell wall suggesting the potent anti-mycobacterium activity of excipients at their MIC value (shown by arrows). The cell wall of M. tuberculosis plays a crucial role in the pathogenesis of tuberculosis involving several proteins and enzymes. Therefore, the most commonly used first-line anti-tubercular drugs were developed by paying a considerable attention on molecular architecture of this cell wall. These established drugs mainly act on the cell wall. A recent bioinformatic study suggested that the GlmM protein responsible for cell wall growth present in M. smegmatis was highly homologous (~79 %) to GlmM protein found in M. tuberculosis. The fast growing ability, non-pathogenic nature, common homologous protein, similar cell wall architecture and same glmM gene are positive attributes of M. smegmatis making it an appropriate surrogate model of M. tuberculosis (Kang et al. 2013). Capmul MCM C-8 is a lipid with high caprylic acid content (>95 %w/w). This is chemically glycerol monocaprylocaprate with medium chain fatty acid. We noticed that capmul MCM C-8 showed extensive damage (shown by arrows) to both bacterial strains as compared to labrasol and acconon C-80 (Fig. 3b, f). Morphological evaluation of capmul MCM C8 treated cells (Fig. 3b, f) indicated the complete cell lysis with wrinkled and abnormal cell-wall. Few cells were apparently collapsed due to the release of cellular content construing loss of osmotic pressure and extensive damage to cell-wall integrity. Capmul MCM C-8 is a lipophilic diglycerides with low HLB value (4.7) that might be capable of interacting with lipophilic bacterial cell wall causing cell wall perturbation. The results are in accordance with previous findings of cytoplasmic release study and time dependent killing of bacteria (Singh et al. 2013). In another study, similar morphological damage was observed when M. smegmatis were treated with lysozyme (Yang et al. 2014). Labrasol (caprylocaproyl macrogolglycerides) and acconon C-80 (ethoxylated coconut glycerol ester) are hydrophilic surfactants which also caused serious cell-wall damage to MS-995 (Fig. 3c, g) and MS 942 (Fig. 3d, h) (shown by arrows) due to presence of various fatty acids (lauric acid, capric acid, caprylic acid etc.). Cell-wall contains some Mycobacterial porins through which poorly soluble hydrophilic agents diffuse. This process may further be augmented by using labrasol and acconon C-80 and allowing the passage of anti-tubercular drugs/lipids into the cell wall at toxic level. Conclusively, these two surfactants may be act synergistically with the anti-tubercular drug/lipid leading to reduced dose and dosing frequency of drugs.
Scanning electron microscopy of control and excipient treated M. smegmatis strains. a–d represent photomicrograph of control, capmul MCM C-8 treated, labrasol treated and acconon C-80 treated MS-995. e–h represents photomicrograph of control, capmul MCM C-8 treated, labrasol treated and acconon C-80 treated MS-942
Transmission electron microscopy
The ultra-structure of both strains of M. smegmatis (control and excipients treated) was visualized using transmission electron microscopy (TEM) to study the probable mechanism of cell lysis. Both strains were treated with excipients separately at two different time point (15 and 120 min) and their TEM photomicrographs are presented in the Fig. 4. TEM images of MS-995 (controls and treated) are portrayed in the Fig. 4a–h while TEM images of MS-942 (control and treated) are shown in the Fig. 4i–p. The control groups did not show any adverse changes in cell wall of Mycobacterium at 15 and 120 min. The cells appeared as normal rod-shaped with intact cellular contents. Figure 4c, d show the MS-995 treated with acconon C-80 at its MIC value at 15 and 120 min, respectively. It was observed that increased exposure time caused enhanced cellular damage. Similar finding was observed with other excipients (capmul MCM C8 and labrasol) against both the strains. However, extent of killing and cells fragmentation were quite different in each excipients. Capmul MCM C-8 (Fig. 4e, f for 15 and 120 min, respectively) and labrasol (Fig. 4g, h for 15 and 120 min, respectively) showed augmented morphological damage than acconon C-80 (Fig. 4c, d) against MS-995. On the other hand, capmul MCM C-8 (Fig. 4m, n for 15 and 120 min respectively) and labrasol (Fig. 4o, p for 15 and 120 min respectively) showed greater activity than acconon C-80 (Fig. 4k–l) against MS-942. The results are in accordance with SEM analysis. Conclusively, it can be inferred that the maximum fragmentation was observed with capmul MCM C-8 as compared to labrasol and acconon C-80 against both the strains. Similar observations were reported using TEM on M. smegmatis after treatment with ethambutol (Jiang et al. 2011).
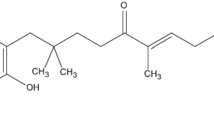
In order to explain the probable mechanism of anti-mycobacterial activity of lipid (Capmul MCM-C8) and surfactants (labrasol and acconon C-80), we reviewed research papers of other authors. Anti-mycobacterium activity of a long chain hydrocarbon was mainly attributed to the degree of unsaturation and presence of hydroxyl group at the terminal end of molecule (Jiang et al. 2011). In addition, it was reported that chain length, hydrophobicity and partition coefficient of the molecules played a significant role in anti-Mycobacterium activity. Chemically, capmul MCM C8 contains capric and caprylic acids with carboxylic groups at one terminal end and other terminal has short chain hydrocarbon (saturated) which provides hydrophobicity to the molecule (Fig. 5a, b). Therefore, there is a proper balance between two parts of molecule with HLB value of about 4.7. It was reported that on increasing lipophilicity of alkanol (up to decanol) caused a sharp reduction in MIC value against M. smegmatis mc2155 and M. tuberculosis H37RV. In further study, finding was contradictory and on increasing the number of carbon atom in the hydrocarbon portion of the molecule led to marked increase in MIC values with no obvious anti-Mycobacterium activity. This was possibly due to poor partitioning of higher alkanol from water to lipid part of Mycobacterium cell wall (Mukherjee et al. 2013). Acconon C-80 is a chemically ethoxylated coconut glycerol esters containing three long hydrocarbon chain. Each hydrocarbon chain contains one double bond and one hydroxyl (–OH) group that makes the molecule anti-mycobacterium. Moreover, labrasol is hydrophilic surfactant (HLB = 14) and contains mixtures of mono-, di- and triglycerides esters (Fig. 5g–k). It is also mono-fatty acid and di-fatty esters of polyethylene glycol. Furthermore, unsaturation along with hydroxyl group at the terminal end of long chain aliphatic hydrocarbon in labrasol (Fig. 5j), hexane-1-ol (Fig. 5e) and optimum HLB values are the major factors which contribute to anti-mycobacterium activity. It was also reported that hexene-1-ol showed greater activity than normal hexanol and no activity in hexene (Fig. 5d).
Compounds with hydroxyl groups and un-saturation exhibiting anti-mycobacterium activity: a Caprylic acid (C8:0) of capmul, b capric acid (C10:0) of capmul, c hexanol, d hexene, e hexene-1-ol, f cremophor-EL (x + z+z = 35) without activity. g–k represented structural moieties present in Labrasol in which (j) may be responsible for anti-mycobacterium activity. In labrasol X = 6 or 8 and Y = 8
Atomic force microscopy
Anti-mycobacterial activities of excipients against MS-995 and MS-942 were further examined using AFM to corroborate the findings of SEM/TEM and to elucidate the probable mechanism of bacterial lysis. The collated images of AFM of the untreated strain MS-995 and MS-942 are illustrated in the Figs. 6a–d and 7a–d respectively. The image Fig. 6a, b are the representative images of 2-dimensional and 3-dimensional profile respectively. The Fig. 6c, d represent the contour profile and dimensional analysis (DA) of the same untreated MS-995. Similarly, the images Fig. 7a, b depicted 2-dimensional and 3-dimensional profile of the control group of MS-942, respectively. The Fig. 7c, d represent the contour surface profile and 2-dimensional analysis (DA) of the same untreated MS-942 bacteria. In these control groups, the collated images of AFM demonstrated normal rod shaped bacilli without obvious structural abnormalities. The cells were found to be apparently smooth, evenly surface topology and homogeneous outer cell wall surface in all direction (as shown by white arrows in controls). Analysis of images using AFM software, average surface roughness (nm), and root mean square (nm) are the basic parameters used to ascertain comparison among them. The values (obtained from different region of bacteria as shown by small black square) of average roughness (ar) and root mean square (rms) were 5.2 ± 2.5 and 6.51 ± 2.9 nm respectively for MS-995. Accordingly, ar and rms of MS-942 were 4.09 ± 0.6, and 5.68 ± 1.1 nm respectively suggesting no considerable differences in these parameters in both control groups. Thus, approximate value of these parameters as evidenced with dimensional analysis (DA) images and contour profile justified the comparable surface nature and morphology without hampering the bacterial cells during sample preparation.
The representative AFM images of bacterial cells treated with the individual excipient have been portrayed in the Fig. 6e–p. The 2-D and 3-D images of MS-995 when treated with the Capmul MCM C8 are depicted in the Fig. 6e, f respectively. The values of average roughness and root mean square were profoundly increased from 5.2 ± 2.5 to 21.37 ± 1.17 nm and 6.51 ± 2.9 to 26.45 ± 1.54 nm, respectively after exposure to Capmul MCM C8. The ar and rms values were calculated from different locations as indicated by small black squares in the Fig. 6g. The enhanced and potentiated cell damage followed with fragmentation resulted from binding and accumulation of lipid into the cell membrane leading to progressive erosion of peptidoglycan layer. Generally, the thickness of the cell wall of M. smegmatis is approximately 7.1 nm which get diminished after treatment (Zuber et al. 2008; Singh et al. 2015). The copious peaks and troughs present in DA curve (Fig. 6h) indicated increased damage, destruction, depressions and cell fragmentation which could lead to release of the cytoplasmic content. The analysis of these images displayed the extensive exudation and oozing of the bacterial cellular material and these findings are in accordance with the TEM reports.
The Fig. 6i–l portrayed the AFM images of MS-995 treated with the Labrasol. The 2-D and 3-D images are presented in the Fig. 6i, j respectively, with substantially increased average roughness (27.25 ± 3.23 nm) and root mean square value (33.48 ± 3.9 nm). There were numerous damaged cells as shown by arrows and fragmented cells (black circle in the Fig. 6i) where the increased rms value was substantiated by the contour profile analysis. The analysis report recommended the extensive cell damage was apparent with cell flattening and comparatively more occurrence of cell fragmentation as compared to control.
The Fig. 6m–p represented the AFM images of MS-995 treated with the acconon C-80. The 2-D and 3-D images are portrayed in the Fig. 6m, n respectively, with considerably augmented average roughness (19.11 ± 4.5 nm) and root mean square value (21.77 ± 5.0 nm) as compared to control group. There were numerous damaged and amalgamated cells as shown by arrows (black circle in the Fig. 7m) where the enhanced rms value was substantiated by the contour profile analysis. Both labrasol (chemically defined earlier) and acconon C80 are hydrophilic excipients in the present study. On the other hand, capmul MCM C8 is lipophilic with low HLB value (4.7) and chemically glyceryl monocaprylate. It is well known fact that molecule of similar nature are easily solubilised such as “like dissolve like”. Therefore, it is essential need of a molecule sought to be penetrated into the cytoplasmic content or in the cell wall of bacteria with the optimum value of HLB. Generally, the cell wall of bacteria is composed of hydrophilic component (ethanolamine) and lipophilic component (fatty acid domains) (Singh et al. 2015). Thus, lipophilic capmul are capable to be accumulated in the lipophilic domain of cell wall responsible to cause significant toxicity. Similarly, hydrophilic labrasol and acconon C80 are responsible to be concentrated into the hydrophilic domain of the layer and supposed not to move further across the non-polar region. In addition, labrasol are considered as P-gp efflux inhibitor which might be responsible to reduce the development of resistance in bacteria (Singh et al. 2015).
The representative AFM images of bacterial cells (MS-942) treated with the same excipients have been collectively depicted in the Fig. 7e–p. The 2-D and 3-D images of MS-942 are illustrated after treatment with the Capmul MCM C8 in the Fig. 7e, f respectively. The average roughness and root mean square values were extremely enhanced from 5.2 ± 2.5 to 27.86 ± 16.0 nm and 6.51 ± 2.9 to 31.77 ± 19.12 nm, respectively when exposed to the lipid Capmul MCM C8 demonstrated increased cellular roughness. The Fig. 7e, f are representative images of 2D and 3D images which revealed numerous collapsed and damaged cells (black circle) with deciphered outline of the bacterial cell as shown in red circle of the Fig. 7g. This might be due to progressive accumulation into the lipophilic domain of the cell wall leading to augmented pore development and significant conformational variation in the same domain. These reasonable reasons corroborated the detrimental and irreparable damage caused by the lipid for irreversible permanent cell death. Similarly, 2D and 3D images of AFM after exposing with Labrasol are elicited in the Fig. 7i, j. These images clearly demonstrated enhanced fragmented bacterial cells with uneven surface as evidenced with increased values of average surface roughness (10.78 ± 0.43 nm) and root mean square (12.21 ± 0.56 nm) as compared to control MS-942 which revealed 4.09 ± 0.6 and 5.68 ± 1.1 nm for ar and rms value respectively. The 2-dimensional contour profile curve showed significant irregular surface with copious fissure, depression and cracks on the bacterial surface (Fig. 7k, l). Furthermore, the collapsed apical end of the cell was observed with MS-942. However, such observations were not apparently present in other treated cells of MS-995. Similar pattern of bacterial killing and cell lysis were caused by the surfactant acconon C80 as shown in the Fig. 7m–p. All the treated cells showed a dramatically decline in size suggesting the exudation of cellular content and genetic material for irreparable damage which ultimately resulted into cell death.
In vitro hemolysis study
Excipients revealed no significant hemolytic activity at MIC and 5 × MIC value (Supplementary Fig S1). Considering 100 percent hemolysis of positive control Triton X-100 (0.1 %v/v), labrasol (2.5 %v/v), capmul MCM C-8 (1.25 %v/v) and acconon C-80 (1.25 %v/v) displayed 18.6 ± 0.5, 13.3 ± 0.2 and 22.8 ± 1.0 % hemolysis respectively, at their MIC value over period of 24 h. Similarly, at 5xMIC of labrasol, capmul MCM C-8 and acconon C-80, these excipients showed 24.8 ± 0.8, 16.7 ± 0.2 and 26.2 ± 1.2 % hemolysis, respectively. Furthermore, the hemolysis potential of the surfactants (labrasol and acconon C-80) was somewhat higher than that of capmul MCM C-8 at same concentration. However, the extent of hemolysis was insignificant when compared to positive control. The negative control (phosphate buffer saline, PBS) showed the 12.2 ± 0.2 % hemolysis while cremophor EL (6.25 %) showed 20.5 ± 0.8 % hemolysis (Supplementary Fig S1). Importantly, we recognized these selected excipients in varied concentration with minimum hemolysis as compared to positive control in studied time. This reflected the ability of explored excipients which could be employed in lipid based carrier system for oral delivery of anti-tubercular drugs.
Excipients susceptibility test against M. tuberculosis H37 Rv
All three excipients were assessed for anti-tubercular activity against experimental tubercular strain H37 Rv. After 6 weeks of incubation period, the growth of tested vials was visualized for growth of any colonies for a range of diluted samples. Both hydrophilic surfactant Labrasol and acconon C80 were prepared in water while capmul MCM C8 was emulsified with cremophor-EL. The result showed that number of colonies was dramatically declined with increase in concentration of excipients. The findings suggested us that the explored excipients have inherent anti-mycobacterium activity against pathogenic strain which could be employed in formulation as excipient to reduce the dose and for synergistic performance with anti-tubercular drugs. Similar type of synergy concept was reported by Singh et al. (2015) using the same lipid and surfactant labrasol against P. aeroginosa. The Fig. 8 showed absence of colonies in vials containing excipients as compared to control group vial with copious buff coloured colonies of Rv. The Capmul MCM C8, Acconon C80 and Labrasol showed complete absence of colonies at 18.4 ± 1.5, 15.6 ± 3.2 and 30.8 ± 7.4 mg/ml respectively as shown in the Fig. 8.
In vivo biosafety evaluation
Conventional formulations containing anti-tubercular drugs exhibit side effects such as hepatotoxicity, neurotoxicity, and others dose dependent toxicities. In this study, we compare the toxicological profile of the excipients at their 10 %w/v concentration. Acute toxicity of lipid-emulsions and surfactant solution were carried out in mice model for 14 days and the results have been portrayed in the Fig. 9. There was no any observed death of mice in treated group as well as control group after oral administration, indicated non-toxic nature of selected excipients. These excipients belong to the category of generally regarded as safe. The finding was further corroborated with histopathological assessment of major visceral organs after 14th day of study. In single dose toxicity study, the surviving mice were sacrificed for organ biopsy. Safety profile of the excipients with inherent anti-mycobacterium activity was established with histopathological examination of vital organs like lungs, kidney, brain, heart and liver after 14 days of treatment. The organs of control group were compared with treated group for any alteration in gross anatomy of organs/tissue. As revealed in the Fig. 9, the stained lungs tissue depicted normal alveolar parenchyma as anatomical structure in treated groups as well as control group. Anatomically, neither cellular infiltration nor tissue fibrosis with alveolar walls was displayed similar to control. Similar finding was also reported with risedronate delivery using pulmonary route in liposomal carrier (Nasr et al. 2013). The brain parenchyma cells from excipients treated groups were of normal histopathology similar to control Fig. 9. The representative images of brain of mice depicted normal parenchymal cells with white matter. Absence of focal infiltrates in parenchyma, vacuolation and glial node in white matter corroborated the normal anatomy in all groups as shown in the Fig. 9. Histopathology study of liver specimen of mice treated with excipient was performed to identify and compare with control group. The results demonstrated normal lobular architecture with central vein and hepatocytes as observed in control. In order to observe any probable toxicity in cardiac tissue, we carried out the biopsy of cardiac tissue. There was absence of collagen deposition, myocardium damage and mononuclear cell infiltration in treated groups and no considerable difference were observed as compared to control group (Fig. 9). To assess the renal anatomy, the histopathological examination revealed that there were no differences in renal anatomy of excipient treated mice as compared to control. Inner medulla, outer medulla and peritubular space were apparently normal.
Conclusions
Based on the preceding results and discussions of in vitro anti-mycobacterium assay, MIC determination, electron microscopy, in vitro hemolysis study and in vivo biosafety evaluation, we concluded coherently for the objectives. Initially, three best excipients capmul MCM C-8 as lipid, labrasol and acconon C-80 were screened with excellent anti-mycobacterium activity exclusive of any anti-tubercular drug. The DNA/RNA content released from the treatment group at different time interval suggested the anti-mycobacterium activities of selected excipients. The most versatile technologies used for morphological studies on the control and treated cells were electron microscopy and atomic force microscopy. Results of these microscopy studies further confirmed for pronounced damage, deformation and disruption caused after treatment with these excipients. Additionally, the result of in vitro hemolysis study of excipients at varied concentration did not display any significant toxic potential on human erythrocytes. This will be a deciding factor for the formulation development with minimum dose that finally may lead to development of the formulation with minimum toxicity and maximum therapeutic potential. Present study suggested that excipients with potential anti-mycobacteium activity can be explored for formulation development for safe and effective delivery of anti-tubercular drugs with effective detrimental effect.
References
Arnold YE, Imanidis G, Kuentz M (2012) In vitro digestion kinetics of excipients for lipid-based drug delivery and introduction of a relative lipolysis half life. Drug Dev Ind Pharm 38(10):1262–1269
Barry III (2001) Mycobacterium smegmatis: an absurd model for tuberculosis? Trends Microbiol 9(10):472–473
Beg S, Jena SS, Patra CN et al (2013) Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Coll Surf B Biointerfaces 101:414–423
Bleck CK, Merz A, Gutierrez MG et al (2010) Comparison of different methods for thin section EM analysis of Mycobacterium smegmatis. J Microsc 237:23–38
Chang T, Benet LZ, Hebert MF (1996) The effect of water-soluble vitamin E on cyclosporine pharmacokinetics in healthy volunteers. Clin Pharmacol Ther 59:297–303
Dahl JL (2005) Scanning electron microscopy analysis of aged Mycobacterium tuberculosis cells. Can J Microbiol 51:277–281
Dudeja PK, Anderson KM, Harris JS et al (1995) Reversal of multidrug resistance phenotype by surfactants: relationship to membrane lipid fluidity. Arch Biochem Biophys 319:309–315
Etienne G, Laval F, Villeneuve C et al (2005) The cell envelope structure and properties of Mycobacterium smegmatis mc2155: is there a clue for the unique transformability of the strain? Microbiology 151:2075–2086
Falamarzian A, Lavasanifar A (2010) Chemical modification of hydrophobic block in poly(ethyleneoxide) poly(caprolactone) based nanocarriers: effect on the solubilization and hemolytic activity of amphotericin B. Macromol Biosci 10:648–656
Fujiwara N, Naka T, Ogawa M et al (2012) Characteristics of Mycobacterium smegmatis J15cs strain lipids. Tuberculosis 92:187–192
Global Tuberculosis Report (2012) World Health Organization (WHO). www.who.int/tb. Accessed 29 Nov 2012
Global Tuberculosis Report (2013) World Health Organization (WHO). www.who.int/tb. Accessed on 24 Dec 2013
Global Tuberculosis Report (2014) World Health Organization (WHO). www.who.int/tb. Accessed 25 Nov 2014
Gopalaswamy R, Narayanan S, Jacobs WR Jr et al (2008) Mycobacterium smegmatis biofilm formation and sliding motility are affected by the serine/threonine protein kinase PknF. FEMS Microbiol Lett 278:121–127
Gupta R, Bandana Thakur B, Singh P et al (2010) Anti-tuberculosis activity of selected medicinal plants against multi-drug resistant Mycobacterium tuberculosis isolates. Indian J Med Res 131:809–813
Hanh ND, Mitrevej A, Sathirakul K et al (2013) Development of phyllanthin-loaded self-microemulsifying drug delivery system for oral bioavailability enhancement. Drug Dev Ind Pharm: doi:10.3109/03639045.2013.858732
Hassan A, Fattouh M, Atteya I, Mohammadeen H, Ahmed H (2014) Validation of A rapid tuberculosis PCR assay for detection of MDR-TB patients in sohag University Hospital. J Appl Environ Microbiol 2(3):65–69
Hussain A, Samad A, Nazish I et al (2013) Nanocarrier-based topical drug delivery for an antifungal drug. Drug Dev Ind Pharm 40(4):527–541
Immanuel G, Sivagnanavelmurugan M, Palavesam A (2011) Antibacterial effect of medium-chain fatty acid: caprylic acid on genotobiotic Artemia franciscana nauplii against shrimp pathogens Vibrio harveyi and V. parahaemolyticus. Aquac Int 19:91–101
Jain S, Jain P, Umamaheshwari RB, Jain NK (2003) Transfersomes a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Drug Dev Ind Pharm 29:1013–1026
Jain S, Kaur A, Puri R et al (2010) Poly propyl ether imine (PETIM) dendrimer: a novel non-toxic dendrimer for sustained drug delivery. Eur J Med Chem 45(11):4997–5005
Jiang T, He L, Zhan Y (2011) The effect of MSMEG_6402 gene disruption on the cell wall structure of Mycobacterium smegmatis. Microb Pathog 51:156–160
Kamdem SS, Guerzoni ME, Baranyi J et al (2011) Effect of capric, lauric and α-linolenic acids on the division time distributions of single cells of Staphylococcus aureus. Int J Food Microbiol 128(1):122–128
Kang J, Xu L, Yang S et al (2013) Effect of phosphoglucosamine mutase on biofilm formation and antimicrobial susceptibilities in M. smegmatis glmM gene knockdown strain. Plos One 8(4):e61589. doi:10.1371/journal.pone.0061589
Lin Y, Shen Q, Katsumi H et al (2007) Effects of Labrasol and other pharmaceutical excipients on the intestinal transport and absorption of rhodamine 123, a P-glycoprotein substrate, in rats. Biol Pharm Bull 30:1301–1307
Malik M, Lu T, Zhao X et al (2005) Lethality of quinolones against Mycobacterium smegmatis in the presence or absence of chloramphenicol. Antimicrob Agents Chemother 49(5):2008–2014
Mukherjee K, Tribedi P, Mukhopadhyay B et al (2013) Antibacterial activity of long-chain fatty alcohols against mycobacteria. FEMS Microbiol Lett 338(2):177–183
Nasr M, Taha I, Hathout RM (2013) Suitability of liposomal carriers for systemic delivery of risedronate using the pulmonary route. Drug Deliv 20(8):311–318
Pandey R, Sharma S, Khuller GK (2006) Oral poly (lactide co-glycolide nanoparticle based antituberculosis drug delivery: toxicological and chemotherapeutic implications. Indian J Exp Biol 44:459–467
Perkins WR, Richard B et al (1997) Combination of antitumor ether lipid with lipids of complementary molecular shape reduces its hemolytic activity. Biochim Biophys Acta 1327:61–68
Rahman MA, Hussain A, Hussain MS et al (2013) Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev Ind Pharm 39(1):1–19
Rege BD, Kao JPY, Polli JE (2002) Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci 16:237–246
Schneider WC (1957) In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol Ill. Academic Press, New York, p 680
Shen Y, Lu Y, Jv M et al (2011) Enhancing effect of labrasol on the intestinal absorption of ganciclovir in rats. Drug Dev Ind Pharm 37(12):1415–1421
Singh N, Verma SM, Singh SK et al (2013a) Antibacterial action of lipidic nanoemulsions using atomic force microscopy and scanning electron microscopy on Escherichia coli. J Exp Nanosci 10(5):381–391
Singh N, Verma SM, Singh SK et al (2013b) Antibacterial activity of cationised and non-cationised placebo lipidic nanoemulsion using transmission electron microscopy. J Exp Nanosci 10(4):299–309
Singh N, Verma SM, Singh SK et al (2014) Consequences of lipidic nanoemulsions on membrane integrity and ultrastructural morphology of Staphylococcus aureus. Mater Res Express 1(02540):1. doi:10.1088/2053-1591/1/2/025401
Singh N, Verma SM, Singh SK et al (2015) Evidence for bactericidal activities of lipidic nanoemulsions against Pseudomonas aeroginosa. Antonie Van Leeuwenhoek 107(6):1555–1568
Su HN, Chen Z-H, Song X-Y et al (2012) Antimicrobial peptide trichokonin VI-induced alterations in the morphological and nanomechanical properties of Bacillus subtilis. PLoS One 7(9):e45818
Verma S, Singh SK, Verm PRP et al (2014) Formulation by design of felodipine loaded liquid and solid self nanoemulsifying drug delivery systems using Box–Behnken design. Drug Dev Ind Pharm 40(10):1–13
Woodcock DM, Linsenmeyer ME, Chojnowski G et al (1992) Reversal of multidrug resistance by surfactants. Br J Cancer 66:62–68
Yang S, Zhang F, Kang J et al (2014) Mycobacterium tuberculosis Rv1096 protein: gene cloning, protein expression and peptidoglycan deacetylase activity. BMC Microbiol 14:174
Zhang H, Yao M, Morrison RA et al (2003) Commonly used surfactant, Tween® 80, improves absorption of P-glycoprotein substrate, digoxin, in rats. Arch Pharm Res 26:768–772
Zhang H, Cui Y, Zhu S et al (2010) Characterization and antimicrobial activity of a pharmaceutical emulsion. Int J Pharm 395:154–160
Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, Daffé M (2008) Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol 190:5672–5680
Acknowledgments
The authors thank the Birla Institute of Technology, Mesra, Ranchi and Government of Jharkhand, for providing the facilities and encouragement. We are also grateful to Mr. Babar Iqbal, formulation and development officer, Unicure India Pvt Ltd., New Delhi for providing drugs as gift samples. Authors are highly thankful to Department of Biotechnology (DBT), New Delhi to provide Senior Research Fellow under DBT Project No. BT/PR5653/MED/29/561/2012) for financial assistance for carrying out this work. The constructive comments and suggestion of anonymous reviewers is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
This article does not contain any studies with human participants by any of the authors. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, A., Singh, S.K. Evidences for anti-mycobacterium activities of lipids and surfactants. World J Microbiol Biotechnol 32, 7 (2016). https://doi.org/10.1007/s11274-015-1965-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-015-1965-4