Abstract
Resveratrol is an important antioxidant that confers several beneficial effects on human health. 4-coumarate coenzyme A ligase (4CL) and resveratrol synthase (RS) are key rate-limiting enzymes in the biosynthetic pathway of resveratrol. Using gene fusion technology, the fusion gene, 4CL::RS, was constructed by the 4CL gene from Arabidopsis thaliana and RS gene from Arachis hypogaea. DNAMAN analysis showed that the fusion gene encoded a 964-amino acid protein with an approximate weight of 104.7 kDa and a pI of 5.63. A prokaryotic expression vector containing Nco-I and EcoR-I restriction sites, pET-30a/4CL::RS, was identified by liquid culture bacterial PCR, enzyme digestion, and sequencing, and then used in the induction of expression. Subsequently, a biosynthetic pathway of resveratrol was constructed in Escherichia coli BL21(DE3) that harbored pET-30a/4CL::RS. The recombinant strains were induced to express the fusion protein at 28 °C for 8 h. After bacterial cells were disrupted by hypothermic ultrasonication, the 4CL::RS fusion protein was thoroughly separated from tags using Ni–NTA affinity chromatography, and then detected by SDS-PAGE analysis. When the recombinant strains expressed the fusion protein, the precursor, p-coumaric acid, was converted to resveratrol. In the present study, the final concentration of resveratrol derived from 1 mM p-coumaric acid was 80.524 mg/L, with a 35.28 % (mol/mol) conversion yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
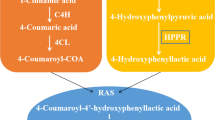
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is an important plant secondary metabolite of Arachis hypogaea and Vitis vinifera (Doré 2005). In the past few years, reports have described the beneficial effects of resveratrol on human health, which include anti-coagulant, anti-oxidation, anti-bacterial, anti-inflammatory, anti-cancer, cardiovascular protection, and neuroprotective properties (Sun et al. 2010; Athar et al. 2007; Li et al. 2012). Resveratrol has been detected in 72 plant species, representing 21 families and 31 genera (Burns et al. 2002). However, the content of resveratrol in plants is generally very low, making it difficult to meet market demands and in turn, rendering resveratrol biosynthesis as a highly interesting topic. The identification of a method of efficiently synthesizing resveratrol would not only address the problems of ecological destruction and resource scarcity associated with extracting resveratrol from plants, but also resolve problems relating to environmental pollution. The biosynthetic pathway of resveratrol involves the metabolic pathway of phenylpropanoid (Vogt 2010; Ferrer et al. 2008; Noel et al. 2005; Winkel 2004). A diagrammatic representation of the resveratrol pathway is presented in Fig. 1 (Donnez et al. 2009; Chong et al. 2009). In this pathway, the intermediate, p-coumaric acid, is generated through one-step and two-step enzymatic reactions involving phenylalanine and tyrosine, respectively (Wang et al. 2010). p-coumaric acid is ultimately converted into resveratrol through the catalyses of 4CL and RS. To increase the production of resveratrol in microorganism, the 4CL gene and RS gene were fused and integrated into a single expression box using gene fusion technology, thus resulting in the construction of a bifunctional enzyme. Based on these methods, the resveratrol was synthesized from the p-coumaric acid.
Bülow previously constructed bifunctional enzymes and analyzed multifunctional-enzyme molecules using a fusion gene (Bülow 1990; Bülow and Mosbach 1987). The fusion proteins not only retained enzyme activity but also imparted a detectable proximity effect on successive catalytic reactions. Studies have shown that when two or more different genes were fused, the activity of the fusion enzyme was directly influenced by the type and length of amino acids of the linker (Zhang et al. 2009). Because of the simple structure and various conformations of non-polar hydrophobic amino acids such as glycine (Gly, G) and serine (Ser, S), its potential application as the component of the linker was considered. The length of the amino acids in the linker ranged from 3 to 59 arbitrary units (A.U) (Robinson and Sauer 1998). For example, the extensive length of the linker renders the fusion protein to be easily biodegradable, whereas a very short linker induces instability due to higher-order structure folding and a low level of enzyme activity of the fusion proteins (Arai et al. 2001; Xue et al. 2004; LaVallie and McCoy 1995).
To construct the 4CL::RS fusion gene in the present study, a 15-amino acid linker was inserted between the 4CL gene from Arabidopsis thaliana and the RS gene from A. hypogaea using gene fusion technology. The BamH-I restriction site was introduced in the linker, and then the 4CL gene and RS gene were fused at this particular restriction site. A prokaryotic expression vector, pET-30a/4CL::RS, was constructed and then expressed. The fusion protein was isolated and purified using Ni–NTA affinity chromatography. Finally, the activity of the fusion protein was identified based on the quantity of the resveratrol derived from p-coumaric acid.
Materials and methods
Bacterial strains and plasmids
Escherichia coli TOP10 (Biomad Co., Ltd., Beijing, China) and E. coli BL21(DE3) (TransGen Biotech, Beijing, China) were used as host strains for cloning and expressing the fusion gene. The pMD19-T vector (TaKara, Tokyo, Japan) was used as cloning vector, whereas pET-30a(+) (Invitrogen) was used as expression vector. pMD18-T/4CL and pMD18-T/RS were kindly provided by Professor Xuefeng Guo of the International Centre for Bamboo and Rattan, China.
Design of the flexible linker
To increase total enzymatic efficiency, a 15-amino acid flexible linker was designed to connect the 4CL gene and RS gene. It has a certain flexibility, which ensures the activity of the two enzymes. The sequences of the linker were designed as GGGGSGGGGSGGGGS. According to the sequences of the 4CL gene and RS gene, as well as that of the BamH-I restriction site, the nucleotide sequences of the flexible linker were designed as follows: ggtggaggcggatcc ggcggaggtggctct ggcggtggcggatcg (the underlined sequences represent the BamH-I restriction site). The BamH-I restriction site was introduced to facilitate the ligation of the 4CL gene and RS gene.
Primer design
In the present study, primers were used to introduce the nucleotide sequences of the linker into the fusion gene. When the nucleotide sequences after the BamH-I restriction site of the linker were placed in the forward primer of the second gene (RS gene), this primer would generate a fragment approximately 63 bp in size, which in turn would reduce its specificity. Therefore, this primer was designed as two parts. Five primers are listed in Table 1 using Primer Premier 5.0. Primers F1 and R1 were the upstream and downstream primers of the first gene (4CL gene), respectively. The Nco-I restriction site, CCATGG, was introduced into F1. The BamH-I restriction site was introduced into R1, and the termination codon (TAG) of the 4CL gene was removed. F21, F22, and R2 were the primers of the RS gene. The BamH-I restriction site was introduced into F22, and the initiation codon (ATG) of the RS gene was eliminated. The EcoR-I restriction site, GAATTC, was introduced into R2. Primers were synthesized and genes were sequenced by Beijing Biomad Co., Ltd.
Construction of the 4CL::RS fusion gene
Gene fragment I, (Nco-I) CCA TGG + 4CL (-TGA) + ggt gga ggc gga tcc (BamH-I), was synthetized using pMD18-T/4CL as template, and F1 and R1 as primers.
The gene was amplified using pMD18-T/RS as a template and F21 and R2 as primers, and then recovered. The gene fragment II, (BamH-I) gga tcc ggc gga ggt ggc tct ggc ggt ggc gga tcg + RS (-ATG) + GAA TTC (EcoR-I), was synthesized with the recovered product as a template, and F22 and R2 as primers.
After recovering gene fragments I and II, enzyme digestion of BamH-I was conducted. The products were recovered using an extraction kit and ligated at 1:1 by the T4 DNA ligase. The ligation efficiency was not high and the ligation product was not pure. Therefore, a PCR amplification experiment was performed using the final product of the ligation as template, and F1 and R2 as primers. The amplified fragment was recovered and ligated into a pMD19-T vector. The final plasmid, pMD19-T/4CL::RS, was transformed into E. coli TOP10. A single colony was selected and inoculated into the fresh medium and cultured for 12 h at 37 °C and 200 rpm, then sequenced.
Construction of pET-30a/4CL::RS
The plasmids extracted from the positive strains and pET-30a(+) were digested by Nco-I and EcoR-I, and then ligated by the T4 DNA ligase. The recombinant plasmid, pET-30a/4CL::RS, was constructed and transfected into E. coli BL21(DE3) cells.
Expression of pET-30a/4CL::RS
Recombinant strains harboring pET-30a/4CL::RS were inoculated into liquid LB broth (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) supplemented with kanamycin sulfate (30 mg/L) at a ratio of 1:50. After growing for 4 h (to an OD600 of approximately 0.5), the cultures were induced by adding 0.3 mM IPTG (Isopropyl β-D-1-thiogalactopyranoside) and incubated for another 8 h at 28 °C. The bacterial cells expressing the desired enzyme were collected and lysed. Then inducible proteins were purified using Ni–NTA affinity chromatography and then detected using SDS-PAGE.
Measurement of 4CL::RS bioactivity
Overnight cultures of recombinant strains with pET-30a/4CL::RS were inoculated at a ratio of 1:100 into 50 mL of LB containing kanamycin sulfate (30 mg/L) in 250 mL flasks at 37 °C and 200 rpm. When the OD600 reached 0.2, 1 mM p-coumaric acid in 200 μL DMSO and 0.3 mM IPTG were added to the cultures, which were then allowed to grow for an additional 48 h at 28 °C and 200 rpm. The blank control group was designed with strains harboring pET-30a and using the same procedure.
Sample preparation prior to HPLC (high performance liquid chromatography) analysis was performed as previously described (Watts et al. 2006). Briefly, 1 mL of the culture was centrifuged at 13,000 rpm for 5 min and the supernatant was transferred into a fresh 1.5 mL tube. Next, 50 μL of 1 M hydrochloric acid was added to the supernatant, and then the mixture was frozen overnight at −20 °C. Subsequently, the mixture solution was thawed out at room temperature and extracted three times with an equal volume (1 mL) of pure ethyl acetate. After removing the solvent with nitrogen gas, the dried residues were dissolved in 1 mL of methanol.
Resveratrol bioconversions were analyzed by HPLC (Waters 2695 separations module) by using a Zorbax SB-C18 column (5 μm particle size, 4.6 × 250 mm, Agilent Technologies, Palo Alto, CA, USA) and a Waters 2996 photodiode array detector (PDA). Resveratrol was eluted with an isocratic mobile phase consisting of 40 % methanol and 60 % water containing 0.1 % trifluoroacetic acid. The injection volume was 10 μL, the flow rate at the mobile phase was 1.0 mL/min, and chromatography was performed at 25 °C. The identification of resveratrol was conducted by comparing the retention time and UV spectrum against known standards. The standard curve of resveratrol was constructed by using six standard solutions (1, 5, 10, 50, 100, and 200 mg/L) (Sigma-Aldrich, Saint Louis, MO, USA) following the same procedure.
Results
Construction of the 4CL::RS fusion gene
PCR products of gene fragments I and II were detected by agarose gel electrophoresis (Fig. 2). Agarose gel electrophoresis showed that gene fragment I was approximately 1700 bp in size. This was similar to the size of the 4CL gene that lacked the termination codon, TGA, and contained the Nco-I restriction site, as well as the 15-bp section linker (containing the BamH-I restriction site). On the other hand, gene fragment II was approximately 1200 bp in size, which was consistent the size of the RS gene lacking the initiation codon, ATG, and containing the other 30-bp section linker (also containing the BamH-I restriction site), and the EcoR-I restriction site. The two gene fragments were recovered, and then ligated overnight after digestion with BamH-I. Finally, the target gene was amplified with the final ligation product used as template, and the F1 and R2 as primers, and then detected by agarose gel electrophoresis (Fig. 2). The obtained fragment was 2913 bp in size, which was similar to that of the theoretical prediction of the 4CL::RS sequence.
Analysis of the 4CL::RS fusion gene
Sequence analysis showed the full length of the 4CL::RS fusion gene was 2895 bp, which included the 4CL gene (1693 bp) without the termination codon, the RS gene (1167 bp) without the initiation codon, and the nucleotide sequence of the flexible linker (45 bp), which were similar to the details of the original design. The sequences of the fusion gene and the encoded amino acids were analyzed using DNAMAN (Fig. 3). Analyses showed that the fusion gene encoded for a 964-amino acid protein of approximately 104.7 kDa in size and a pI of 5.63.
Identification of pET-30a/4CL::RS
Liquid culture bacterial PCR
The single colony was inoculated into 1 mL of liquid LB containing kanamycin sulfate (30 mg/L) and cultured for 12 h at 37 °C and 200 rpm. In the present study, the negative control, pET-30a(+) was used as template, and the positive control, in which the final ligated product was used as template, were designed. The T7 primers were selected for liquid culture bacterial PCR (Table 1). The results were detected using agarose gel electrophoresis (Fig. 4). The amplified fragment of the negative control was 410 bp in size, which indicated that the nucleotide sequences of the forward and reverse primers of pET-30a(+) were 410 bp in size. The amplified fragment from liquid culture bacterial PCR was 3381 bp in size, which was similar to that observed in the positive control. This finding indicated that pET-30a/4CL::RS has been successfully constructed.
Restriction endonuclease digestion
The plasmids were extracted from positive strains that were verified by bacterial PCR, and treated with Nco-I, EcoR-I, and double enzymes. The results were detected by agarose gel electrophoresis (Fig. 5). The recombinant plasmids after single-enzyme digestion were 8303 bp in size, whereas after double digestions were 5408 and 2895 bp in size, respectively. The product of the 5408 bp fragment was consistent with that observed when pET-30a(+) was digested with same enzymes, and the 2895 bp fragment was consistent with the size of the fusion gene fragment. The structure of the prokaryotic expression vector was therefore further verified.
Sequencing
Bacteria that had been identified as positive were sequenced. Sequence analyses showed that the 4CL::RS fusion gene had been successfully ligated to the pET-30a by the Nco-I and EcoR-I restriction sites.
Induced expression of the fusion protein
The 4CL::RS fusion protein was expressed by exogenously added IPTG and detected by SDS-PAGE (Fig. 6). Electrophoresis showed that the molecular weight of the expressed protein was approximately 105 kDa, which was consistent to its predicted molecular weight. The bacterial cells were collected and lysed by using hypothermic ultrasonication. The suspension was then centrifuged at 13,000 rpm for 30 min at 4 °C. The supernatant was purified using Ni–NTA affinity chromatography and detected by SDS-PAGE (Fig. 6). The results showed that fusion protein was purified after nickel affinity chromatography because the fusion protein contained a His-tag.
SDS-PAGE analysis of the fusion protein. 4CL::RS fusion proteins were obtained from E. coli BL21 (DE3) cells with or without Ni–NTA purification, and were analyzed on 8 % SDS-PAGE gels. Broad-range protein molecular weight markers (Biomad Co., Ltd.) were used as protein standard for the determination of molecular weights. Lane M protein marker, Lane 1 total cell proteins of E. coli BL21 (DE3) containing pET-30a/4CL::RS, Lane 2 purified protein
Assay of 4CL::RS bioactivity
HPLC analyses of the product generated from recombinant strains with pET-30a/4CL::RS showed a peak with a retention time of 13 min and a UV spectrum of 305.7 nm, which were identical to that of authentic resveratrol (Fig. 7). Control cultures of E. coli containing pET-30a(+) did not show the same peak (data not shown). In the present study, the standard curve of resveratrol was calculated as follows:
wherein Y = peak area and X = compound concentration.
HPLC analysis of resveratrol produced by recombinant E. coli BL21(DE3) cells with pET-30a/4CL::RS. A shows the chromatogram of authentic resveratrol (Sigma-Aldrich), and B represents the samples produced by recombinant strains expressing 4CL and RS in LB media with p-coumaric acid in a shaking flask culture at 28 °C. The peaks 1 and 3 at 13 min represent resveratrol, which was monitored using a PAD (photodiode array detector) at a wavelength of 305.7 nm (C). Peak 2 at 8 min shows an absorption wavelength of 310.4 nm (D), representing p-coumaric acid
The correlation coefficient (R2) of this regression equation was 0.9998. After 48 h of growth, 80.524 mg/L resveratrol was detected in the culture media of the experimental group with 1 mM p-coumaric acid, which was equivalent to a 35.28 % conversion yield (mol/mol). Taken together, the results indicated that the fusion protein expressed from the recombinant strain possessed the activities of the 4CL and RS enzymes.
Discussion
A fusion gene was designed using a flexible peptide containing 15 neutral amino acids (G, S) that was inserted between the RS gene and the 4CL gene. This flexible peptide is easy to bend and is elastic, and this property may facilitate the correct folding of the two proteins so that their original functions are not affected.
Previous studies on the microbial production of resveratrol showed that the RS gene of A. hypogaea, V. vinifera, Pinus massoniana, and Pinus strobus L. encodes an active proteins in E. coli, and the activity of RS from A. hypogaea and V. vinifera was significantly higher than that observed in the other species (Lim et al. 2011). To increase resveratrol yield, a fusion protein, 4CL::RS, was constructed using 4CL from A. thaliana and RS from V. vinifera, which increased resveratrol production in yeast to up to 5.35 mg/L (32.11 % mol/mol) (Zhang et al. 2006). On the other hand, a recombinant plasmid, pUC-Vvsts-At4cl1, which co-expressed the 4CL gene from A. thaliana and the RS gene from V. vinifera, yielded a final concentration of resveratrol of up to 2340 mg/L, with a 68.35 mol% conversion yield (Lim et al. 2011).
In the present study, the fusion gene was successfully constructed by the BamH-I site and the fusion protein displayed the bioactivities of the both enzymes. This research results were different from the previous report (Watts et al. 2006). No studies on building a 4CL::RS fusion gene using this method have been reported to date. Using our experimental conditionals, our recombinant strain produced 80.524 mg/L of resveratrol within 48 h. The present study provides baseline information that could be utilized in future studies on the optimization of resveratrol fermentation, which in turn could potentially increase the productivity of resveratrol.
References
Arai R, Ueda H, Al Kitayama, Kamiya N, Nagamune T (2001) Design of the linkers which effectively separate domains of a bifonctional fusion protein. Protein Eng 14(8):529–532
Athar M, Back JH, Tang XW, Kim KH, Kopelovich L, Bickers DR, Kim AL (2007) Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmcol 224(3):274–283
Bülow L (1990) Preparation of artificial bifunctional enzymes by gene fusion. Biochem Soc Symp 57:123–133
Bülow L, Mosbach K (1987) Preparation of bifunctional enzyme complexes by fusion of two genes. Ann NY Acad Sci 501:44–49
Burns J, Yokota T, Ashihara H, Lean MEJ, Crozier A (2002) Plant foods and herbal sources resveratrol. J Agric Food Chem 50(11):3337–3340
Chong J, Poutaraud A, Hugueney P (2009) Metabolism and roles of stilbenes in plants. Plant Sci 177(3):143–155
Donnez D, Jeandet P, Clément C, Courot E (2009) Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol 27(12):706–712
Doré S (2005) Unique properties of polyphenol stilbenes in the brain: more than direct antioxidant actions; gene/protein regulatory activity. Neurosignals 14:61–70
Ferrer JL, Austin MB, Stewart CJ, Noel JP (2008) Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem 46(3):356–370
LaVallie ER, McCoy JM (1995) Gene fusion expression systems in Escherichia coli. Curr Opin Biotechnol 6(5):501–506
Li H, Xia N, Förstermann U (2012) Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 26(2):102–110
Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MA (2011) Hight-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol 77(10):3451–3460
Noel JP, Austin MB, Bomati EK (2005) Structure-Function relationships in plant phenylpropanoid biosynthesis. Curr Opin Plant Biol 8(3):249–253
Robinson CR, Sauer RT (1998) Optimizing the stability of single-chain protein by linker length and composition mutagenesis. Proc Natl Acad Sci USA 95(11):5929–5934
Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41:375–383
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3(1):2–20
Wang Y, Chen H, Yu O (2010) Metabolic engineering of resveratrol and other longevity boosting compounds. BioFactors 36(5):394–400
Watts KT, Lee PC, Schmidt-Dannert C (2006) Biosynthesis of plant-specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol 6:22
Winkel BSJ (2004) Metabolic channeling in plants. Annu Rev Plant Biol 55:85–107
Xue F, Gu Z, Feng J (2004) LINKER: a web server to generate peptide sequences with extended conformation. Nucleic Acids Res 32(suppl 2):W562–W565
Zhang Y, Li SZ, Li J, Pan X, Cahoon RE, Jaworski JG, Wang X, Jez JM, Chen F, Yu O (2006) Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and mammalian cells. J Am Chem Soc 128(40):13030–13031
Zhang J, Yun J, Shang Z, Zhang X, Pan B (2009) Design and optimization of a linker for fusion protein construction. Prog Nat Sci 19(10):1197–1200
Acknowledgments
This work was supported by a grant from the State Forestry Administration 948 Project (Program No. 2013-4-07), P. R. China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Erhao Zhang and Xuefeng Guo have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, E., Guo, X., Meng, Z. et al. Construction, expression, and characterization of Arabidopsis thaliana 4CL and Arachis hypogaea RS fusion gene 4CL::RS in Escherichia coli . World J Microbiol Biotechnol 31, 1379–1385 (2015). https://doi.org/10.1007/s11274-015-1889-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-015-1889-z






 was the initiation codon of the 4CL::RS fusion gene. A 15-amino-acid with frame served as the sequences of the linker. CCATGG and GAATTC indicated the Nco-I and EcoR-I restriction sites, respectively
was the initiation codon of the 4CL::RS fusion gene. A 15-amino-acid with frame served as the sequences of the linker. CCATGG and GAATTC indicated the Nco-I and EcoR-I restriction sites, respectively


