Abstract
With the growing microbial resistance to conventional antimicrobial agents, the development of novel and alternative therapeutic strategies are vital. During recent years novel peptide antibiotics with broad spectrum activity against many Gram-positive and Gram-negative bacteria have been developed. In this study, antibacterial activity of CM11 peptide (WKLFKKILKVL-NH2), a short cecropin–melittin hybrid peptide, is evaluated against antibiotic-resistant strains of Klebsiella pneumoniae and Salmonella typhimurium as two important pathogenic bacteria. To appraise the antibacterial activity, minimal inhibitory concentration (MIC), minimal bactericidal concentration (MBC) and bactericidal killing assay were utilized with different concentrations (2–128 mg/L) of peptide. To evaluate cytotoxic effect of peptide, viability of RAJI, Hela, SP2/0, CHO, LNCAP cell lines and primary murine macrophage cells were also investigated with MTT assay in different concentrations (3–24 and 0.5–16 mg/L, respectively). MICs of K. pneumoniae and S. typhimurium isolates were in range of 8–16 and 4–16 mg/L, respectively. In bactericidal killing assay no colonies were observed at 2X MIC for K. pneumoniae and S. typhimurium isolates after 80–90 min, respectively. Despite the fact that CM11 reveals no significant cytotoxicity on RAJI, Hela, SP2/0, and CHO cell lines beneath 6 mg/L at first 24 and 48 h, the viability of LNCAP cells are about 50 % at 3 mg/L, which indicates strong cytotoxicity of the peptide. In addition, macrophage toxicity by MTT assay showed that LD50 of CM11 peptide is 12 μM (16 mg/L) after 48 h while in this concentration after 24 h macrophage viability was about 70 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial peptides (AMPs) are important members of the host defense system in eukaryotes with broad range of functions in inflammation, wound repair, regulation of the adaptive immune system and have a role as endogenous antibiotics (Wiesner and Vilcinskas 2010; Brown and Hancock 2006; Cederlund et al. 2011; Zasloff 2002c). They have a broad ability to kill microorganisms with different mechanisms. The large antimicrobial peptides with more than 100 amino acid, are often lytic enzymes, nutrient-binding proteins or contain sites that target specific microbial macromolecules. While small antimicrobial peptides as a major group of these peptides, act greatly through disrupting the structure or the function of microbial cell membranes or interact with ATP and directly inhibit the action of certain ATP-dependent enzymes (Brogden 2005; Epand and Vogel 1999; Zasloff 2002b; Akhtar et al. 2011). These peptides, demonstrating potent antimicrobial activity, are rapidly mobilized to neutralize a broad range of microbes, including viruses, bacteria, protozoa, and fungi (Zhang and Falla 2006; Butu 2011). The most antibacterial peptides are cationic peptides with 12–50 amino acid and can adopt amphipathic conformations with spatially separated hydrophobic and charged regions (Brogden 2005; Brown and Hancock 2006; Hancock and Patrzykat 2002; Wilcox 2004). These structures have both hydrophobic and hydrophilic regions capable of interacting strongly with different regions in biological membranes. The main advantage of such peptides, over other defensive molecules (i.e. antibiotics), is broad-spectrum activity with rapid onset of killing and low levels of induced resistance compared with conventional antibiotics (Gordon et al. 2005). Moreover, since the AMPs are small, they can be readily modified through substitutions, chain elongation or deletions of amino acids to improve their efficiency in specific host–pathogen interactions (Epand and Vogel 1999; Hancock and Patrzykat 2002; Zasloff 2002b).
To target the cell membrane, the initial adsorption of peptides on negative charge of cell membrane could be primary mechanism for subsequent probable translocation into cytoplasm and interrupting vital cellular processes. They may act also by interfering with external molecular targets on the microbial envelope, such as lipids involved in cell–wall synthesis or autolytic enzymes required for cellular division and remodeling, which would also contribute to cellular damage (Chou et al. 2010; Christensen et al. 1988; Zasloff 2002b).
In recent decades, with growing microbial resistance to conventional antimicrobial agents, unconventional therapeutic options are urgently needed. According to the characteristics of cationic peptides, these molecules are one of the best new alternative antibiotic agents as an innovative response to the increasing problem of multi-drug-resistances, but the size of some peptides and side effects such as toxicity are a major problem (Gordon et al. 2005). To overcome these problems, hybrid and short peptide analogs have been designed (Andreu et al. 1992; Gordon et al. 2005; Hancock and Patrzykat 2002; Hassan et al. 2012; Park et al. 2011; Zasloff 2002a). However, of the many hundreds of these peptides, synthetic hybrids of the insect peptide cecropin and the bee venom peptide melittin are among the most potent and well-studied (Andreu et al. 1992; Cao et al. 2010; Bhargava and Feix 2004).
Our previous studies in this field led to the identification of CM11 peptide as a hybrid peptide derived from N-terminal domain residues of cecropin A (2–8 residues) and hydrophobic C-terminal domain residues of melittin (6–9 residues) with effective antibacterial activity against some of Gram-negative and Gram-positive pathogenic bacteria (Moosazadeh et al. 2012). This peptide is a shorter sequence analog from a primary hybrid peptide, derived from 2 to 8 cecropin A residues and from 6 to 9 residues of melittin which is known as CM15 peptide. (Cavallarin et al. 1998; Montesinos 2007) (Table 1).
Owing to the importance of other pathogenic bacteria in the incidence of antibiotic-resistant infections, in this paper we investigated antibacterial activity CM11 peptide on two important infectious bacteria, Klebsiella pneumoniae and Salmonella typhimurium. Pneumonia is the most common infection caused by Klebsiella bacteria outside the hospital, typically in the form of bronchopneumonia and also bronchitis (Podschun and Ullmann 1998). Klebsiella can also cause infections in the urinary tract, lower biliary tract, and surgical wound sites and is one of the most carbapenem-resistant enterobacteriaceae emerging as an important challenge in health. Carbapenem-resistant K. pneumoniae is resistant to almost all available antimicrobial agents, and infections with this bacteria cause high rates of morbidity and mortality (Munoz-Price et al. 2010; Patel et al. 2008; Andrew 2009). Moreover, Salmonella enteric serovar typhimurium, the etiological agent of typhoid fever and gastroenteritis in humans, is among the most antibiotic resistant bacteria pathogens known. For example, multidrug-resistant S. typhimurium phage type DT104 has emerged during the last decade as a global health problem because is mostly resistant to ampicillin, chloramphenicol, streptomycin, sulfonamides and tetracyclines (Kingsley et al. 2009; Leegaard et al. 2000; Baliga 2007).
The determination of the cytotoxicity of antimicrobial peptides is an essential step to warrant their safe use; therefore, in this study we investigated the in vitro cytotoxicity of CM11 peptide on eukaryotic cells.
Materials and methods
Peptide synthesis
In order to synthesis CM11 peptide, solid-phase synthesis method was utilized on a Rink p-methylbenzhydrylamine resin (Badosa et al. 2007). The peptide was purified with reversed-phase semi preparative HPLC on C18 Tracer column. >95 % purity was obtained. Electrospray ionization mass spectrometry was used to confirm peptide identity.
Bacterial strains
Klebsiella pneumoniae (ATCC 13883) and Salmonella typhimurium (ATCC 14028) strains with 30 and 20 clinical isolates, respectively, were gathered. The isolates were retrieved from clinical diagnostic laboratories (In Khatam-al-Anbia and Shahid Motahari hospitals), which were confirmed with standard microbial laboratory tests.
Selection of isolates with the highest rates of antibiotic resistance
The agar disk diffusion test was applied to investigate the antibiotic resistance on Mueller–Hinton agar using 0.2 mL inoculums (108 cells/mL). Specific antibiotics were selected according to Clinical and Laboratory Standards Institute [CLSI 2010, formerly National Committee for Clinical Laboratory Standards (NCCLS)] guidelines (Table 2).
(NCCLS 2010). Screening of resistant bacteria isolates was accomplished after anti-biogram test. The Antibiotic resistance rates were determined by measuring the diameter of inhibition zones. Accordingly, among the bacteria samples taken from clinical labs we have selected isolates with the highest antibiotic resistance rates.
MIC and MBC determinations
Peptides solubilized in saline phosphate-buffer (pH 7.2) to achieve 1 mg/mL concentration. To measure antibacterial activity of peptide, minimal inhibitory concentration (MIC) was determined using a broth microdilution method with Mueller–Hinton broth and an initial inoculum of 1.5 × 108 CFU/mL according to the procedures outlined by the CLSI (NCCLS 2010). Bacterial cultures with different peptide concentrations from 2 to 128 mg/L were incubated in a shaking bath for 18 h at 37 °C. The lowest peptides concentration that inhibited bacterial growth was considered MIC. The minimal bactericidal concentration (MBC) was taken as the lowest concentration of each drug that resulted in more than 99.9 % reduction in the initial inoculums. Experiments were performed in triplicate.
Bacterial killing assay
Test tubes containing freshly prepared Mueller–Hinton broth supplemented with 0.5X, 1X, and 2X MIC of peptide, were inoculated with 1.5 × 108 CFU/mL of each strain and incubated at 37 °C. Aliquots were sampled after 0, 5, 10, 15, 20, 30, 40, 50, 60–120 min. Samples were diluted and cultured on Mueller–Hinton agar plates for cell counts.
Cell line cultures
Cytotoxicity effect of CM11 peptide was investigated on Hela (Human Cervix Tissue), LNCAP (Human Prostate Tissue), RAJI TK+ (Human Lymphoblast Tissue), SP2/0 (Mouse Spleen Tissue) and CHO (Chinese Hamster Ovary Tissue) cell lines at 3–24 mg/L peptide concentrations (National Cell Bank of Iran). These cells were selected according to some parameters such as suspended or adhesive in media culture and tissue source. Cell lines were cultured in 75 cm2 flasks. Followed by incubation, cells were detached and centrifuged for 5 min at 1,100 g and suspended in fresh medium. Cell concentration was adjusted to 105 cells/mL. 100 μL aliquots of the suspension were diluted in each well of 96-well cell culture plate containing RPMI-1640 medium (Cambrex Bioscience, USA) supplemented with l-glutamine (2 mM, Gibco), penicillin–streptomycin (100 IU/mL penicillin, 100 mg/mL streptomycin) (Gibco, Germany) and heat inactivated fetal bovine serum (Biowest, France) 10 % (v/v). Cultures were incubated at 37 °C in a humidified incubator with 5 % CO2 atmosphere for 24 and 48 h.
Cell line toxicity by MTT assay
To determine the cell viability and the cytotoxic effect of CM11 peptide on selected cell lines, colorimetric MTT (3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide) metabolic activity assay was used (Vaucher et al. 2010). MTT solution (USB Corporation, USA) was added to each well and the plates were incubated for 4 h at 37 °C. MTT solution was removed and DMSO was added to dissolve formazan crystals. Absorbance was read at 540 nm in a 680 microplate reader (Bio-Rad Laboratories, Hercules, USA). The viability percentage was calculated as AT/AC × 100; where AT and AC are absorbance of treated and control cells, respectively.
Isolation of primary murine macrophage cells
We isolated primary macrophage cells from the peritoneal cavity of mouse according to basic protocol 1 described by Zhang et al. (2008).
Macrophage toxicity by MTT assay
After isolation and culturing primary macrophage cells we performed MTT assay according to Heather and Feix protocol (Heather and Feix 2011).
Hemolytic activity assay
The hemolytic activity of peptides was evaluated on human erythrocytes. The 20 % (v/v) solution of erythrocytes in phosphate-buffered saline (PBS) was preincubated for 15 min at 37 °C, and then solution was diluted to 10 % by adding 2–128 mg/L peptide concentration solutions. After additional 15 min at 37 °C, the cells were centrifuged. The absorption of supernatant was measured at 415 nm. The hemolysis percentage was calculated as Apeptide − Amedia/A100 − Amedia × 100; where A100 is the absorbance of erythrocytes suspension with 100 % hemolysis. Complete lysis was obtained by suspending erythrocytes in PBS containing 0.2 % Triton X-100 (Ryadnov et al. 2002). To analyze dose–response relationships in hemolysis, the 50 % hemolytic dose (HD50) was determined following the procedure as previously described for the analysis of antibacterial activity.
Statistical analysis
Statistical analysis was accomplished by SPSS 15.0 (SPSS Inc,Chicago,IL). The data in each figure was a representative of three independent experiments expressed as the mean ± standard deviation (SD). The significance level was determined at p < 0.05.
Results
Selection of isolates with the highest rates of antibiotic resistance
Anti-biogram test was used to evaluate the antibiotic resistance of clinical isolates. The inhibitory effect of antibiotics can be identified by measuring the inhibition zone diameter of each antibiotic disk. Results were analyzed according to CLSI standards and among all K. pneumonia and S. typhimurium samples 20 and 30 isolates were selected with the highest antibiotic resistance pattern (Table 2).
MIC and MBC determination
The CM11 peptide demonstrated an inhibitory range of 4–16 and 8–16 mg/L against antibiotic-resistant isolates of S. typhimurium and K. pneumonia, respectively. For all isolates the MIC90 and MBC90 (minimum concentration needed to inhibit growth and recovery by 90 % of tested isolates) of CM11 peptide were 8 and 16 mg/L, respectively (Table 3).
Bacterial killing assay
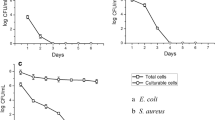
Viable counts of K. pneumoniae and S. typhimurium treated with MIC and MBC of CM11 peptide are shown in Fig. 1a, b. Time-killing curve was used to determine the survival of bacterial strains treated with 0.5X, 1X and 2X MIC of CM11. At 2X MIC, CFUs were decreased during first 5 min and reached a plateau afterward. In bactericidal killing assay no colony was observed in MIC for K. pneumoniae and S. typhimurium isolates after 80 and 90 min, respectively. There was a statistically significant difference between 1X and 2X MIC treated and control groups.
Time-kill determinations for K. pneumonia (a) and S. typhimurium (b) strains, after treatment with 0.5X MIC, MIC and 2X MIC of the CM11 peptide concentrations. Horizontal and vertical-axis show the killing time and the percentage of bacterial survival, respectively. Survival counts were performed three times in different days, and the means and the standard deviations indicates statistically significant difference survival rates (p < 0.05)
Cytotoxicity effect of peptide on cell lines
Cytotoxicity of CM11 was appraised with MTT assay. Dose-dependent results of assay (after 24 and 48 h) are presented (Fig. 2a, b). After early 24 h, the peptide shows approximately little and same influence on the viability of RAJI, SP2/0, Hela, and CHO cell lines (80 % alive at 6 mg/L), while the viability of LNCAP cells are drastically decreased (<50 %). CM11 induced a significant decrease on cell viability for RAJI, CHO (<65 %) and LNCAP (<30 %) at concentration of 12 mg/L. Also at 24 mg/L results indicated a rapid reduction in all cell lines viability (<10 %). Viability pattern after 48 h resembled with 24 h period, except significant reduction of SP2/0 cells.
The cytotoxicity of CM11 peptide on some eukaryotic cell lines (RAJI, SP2/0, Hela, CHO, and LNCAP) using MTT assay. Cells were cultured for 24 (a) and 48 h (b) with the indicated concentrations of peptide. The cytotoxic effect of peptide was determined based on percentage of viable cells in culture after 24 and 48 h by measuring absorption of cell cultures at 540 nm (p < 0.05)
Cytotoxicity effect of peptide on murine macrophage cells
In order to assessment of CM11 peptide toxicity on eukaryotic cells, also we used primary murine macrophages as natural cell model. This peptide showed a LD50 value after 48 h at 16 mg/L peptide concentration (12 μM), while after 48 h at 8 mg/L concentration cell viability was >70 % (Fig. 3).
Hemolysis effect of peptide on erythrocytes
We investigated the hemolytic activity of CM11 peptide on human erythrocytes. Dose-dependent hemolysis results are presented (Fig. 4). The HD50 value (the 50 % hemolytic dose) of peptide was 64 mg/L (45 μM whereas the hemolytic activity of peptide in MIC range was very weak with about 10 % cytotoxicity on blood red cells.
The cytotoxicity of CM11 peptide on erythrocyte cells using hemolysis assay. Dose-dependent hemolysis was assessed with the indicated concentrations of peptide. The hemolysis percentage was determined by measuring of human erythrocyte supernatant absorption at 415 nm in compare with control sample (100 % hemolysis) (p < 0.05)
Discussion
In this study, antimicrobial activity of CM11 hybrid peptide has been determined against clinical antibiotic-resistant strains of K. pneumoniae and S. typhimurium. For the first time, Giacometti et al. (2004) investigated the antibacterial activity of CM15 peptide on clinical isolates of Staphylococcus aureus as a pathogenic bacterium; results showed MICs between 1 and 16 mg/L. In continuance, Rodriguez-Hernandez et al. (2006) and Saugar et al. (2006) investigated the antibacterial activity of this peptide against clinical isolates of Acinetobacter baumannii, their results showed a MIC range between 4 and 64 mg/L. In 2006, Ferre et al. studied the antibacterial activity of CM11 and 22 analogs of this peptide against plant phytopathogenic bacteria: Erwinia amylovora, Xanthomonas vesicatoria and Pseudomonas syringae. He reported 10–14 mg/L as MIC of CM11 for these bacteria (Ferre et al. 2006). Based on these studies, for the first time we evaluated antibacterial activity of CM11 and CM15 peptides against antibiotic-resistance isolates of five pathogenic bacteria including Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae, Acinetobacter baumannii, and Escherichia coli (Moosazadeh et al. 2012). Our studies demonstrated the effective antibacterial activity of these peptides against five pathogenic bacteria with the same ranges of inhibitory values (MIC 8 mg/L and MBC 32 mg/L) in early 24 h but after 48 h the MIC and MBC remained constant for CM11 peptide, while for CM15 peptide these parameters were not stable. Results highlighted bacteriostatic potential of CM11 peptide even after 48 h which specifies a pleasurable stability of it against the hydrolyzing environment of the experiments. This stability seems to be because of structural modifications in sequence compared with CM15. According to these studies, we investigated the antibacterial activity of this peptide against two other important pathogenic bacteria, K. pneumoniae and S. typhimurium. Results showed a similar MICs range against antibiotic-resistant isolates of these bacteria compared with other pathogenic bacteria in our previous study.
Based on our results bacterial killing by peptide was also completed at 8 mg/L concentration after 80 and 90 min for K. pneumoniae and S. typhimurium isolates, respectively, while previous study showed that the bactericidal activity of CM11 peptide are completed after 30 min at 4 mg/L concentration for Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae and Acinetobacter baumannii, but for Acinetobacter baumannii and Escherichia coli time-killing was 40 min. Therefore, in comparison with these bacteria, the bactericidal effect of CM11 peptide on K. pneumoniae and S. typhimurium occurs on a longer time.
According to studies done by Shai and Ferre et al., it seems that CM11 peptide kills bacteria based on a “carpet-like” mechanism (Ferre et al. 2006; Shai 2002). In this mechanism, after carpeting and thinning the bacteria membrane by peptide, at a critical threshold concentration peptide forms toroidal transient holes in the membrane and above this concentration; the membrane disintegrates and forms micelles after disruption of the bilayer curvature (Lee et al. 2008; Madani et al. 2011; Papo and Shai 2005).
Despite the broad spectrum antimicrobial activity of CM11 peptide in vitro, in vivo applications could be limited by peptide cytotoxicity effects on host cellular membranes (Pacor et al. 2002). In general, the cytotoxicity and the damaging effects of AMPs are dependent to the charge, hydrophobicity, amphipathicity, stereochemistry, and propensity of peptides to form barrels. Sensitivity to peptides and differences in viability of eukaryotic cells are also dependent on variations in membrane lipid compositions, hydrophobicity, and the metabolic activity of cells (Pacor et al. 2002; Vaucher et al. 2010). Our peptide cytotoxicity studies showed that after 48 h, at 12 mg/L peptide concentration higher cytotoxicity was for LNCAP and SP2/0 cell lines with fewer than 30 % cell viability, while for other cell lines viability was about 50 %. This differential sensitivity to the peptide is probably related to differences in the amount of acidic phospholipids and sterols in cell membrane composition and the orientation of phospholipids compounds in membrane bilayers. The lower amount of acidic phospholipids and higher sterol compounds in membrane structures reduce the susceptibility of eukaryotic cells to lytic peptides due either to stabilization of the lipid bilayer or to interactions between sterol and the peptide. The low amount of acidic phospholipids also reduces negative charge on membrane cell, leading to a weakening interaction between peptide and cell membrane (Zasloff 2002b). Therefore, the lytic power of peptide depends strongly on phospholipid composition of bilayer, the presence of sterol and charge membrane; although a recent study indicates that the negative surface charge linked to glycosylated membrane proteins also can be effective in sensitivity of eukaryotic cells to cationic AMPs (Sverre et al. 2013).
In this study the 50 % hemolytic dose of peptide was at 64 mg/L (45 μM) but Ferre et al. (2006) reported HD50 at 104 μM that is twofold our result. This difference in results may be due to methodology or the presence of enhancer/inhibitor agents (such as divalent cations) in the experiment conditions (Rudenko and Madanat 2005). Also in MICs range, sensitivity of erythrocyte cells to peptide was low in compare with cell lines that may relate to high cholesterol content in human erythrocyte cell membranes (Yeagle 1985). On the other hand, the CM11 peptide demonstrates little hemolytic activity compared with the CM15 peptide as its ancestor, because Heater and Fiex reported a significant lysis for CM15 peptide with 64 μM concentration causing nearly 80 % RBC lysis. In their study they used RAW264.7 murine macrophages as a eukaryotic cell model for investigation of peptide cytotoxicity. Similar to RBC hemolysis, CM15 significantly decreased macrophage viability with a LD50 of 3.8 μM (Heather and Feix 2011), but our studies showed that for these cells LD50 of CM11 peptide is 12 μM (16 mg/L) after 48 h, while in this concentration after 24 h viability of macrophages was about 70 %.
Previous studies indicated a relationship between helical content in peptide structure and its cytotoxic effect on eukaryotic cell. According to these studies, disruption of secondary structure reduces both antimicrobial activity and eukaryotic cell toxicity, but this reduction is more pronounce for toxicity. Circular dichroism (CD) studies for CM15 and CM11 peptides by Ferre et al. (2006) and Heather and Feix (2011) indicated α-helical contents approximately 85 and 23 %, respectively. Based on these predictions and actual different toxicity effect of CM15 (LD50 of 3.8 μM) (Heather and Feix 2011) and CM11 (LD50 of 12 μM) peptides on macrophage cells, we can be observe this relationship between helical content of CM15 and CM11 peptides and their cytotoxic effect.
This present study demonstrated that short cationic peptide CM11 have significant activity against clinical isolates K. pneumoniae and S. typhimurium in vitro. We hope that these findings will lead to new treatment strategists to eradicate resistance hospital infections. Recently, significant efforts have been also applied to use such peptides as novel compounds against cancerous cells. The higher cytotoxic effect on LNCAP than other cell lines represents that CM11 peptide could be effective on prostate cancer but more studies are needed.
References
Akhtar MS, Nadeem MA, Shahid A (2011) Antimicrobial peptides as infection imaging agents: better than radiolabeled antibiotics. Int J Pept 2012:1–19
Andreu D, Ubach J, Boman A, Wahlin B, Wade D, Merrifield RB, Boman HG (1992) Shortened cecropin A–melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett 296(2):190–194
Andrew MRW (2009) Antimicrobial resistance update: Klebsiella pneumoniae carbapenemases. Drug Benefit Trends 21(8)
Badosa E, Ferre R, Planas M, Feliu L, Besalu E, Cabrefiga J, Bardaji E, Montesinos E (2007) A library of linear undecapeptides with bactericidal activity against phytopathogenic bacteria. Peptides 28(12):2276–2285. doi:10.1016/j.peptides.2007.09.010
Baliga K (2007) Drug resistance in Salmonella typhi: tip of the Iceberg. Online J Health 3(4):1–2
Bhargava K, Feix JB (2004) Membrane binding, structure, and localization of cecropin–mellitin hybrid peptides: a site-directed spin-labeling study. Biophys J 86(1 Pt 1):329–336. doi:10.1016/S0006-3495(04)74108-9
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature Rev Microbiol 3(3):238–250. doi:10.1038/nrmicro1098
Brown KL, Hancock RE (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18(1):24–30. doi:10.1016/j.coi.2005.11.004
Butu MB (2011) Antimicrobial peptides—natural antibiotics. Roman Biotech Lett 16(3):6135–6145
Cao Y, Yu RQ, Liu Y, Zhou HX, Song LL, Qiao DR (2010) Design, recombinant expression, and antibacterial activity of the cecropins–melittin hybrid antimicrobial peptides. Curr Microbiol 61(3):169–175. doi:10.1007/s00284-010-9592-7
Cavallarin L, Andreu D, Segundo BS (1998) Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol Plant Microbe Interact 11(3):218–227. doi:10.1094/Mpmi.1998.11.3.218
Cederlund A, Gudmundsson GH, Agerberth B (2011) Antimicrobial peptides important in innate immunity. FEBS J 278(20):3942–3951. doi:10.1111/j.1742-4658.2011.08302.x
Chou HT, Wen HW, Kuo TY, Lin CC, Chen WJ (2010) Interaction of cationic antimicrobial peptides with phospholipid vesicles and their antibacterial activity. Peptides 31(10):1811–1820. doi:10.1016/j.peptides.2010.06.021
Christensen B, Fink J, Merrifield RB, Mauzerall D (1988) Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA 85(14):5072–5076
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta 1462(1–2):11–28
Ferre R, Badosa E, Feliu L, Planas M, Montesinos E, Bardaji E (2006) Inhibition of plant-pathogenic bacteria by short synthetic cecropin A–melittin hybrid peptides. Appl Environ Microbiol 72(5):3302–3308. doi:10.1128/Aem.72.5.3302-3308.2006
Giacometti A, Cirioni O, Kamysz W, D’Amato G, Silvestri C, Simona Del Prete M, Lukasiak J, Scalise G (2004) In vitro activity and killing effect of the synthetic hybrid cecropin A-melittin peptide CA(1–7)M(2–9)NH(2) on methicillin-resistant nosocomial isolates of Staphylococcus aureus and interactions with clinically used antibiotics. Diagn Microbiol Infect Dis 49(3):197–200
Gordon YJ, Romanowski EG, McDermott AM (2005) A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30(7):505–515
Hancock RE, Patrzykat A (2002) Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr Drug Targets Infect Disord 2(1):79–83
Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2012) Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol 113(4):723–736. doi:10.1111/j.1365-2672.2012.05338.x
Heather MK, Feix JB (2011) Effects of D-lysine substitutions on the activity and selectivity of antimicrobial peptide CM15. Polymers-Basel 2011(3):2088–2106
Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, Marsh K, Achtman M, Molyneux ME, Cormican M, Parkhill J, MacLennan CA, Heyderman RS, Dougan G (2009) Epidemic multiple drug resistant Salmonella typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res 19(12):2279–2287. doi:10.1101/gr.091017.109
Lee MT, Hung WC, Chen FY, Huang HW (2008) Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci USA 105(13):5087–5092. doi:10.1073/pnas.0710625105
Leegaard TM, Caugnat DA, Froholm LO, Hoiby EA, Lassen J (2000) Emerging antibiotic resistance in Salmonella typhimurium in Norway. Epidemiol Infect 125(3):473–480
Madani F, Lindberg S, Langel U, Futaki S, Graslund A (2011) Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys 2011:414729. doi:10.1155/2011/414729
Montesinos E (2007) Antimicrobial peptides and plant disease control. FEMS Microbiol Lett 270(1):1–11. doi:10.1111/j.1574-6968.2007.00683.x
Moosazadeh MM, Abolhasani F, Babavalian H, Mirnejad R, Azizi KB, Amani J (2012) Comparison of in vitro antibacterial activities of two cationic peptides CM15 and CM11 against five pathogenic bacteria: Pseudomonas aeruginosa, Staphylococcus aureus, Vibrio cholerae, Acinetobacter baumannii, and Escherichia coli. Probiotics Antimicrob Proteins 4(2):135–139
Munoz-Price LS, Hayden MK, Lolans K, Won S, Calvert K, Lin M, Stemer A, Weinstein RA (2010) Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol 31(4):341–347. doi:10.1086/651097
National Committee for Clinical Laboratory Standards (NCCLS) (2010) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6
Pacor S, Giangaspero A, Bacac M, Sava G, Tossi A (2002) Analysis of the cytotoxicity of synthetic antimicrobial peptides on mouse leucocytes: implications for systemic use. J Antimicrob Chemother 50(3):339–348
Papo N, Shai Y (2005) Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci 62(7–8):784–790. doi:10.1007/s00018-005-4560-2
Park SC, Park Y, Hahm KS (2011) The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int J Mol Sci 12(9):5971–5992. doi:10.3390/Ijms12095971
Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29(12):1099–1106. doi:10.1086/592412
Podschun R, Ullmann U (1998) Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11(4):589–603
Rodriguez-Hernandez MJ, Saugar J, Docobo-Perez F, de La Torre BG, Pachon-Ibanez ME, Garcia-Curiel A, Fernandez-Cuenca F, Andreu D, Rivas L, Pachon J (2006) Studies on the antimicrobial activity of cecropin A–melittin hybrid peptides in colistin-resistant clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother 58(1):95–100. doi:10.1093/Jac/Dkl145
Rudenko SV, Madanat WKM (2005) Modulatory effect of peptide structure and equilibration conditions of cell on peptide-induced hemolysis. Bull Kharkiv Karazin Natl Univ Ser Biol 709(1–2):139–146
Ryadnov MG, Degtyareva OV, Kashparov IA, Mitin YV (2002) A new synthetic all-D-peptide with high bacterial and low mammalian cytotoxicity. Peptides 23(10):1869–1871
Saugar JM, Rodriguez-Hernandez MJ, de la Torre BG, Pachon-Ibanez ME, Fernandez-Reyes M, Andreu D, Pachon J, Rivas L (2006) Activity of cecropin A–melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50(4):1251–1256. doi:10.1128/Aac.50.4.1251-1256.2006
Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66(4):236–248. doi:10.1002/Bip.10260
Sverre LS, Nissen-Meyer J, Sand O, Haug TM (2013) Plantaricin A, a cationic peptide produced by Lactobacillus plantarum, permeabilizes eukaryotic cell membranes by a mechanism dependent on negative surface charge linked to glycosylated membrane proteins. Biochim Biophys Acta 1828(2):249–259
Vaucher RA, Teixeira ML, Brandelli A (2010) Investigation of the cytotoxicity of antimicrobial peptide P40 on eukaryotic cells. Curr Microbiol 60(1):1–5. doi:10.1007/s00284-009-9490-z
Wiesner J, Vilcinskas A (2010) Antimicrobial peptides: the ancient arm of the human immune system. Virulence 1(5):440–464. doi:10.4161/viru.1.5.12983
Wilcox S (2004) The new antimicrobials: cationic peptides. Bio Teach J 2(1):88–91
Yeagle PL (1985) Cholesterol and the cell membrane. Biochim Biophys Acta 822(3–4):267–287
Zasloff M (2002a) Antimicrobial peptides in health and disease. New Engl J Med 347(15):1199–1200. doi:10.1056/Nejme020106
Zasloff M (2002b) Antimicrobial peptides of multicellular organisms. Nature 415(6870):389–395. doi:10.1038/415389a
Zasloff M (2002c) Innate immunity, antimicrobial peptides, and protection of the oral cavity. Lancet 360(9340):1116–1117. doi:10.1016/S0140-6736(02)11239-6
Zhang L, Falla TJ (2006) Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 7(6):653–663. doi:10.1517/14656566.7.6.653
Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. In: Coligan JE et al (eds) Current protocols in immunology, Chapter 14:Unit 14 11. doi:10.1002/0471142735.im1401s83
Acknowledgments
This study was supported and conducted by Applied Biotechnology Research Center. The authors like to thanks Dr. Ali Mohammad Latifi for his kind and generous assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moghaddam, M.M., Barjini, K.A., Ramandi, M.F. et al. Investigation of the antibacterial activity of a short cationic peptide against multidrug-resistant Klebsiella pneumoniae and Salmonella typhimurium strains and its cytotoxicity on eukaryotic cells. World J Microbiol Biotechnol 30, 1533–1540 (2014). https://doi.org/10.1007/s11274-013-1575-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1575-y