Abstract
The diversity of sponge-associated fungi has been poorly investigated in remote geographical areas like Antarctica. In this study, 101 phenotypically different fungal isolates were obtained from 11 sponge samples collected in King George Island, Antarctica. The analysis of ITS sequences revealed that they belong to the phylum Ascomycota. Sixty-five isolates belong to the genera Geomyces, Penicillium, Epicoccum, Pseudeurotium, Thelebolus, Cladosporium, Aspergillus, Aureobasidium, Phoma, and Trichocladium but 36 isolates could not be identified at genus level. In order to estimate the potential of these isolates as producers of interesting bioactivities, antimicrobial, antitumoral and antioxidant activities of fungal culture extracts were assayed. Around 51 % of the extracts, mainly from the genus Geomyces and non identified relatives, showed antimicrobial activity against some of the bacteria tested. On the other hand, around 42 % of the extracts showed potent antitumoral activity, Geomyces sp. having the best performance. Finally, the potential of the isolated fungi as producers of antioxidant activity seems to be moderate. Our results suggest that fungi associated with Antarctic sponges, particularly Geomyces, would be valuable sources of antimicrobial and antitumoral compounds. To our knowledge, this is the first report describing the biodiversity and the metabolic potential of fungi associated with Antarctic marine sponges.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With more than 70 % of the planet’s surface covered by water, oceans are probably the most promising habitat to explore for novel chemicals with biological properties. However, currently is recognized that just a little fraction of these chemicals from marine sources have been studied and identified. In this context, sponges (phylum Porifera) have been described as a rich source of bioactive compounds with biotechnological interest, such as antimicrobial, antitumoral, and others (Wang 2006; Blunt et al. 2007).
One interesting aspect of sponges is that these organisms maintain symbiotic relationships with microorganisms. Sponges are commonly known to harbor diverse microbes and in fact, they can compose up to 50 % of the sponge tissue volume exceeding microorganisms in seawater by 2–4 orders of magnitude (Hentschel et al. 2006). Many compounds from marine macroorganisms bear structural resemblance to those from microbes (for examples see Moore 1999; Schmidt et al. 2000), suggesting that most of the biological activities from marine sponges could be truly produced by the microbiota associated. This fact has drawn the attention of researchers to the study of biological activities produced by sponges-associated microbes, which has led to the obtainment of several clinically important bioactive compounds from these microbes (Thomas et al. 2010).
The study about the diversity of the sponge-associated microbes is still fragmentary. In contrast to prokaryotic microbes associated with sponges, studies about the diversity of sponge-associated fungi are still scarce (Zhou et al. 2011). Fungi associated with sponges have been described mainly in sponges from temperate shallow-water, tropical, and Mediterranean systems, particularly in the Atlantic Ocean (Baker et al. 2009; Menezes et al. 2010), the Mediterranean Sea (Wiese et al. 2011; Paz et al. 2010), the Indian Ocean (Thirunavukkarasu et al. 2012), the South China Sea (Zhou et al. 2011; Liu et al. 2010; Ding et al. 2011) and the Pacific Ocean (Wang et al. 2008; Gao et al. 2008; Li and Wang 2009). On the contrary, studies about sponge-associated fungi from cold marine environments, such as Antarctic seas, are extremely scarce. In many Antarctic benthic communities, sponges are one of the dominant macroinvertebrate organisms (Avila et al. 2007), and the presence of bacteria, archaea and benthic diatoms has been reported in them (Webster et al. 2004). However, to the best of our knowledge, the presence of fungi associated with Antarctic sponges has remained virtually ignored.
Numerous studies have showed that sponge-associated fungi are one of the major marine sources of bioactive compounds (Bugni and Ireland 2004; Raghukumar 2008). Due to the increasing need of new antibiotic drugs with novel mechanisms of action for which bacteria have not developed still resistances, antimicrobial activity is one of the most searched properties in sponge-associated fungi (Zhou et al. 2011; Baker et al. 2009; Liu et al. 2010; Ding et al. 2011). The results of these assays have shown that fungi isolated from marine sponges are good sources of new antibiotics. On the contrary, other bioactivities such as antitumoral and antioxidant have been less studied in sponge-associated fungi. Compounds with antitumoral activity have been described in few fungi associated with sponges (Thomas et al. 2010; Liu et al. 2010). On the hand, a recent study suggests that sponge-associated fungi could be good producers of antioxidant compounds (Thirunavukkarasu et al. 2012), but as antitumoral, this is a poorly explored activity in these organisms.
Taking in account that diversity of fungi associated with Antarctic marine sponges and their potential as producers of interesting bioactivities have been poorly studied, our aim was to study, for the first time, the diversity of cultivable fungi associated with Antarctic sponges, and to describe the potential of these isolated fungi as producers of antimicrobial, antitumoral and antioxidant activities.
Materials and methods
Sponge collection
Fragments of 10–15 cm of eleven marine sponges belonging to genera Dendrilla sp. (two specimens), Tedania sp. (three specimens), Hymeniacidon sp. (three specimens), and 3 unidentified sponges from the order Poecilosclerida (probably from genera Microciona (Clathria) sp. and/or Crella sp.), were collected using scuba diving in Fildes Bay (62°12′0″S 58°57′51″W), King George Island (Antarctica) at a depth of 6 m. Once excised, sponge specimens were transferred directly to sterilized zip-lock bags containing seawater to prevent contact of sponge tissue with air, and cooled on ice. The samples were transported to the laboratory facilities in Professor Julio Escudero Base located by Fildes Bay and processed immediately for fungal isolation.
Isolation of sponge-associated fungi
Sponges were rinsed with sterile solution 0.9 % NaCl. After that, two parallel procedures for the processing of the sponges were used. First, pieces of approximately 1 cm3 of the inner tissues from each sponge were excised under sterile conditions with a scalpel and forceps, and directly spread onto Petri plates containing different culture media (see below). Alternatively, other samples of the inner tissues were homogenized with a minimal volume of 0.9 % NaCl under aseptic conditions by a high-speed pulse-type homogenizer machine. One hundred microliters of each homogenate were spread onto Petri plates as mentioned above.
Culture media used were potato dextrose agar (PDA, Difco), PDAMM (PDA plus 2 g/l NaCl), GPY (1 g/l glucose, 0.1 g/l yeast extract, 0.5 g/l peptone, 15 g/l agar), and GPYMM (GPY plus 3 g/l NaCl), all of them containing the antibiotics benzylpenicillin and streptomycin (100 μg/ml each) to prevent bacterial growth. The plates were incubated at 20 °C for 1–3 weeks. Each fungal isolate obtained was individually picked and transferred onto a new PDA plates containing antibiotics as above and incubated at 20 °C by 12 days. In order to obtain pure axenic cultures, each fungal colony obtained was submitted to serial dilutions (1:1,000, 1:10,000, and 1:100,000) in 0.9 % NaCl and subsequently, 100 μl of each dilution were plated in duplicate onto PDA plates. Plates were incubated as above, colonies obtained were picked, grown onto a new PDA plates, and submitted to serial dilution again. This procedure was repeated until the morphology of all the colonies of the dilutions was undistinguishable.
Molecular identification and phylogenetic analysis of cultivated fungi
All fungi were grown on the following media: CYA, PDA, NO2, CREA, MEA, and Cz-Dox (Frisvad and Samson 2004). Phenotypical traits (morphology and colour of mycelia and conidia) were examined to exclude redundant fungal isolates.
For the extraction of fungal DNA, the fungal isolates were grown in CYA broth at 20 °C and 200 rev/min for 2–4 days. The mycelium was harvested using Nytal filter, washed with solution 0.9 % NaCl, and dried between two layers of paper towel. The resulting mycelial mat was ground using liquid nitrogen. Fungal DNA was extracted using the procedure described by Navarrete et al. (2009). In brief, the mycelial powder was transferred to a 2 ml tube containing 800 μl of Grinding buffer (0.2 M Tris, 10 mM EDTA, 1 % SDS, pH 8.2) and 800 μl of phenol solution. After a brief mixing, the mixture was heated at 50 °C for 30 min. After centrifugation at 14,000 rev/min for 10 min, the aqueous phase was transferred to a new microtube, extracted twice with an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) and then with chloroform/isoamyl alcohol (24:1). Finally, DNA was precipitated by adding two volumes of ethanol and 1/10 volumes of acetate of sodium 3 M pH 5.2. The DNA pellet was washed with 70 % ethanol and re-suspended in 50 μl of sterile water. Each DNA was used as template to amplify the fungal ITS-rDNA fragments using the primers ITS1 (5′-TCCGTAGGTGAACCTGCG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. 1990). PCR conditions were as follows: initial denaturation (94 °C for 5 min), 30 cycles of denaturation (94 °C for 50 s), primer annealing (55 °C for 50 s), and elongation (72 °C for 1 min), with a final elongation at 72 °C for 1 °C min. PCR products were purified using Wizard SV Gel and PCR Clean-Up System (Promega) and used directly for sequencing analysis (Macrogen Inc., Korea).
The sequences obtained were submitted to the BlastN, matching with GenBank database sequences. Multiple alignments were carried out using ClustalX. Data from ClustalX were exported to the Mega 5 package to build phylogenetic trees using the maximum-parsimony method, using close-neighbor-interchange algorithm (search level = 2, random additions = 100). The quality of the consensus tree was examined by bootstrap re-sampling of the data sets with 1,000 replications.
Antimicrobial, antitumoral, and antioxidant assays of isolated fungi
Fungal isolates were cultured in 500 ml Erlenmeyer flasks containing 100 ml of CYA medium. After incubation for 10–12 days at 20 °C and 200 rev/min, the mycelium was separated from the culture medium by filtration. The fermentation broth was extracted with ethyl acetate (3 × 60 ml). This procedure is widely used to extract compounds from fungal culture broths (Wiese et al. 2011). Then, the filtrate was adjusted to pH 2.0 with 1 N HCl and extracted again with ethyl acetate. Finally, the organic phase was dried under vacuum using a rotary evaporator to give a solid or oily extract, which was used to perform bioassays (see below). Since the aqueous fraction resulting from the extraction with ethyl acetate lacks bioactivities (data not shown), this fraction was discarded.
The antimicrobial assay was carried out by agar diffusion assay. Briefly, paper discs were impregnated with 1 mg of crude extracts dissolved in 10 μl of methanol. The methanol was allowed to evaporate and the discs were placed upon agar plates containing bacterial strains Pseudomonas aeruginosa, Staphylococcus aureus ATCC25922, Clavibacter michiganensis 807 and Xanthomonas campestris 833. The plates were incubated at 37 °C (P. aeruginosa and S. aureus) or at 27 °C (C. michiganensis and X. campestris). After 24 h of incubation, inhibition zones were measured. Negative controls were only treated with methanol; positive controls were treated with benzylpenicillin (Calbiochem) and streptomycin (Sigma-Aldrich).
For antitumoral activity, the procedure describes by McLaughlin and Rogers (1998) was followed. Previous to use, potatoes were sterilized by immersing in 1 % NaOCl for 30 min. After this, potato discs (5 mm × 8 mm) were made with cork borer and placed on agar (2 %) plates (5 discs per plate). On the other hand, Agrobacterium tumefaciens At348 was grown for 48 h in Luria broth medium, and used to prepare each inoculation mix. Each inoculation mix was prepared by mixing 2 ml of Agrobacterium culture, 1.5 ml of water and 0.5 ml of the corresponding test extract solution (2 mg of extract in 0.5 ml of DMSO). Then, the surface of each potato disc was inoculated with 50 μl of each inoculation mix. Control discs were prepared in the same way, except that the test extracts were omitted. Camptothecin served as a positive control of inhibition. Fifteen potato discs were used for each test and for the control. Potato discs were incubated at 28 °C for 21 days. After staining the discs with Lugol solution (10 % KI and 5 % I2) for 30 min, the number of tumors per disc was counted and the percent inhibition for each extract was determined using the following formula:
To estimate the antioxidant capacity of fungal extracts, the 3-(4,5-dimethylthiazole-2-yl)-2,5 diphenyltetrazolium bromide (MTT) method was followed (Liu and Nair 2010). This method has proved to be simple, rapid, inexpensive, sensitive and accurate (Muraina et al. 2009). In brief, extracts were dissolved in DMSO (4 mg/ml) and MTT was dissolved in water (1 mg/ml). An aliquot of 570 μl of MTT solution, and 30 μl of dissolved extract were vortexed in a capped glass vial for 1 min. Then, 600 μl of DMSO were added and the solution was vortexed again. The reaction was incubated at 25 °C overnight, and the absorbance was measure at 570 nm. Each sample was assayed in triplicate. Stock solutions (1 mg/ml) of control compounds (lauric acid as negative control and ascorbic acid as positive control) were also used. The antioxidant capacity was determined by comparing the absorbance of ascorbic acid (ODa) with absorbance of each extract (ODe) according to equation:
Nucleotide sequence accession numbers
Fungal rDNA-ITS sequences obtained in this study were deposited in GenBank under accession numbers JX845279–JX845302.
Results
Phylogenetic diversity of cultivable fungi associated with Antarctic sponges
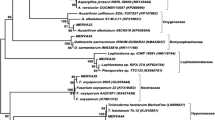
In total, 101 phenotypically different strains were obtained from 11 sponges samples collected in Fildes Bay, Antarctica, which were submitted to DNA extraction and sequence analysis of the ITS1-5.8S-ITS2 region. Table 1 summarizes these results. Interestingly, and although the phenotypic differences among the 101 strains were clearly notorious, only 24 distinctive ITS genotypes were detected (Table 1), indicating that despite the diverse phenotypes observed, several isolates belong to the same fungal species. The sequence analysis revealed that all the fungal isolates belong to the phylum Ascomycota, and they are affiliated to four taxonomic classes (Fig. 1): Leotiomycetes (76 isolates; 12 ITS types), Dothideomycetes (13 isolates; 5 ITS types), Eurotiomycetes (8 isolates; 5 ITS types) and Sordiaromycetes (4 isolates; 2 ITS types).
As shown in Table 1 and the phylogenetic tree (Fig. 1), most of fungal isolates obtained in the class Leotiomycetes belong to genus Geomyces (33 isolates, 32.7 %). Eleven isolates were identified as Geomyces pannorum (Table 1). Interestingly, the other 22 Geomyces isolates could not be identified to species level by comparing their ITS sequences with the databases, and they were closely affiliated (99–100 % identity) with Geomyces sp. (Table 1). Other isolates into the class Leotiomycetes were highly related with an uncultured Pseudeurotium from Himalaya (Gao and Yang 2010) and to Thelebolus sp. from Antarctic soils (Table 1). It is noteworthy that most of the fungi in class Leotiomycetes (33 isolates, 32.7 %) could not be identified to genus level by comparison of their ITS with sequences from database. In this group, collectively named Fungal sp., the ITS sequences were grouped into five ITS types, which were highly related (99–100 %) with unidentified isolates from Antarctic soils (Table 1, Fig. 1). According to their phylogenetic placement (Fig. 1), we think that these isolates probably belong to non identified Geomyces spp. or closer relatives. The formal taxonomy of these isolates will be necessary to confirm this suggestion. Other three non identified isolates are described below.
Class Dothideomycetes includes orders Dothiteales, Tuberculariales, Pleosporales and Capnodiales which are represented by Aureobasidium pullulans (1 isolate, 1 %), Epicoccum sp. (6 isolates, 5.9 %), Phoma herbarum (1 isolate, 1 %) and Cladosporium spp. (5 isolates, 4.9 %), respectively (Fig. 1, Table 1).
Fungal isolates belonging to class Eurotiomycetes were closely affiliated with 2 genera of the order Eurotiales: Penicillium (7 isolates, 6.9 %) and Aspergillus (1 isolate, 1 %). Two isolates were identified as P. polonicum and five were highly related (99–100 % identity) to unidentified Penicillium species from Russia and Antarctica (Table 1). Finally, one isolate was identified as Aspergillus versicolor. The closest relatives of the isolates of Penicillium and Aspergillus obtained by us are strains from cold climates (Table 1).
Finally, four isolates were affiliated to class Sordariomycetes. One of these isolates was highly related (99 % identity) with Trichocladium sp. (order Sordariales) isolated from bat hibernaculum (Table 1). On the other hand, three isolates with identical ITS (ITS type 23) were distantly related to an unidentified isolate from air sample (94 % identity; Table 1). Analyzing the rest of the BLAST report obtained with this ITS, several sequences from Acremonium spp., showing low similarity with ITS type 23, were also observed. Thus, this result suggests that these three isolates belong to the class Sordariomycetes. The phylogenetic placement obtained supports this suggestion (Fig. 1), being these isolates, probably, a non-identified Acremonium species or a closer relative.
Antimicrobial, antitumoral and antioxidant activity of sponge-associated cultivable fungi
From the 101 fungal extracts assayed, 52 extracts (51 %) showed antibacterial activity against, at least, one of the bacteria tested (Table 1). In general, fungal extracts were most active against Gram positive (particularly against Staphylococcus aureus) than Gram negative bacteria (Fig. 2). In particular, 5 isolates (belonging to the genera Geomyces and Epicoccum) exhibited antibacterial activity against P. aeruginosa, 44 isolates (belonging to all the genera found except Aureobasidium, Aspergillus and Phoma) showed antibacterial activity against S. aureus, 11 isolates (belonging to Fungal sp. and genera Geomyces and Penicillium) exhibited antibacterial activity against C. michiganensis, and 22 isolates (belonging to Fungal sp. and genera Geomyces, Penicillium, Epicoccum and Cladosporium) exhibited antibacterial activity against X. campestris (Table 1). Interestingly, several fungal isolates with the same ITS type showed different antimicrobial activity (Table 1).
On the other hand, we found that several of the extracts assayed had potent antitumoral activity. Forty-three isolates (42.6 % of total) showed >50 % of inhibition of the growth of crown gall tumors on potato. Among them, 12 isolates showed >90 %, including three isolates from the genus Geomyces whose extracts totally inhibit growth of crown gall tumors (100 % of inhibition; Table 1). In addition, it is interesting to note that fourteen isolates (13.9 % of total) showed >50 % of stimulation of the growth of crown gall tumors (see negative values in Table 1). In some cases, this stimulation is notorious (>500 % in Thelebolus sp. isolate F09-T14-1; >150 % in Fungal sp. F09-T18-3).
Finally, antioxidant assay showed that from the 101 fungal extracts assayed, four were negative for antioxidant activity. Despite that all the rest showed some antioxidant activity, most of them showed activities ranging from very low to moderate activity (Table 1), and just three isolates showed high antioxidant activities (over 60 % of antioxidant capacity of the ascorbic acid used as control). Two of these isolates, belonging to the genus Epicoccum (F09-T15-3 and F09-T15-6), had high antioxidant capacity (75.9 and 61.2 %). In addition, a Fungal sp. isolate (F09-T18-16) showed 69.4 % of antioxidant capacity.
Discussion
Although sponges are very abundant in Antarctica there is, to our knowledge, only one study about the diversity of microorganisms in Antarctic sponges (Webster et al. 2004). In this study, the detection of non-cultivable eukaryotic microorganisms (including fungi) was attempted using universal eukaryotic primers. The presence of diatoms and dinoflagellates was detected, but not the presence of fungi. Thus, our data of cultivable fungi associated with Antarctic sponges provide, for the first time, a look into the fungal communities living into these Antarctic marine invertebrates.
In our work, 101 fungal isolates phenotypically different were obtained from 11 Antarctic sponges samples. Interestingly, 75.2 % of isolates belong to Leotiomycetes, 12.9 % to Dothideomycetes, 7.9 % to Eurotiomycetes and 4 % to Sordiaromycetes. Compared with the prevalence of fungal classes described in sponges from other latitudes, our results suggest that fungal community of Antarctic sponges is remarkably different. For example, in Mediterranean sponges, Dothideomycetes, Eurotiomycetes and Sordiaromycetes are prevalent (between 29 and 37 % of total isolates), but Leotiomycetes barely represent 1.2 % (Wiese et al. 2011). In South China Sea sponges, Eurotiomycetes (53 %) and Sordiaromycetes (Hypocreales: 38.4 %) are highly prevalent, but Leotiomycetes were not found (Zhou et al. 2011). Finally, in sponges from Brazilian coasts, over 50 % of isolates belong to Sordiaromycetes (mainly genus Trichoderma), being Eurotiomycetes and Dothideomycetes less prevalent (14 and 7 %, respectively). In this case, again, Leotiomycetes were not found (Menezes et al. 2010). Thus, and different to Antarctic marine sponges, Leotiomycetes have been rarely obtained from marine sponges. In addition, some fungal orders commonly found in sponges from other latitudes seem to be underrepresented in Antarctic marine sponges.
Although Geomyces spp. have been mainly described as terrestrial saprophytes that grow at cold temperatures (Blehert et al. 2009), some isolates have been obtained from cold marine environments (Loque et al. 2010; Pivkin et al. 2006). In our case, and except for an isolate closely related to an Arctic strain (Kochkina et al. 2007), all the rest of Geomyces isolates are relatives of Geomyces strains from terrestrial Antarctica (Table 1). Thus, our results suggest that in addition to soil, Antarctic Geomyces would be the most prevalent genus of cultivable fungi associated with Antarctic sponges.
Geomyces is a poorly studied genus, and in fact, the order to which this genus belongs is not clear, being classified as Leotiomycetes incertae sedis (i. s) (Fig. 1). Of the 33 isolated Geomyces, only eleven isolates were identified to species level as Geomyces pannorum (Table 1). G. pannorum has been previously isolated in one sponge from the Sea of Okhotsk, Russia, but its prevalence in this sponge was very low (Pivkin et al. 2006). G. pannorum is widely distributed in soil and non-marine aquatic habitats from terrestrial Antarctica (Bridge and Newsham 2009; Brunati et al. 2009). However, in the Antarctic marine environment, this species has been only found associated to seaweeds (Loque et al. 2010). Our results suggest that G. pannorum could have a broader distribution in the marine Antarctic ecosystem, including marine invertebrates. Interestingly, the other 22 Geomyces isolates could not be identified to species level by comparing their ITS sequences with the databases. To date, the genus Geomyces includes only five known species (Gargas et al. 2009). Thus, our results suggest that the number of species of Geomyces could be greater than heretofore described.
Other isolates into the class Leotiomycetes were highly related with an uncultured Pseudeurotium from Himalaya (Gao and Yang 2010) and to Thelebolus sp. from Antarctic soils (Table 1). As Geomyces, both genera have been commonly reported in terrestrial Antarctica (Bridge et al. 2009). However, to our knowledge, this is the first report of these genera in Antarctic seawater. Interestingly, Thelebolus sp. has been found as the predominant genus in the Antarctic lakes (de Hoog et al. 2005; Brunati et al. 2009). On the other hand, Pseudeurotium has been found only in sponges from the Sea of Okhotsk (Pivkin et al. 2006) while, to our knowledge, this is the first report of Thelebolus sp. in sponges.
The second major group of fungi found (n = 33) includes those not identified isolates, collectively named Fungal sp., which are highly related (99–100 %) with unidentified isolates from Antarctic soils. According to their phylogenetic placement (Fig. 1), these isolates may belong to the class Leotiomycetes. Therefore, Antarctic sponges could be an important reservoir of new fungal species belong to class Leotiomycetes.
The rest of the fungi isolated from Antarctic sponges (25 isolates, 24.7 %) were found with an abundance relative of 7.9 % or less. These fungi belong to the genera Penicillium, Aspergillus, Cladosporium, Epiccocum, Phoma, Aureobasidium, Trichocladium, and Acremonium, which have been found in terrestrial Antarctic environments (Bridge et al. 2009). From these genera, only Penicillium, Aureobasidium, Phoma, Trichocladium and Acremonium have been reported in the Antarctic marine environment (Montemartini Corte et al. 2000; Loque et al. 2010; Grasso et al. 1997; Bridge et al. 2009). Thus, to the best of our knowledge, this work describes for the first time, the presence of Cladosporium, Aspergillus and Epicoccum in the Antarctic marine environment. The closest relatives of Cladosporium, Epiccocum, Phoma and Aureobasidium isolates obtained in this work are strains found in temperate climates (Table 1), which suggest that these isolates could be propagules introduced in the Antarctica. Conversely, the closest relatives of the isolates of Penicillium and Aspergillus obtained by us are strains from cold climates. These genera have frequently been found in soils of Antarctica suggesting that they likely come from terrestrial Antarctica. It is interesting to note that while fungi of order Eurotiales present high diversity in sponges from some other latitudes, including sponges from cold sea from the North Pacific (Zhou et al. 2011; Wiese et al. 2011; Pivkin et al. 2006), its abundance in Antarctic sponges is just 7.9 %.
Finally, three important bioactivities, antimicrobial, antitumoral and antioxidant, were assayed. Antimicrobial assay showed that 51 % of the fungal extracts were positive against, at least, one of the bacteria tested. In other studies where antimicrobial activity from fungi associated with sponges from other latitudes was screened, around 45 % of positive results were obtained (Ding et al. 2011; Zhou et al. 2011). Thus, our results suggest that fungi isolated from Antarctic sponges would be an important potential source of antimicrobial compounds. As was expected, since Leotiomycetes i.s was the most abundant fungal order obtained, its antimicrobial spectrum was more diverse. Consistently, Geomyces was the most prolific fungal genus, with 18 isolates (54.5 % of Geomyces sp.) showing antimicrobial activity (Table 1). Remarkably activity was shown by two isolates of Geomyces (F09-T1-8 and F09-T3-19), which were active against the four bacteria tested, suggesting that this fungal genus could have a high potential as producer of antimicrobial compounds. From the chemical point of view, the genus Geomyces has been poorly studied. To our knowledge, only two compounds with antimicrobial activity from Geomyces strains of terrestrial origin have been described (Li et al. 2008; Parish et al. 2009). Also into the class Leotiomycetes, extracts from the genus Thelebolus have previously shown antimicrobial activity (Brunati et al. 2009), but to our knowledge, this work is the first description of antimicrobial activity in the genus Pseudeurotium. Finally, most of non-identified isolates (named Fungal sp.) also were prolific as producers of antimicrobial activities, which is consistent with the fact that many of them could be Leotiomycetes (see above).
Eurotiales (Penicillium and Aspergillus) are the “classical” producers of antimicrobial activities. Accordingly, all the isolates, excepting for P. solitum and A. versicolor, produced antimicrobial activity. In the case of P. solitum, there are no reports in the literature concerning antibiotic production by this species (Papagianni et al. 2007), which agree with our results. However, the absence of antimicrobial activity in A. versicolor is somewhat surprising, since an A. versicolor strain previously isolated from sponges exhibited antibacterial activity against Gram positive strains, including S. aureus (Lee et al. 2010).
Regarding the rest of fungi, and in agreement with our results, all the Dothideomycetes and Sordariomycetes isolates obtained (Cladosporium, Epicoccum, Phoma, Aureobasidium and Trichocladium) have been previously described as producers of antimicrobial activities by several authors.
To determine the potential of fungi associated with Antarctic marine sponges as producers of antitumoral compounds, we used the crown gall tumor inhibition (potato disc) assay. This is a simple, inexpensive and fast screen for antitumoral activities and it has been validated by several researchers as a first general screen in the search for new antitumor compounds (Coker et al. 2003). We found that several of the extracts assayed had potent antitumoral activity (>50 % of inhibition of the growth of crown gall tumors on potato). To our knowledge, this is the first description of antitumoral activity in Geomyces sp. Thus, in addition to antimicrobial compounds, this fungal genus could have a high potential as producer of antitumoral compounds.
Other good producers of antitumoral activity are A. versicolor, Cladosporium sp., Thelebolus sp., A. pullulans, Trichocladium sp. and Fungal sp. strains (Table 1). Fungi from marine sponges producing antitumoral activities have been described before (Thomas et al. 2010), but most of them belong to other fungal genera than those described in this work. Thus, our results extend the range of fungi associated with sponges capable of producing antitumoral compounds. Interestingly, several fungal extracts stimulate growth of crown gall tumors (see negative values in Table 1). The stimulatory effect of fungal extracts on crown gall tumors has been previously observed (Nano et al. 2002), but to our knowledge, this is the first description of any proliferative activity in sponge-associated fungi. Proliferative compounds have been isolated from fungi (Teles et al. 2005), suggesting the putative presence of compounds with similar activities in sponge-associated fungi.
Finally, the antioxidant potential of the isolates seems to be limited since only thirteen isolates showed moderate antioxidant activity (between 25 and 65 % compared to the antioxidant activity of ascorbic acid). Two isolates from the genus Epicoccum showed good antioxidant activity (75 and 61 % of antioxidant activity of ascorbic acid). It has been described that the antioxidant properties of Epicoccum from marine sponges (and fungi in general) are due to the production of high quantity of extracellular polysaccharides (Sun et al. 2011). Thus, and excepting for these Epicoccum isolates, the potential of the antioxidant activity in fungi associated to Antarctic sponges seems to be moderate. The same conclusion was obtained by Thirunavukkarasu et al. (2012) analyzing fungi from sponges collected in Indian coast.
Taking into account the biosynthetic potential of Geomyces strains isolated in this work, and the poor knowledge of their chemistry, we think that Antarctic sponge-derived Geomyces would be good candidates for deeper studies about the production of natural products.
References
Avila C, Taboada S, Núñez-Pons L (2007) Antarctic marine chemical ecology: what is next? Mar Ecol 29:1–71
Baker PW, Kennedy J, Dobson ADW, Marchesi JR (2009) Phylogenetic diversity and antimicrobial activities of fungi associated with Haliclona simulans isolated from Irish coastal waters. Mar Biotechnol 11:540–547
Blehert DS, Hicks AC, Behr M, Meteyer CU, Berlowski-Zier BM, Buckles EL, Coleman JT, Darling SR, Gargas A, Niver R, Okoniewski JC, Rudd RJ, Stone WB (2009) Bat white-nose syndrome: an emerging fungal pathogen? Science 323:227
Blunt JW, Copp BR, Hu WP, Munro MH, Northcote PT, Prinsep MR (2007) Marine natural products. Nat Prod Rep 24:31–86
Bridge PD, Newsham KK (2009) Soil fungal community composition at Mars Oasis, a southern maritime Antarctic site, assessed by PCR amplification and cloning. Fungal Ecol 2:66–74
Bridge PD, Spooner BM, Roberts PJ (2009). List of non-lichenized fungi from the Antarctic region. www.antarctica.ac.uk/bas_research/data/access/fungi/
Brunati M, Rojas JL, Sponga F, Ciciliato I, Losi D, Göttlich E, de Hoog S, Genilloud O, Marinelli F (2009) Diversity and pharmaceutical screening of fungi from benthic mats of Antarctic lakes. Mar Genomics 2: 43–50
Bugni TS, Ireland CM (2004) Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Nat Prod Rep 21:143–163
Coker PS, Radecke J, Guy C, Camper ND (2003) Potato disc tumor induction assay: a multiple mode of drug action assay. Phytomed 10:133–138
de Hoog GS, Göttlich E, Platas G, Genilloud O, Leotta G, van Brummelen J (2005) Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud Mycol 51:33–76
Ding B, Yin Y, Zhang F, Li Z (2011) Recovery and phylogenetic diversity of culturable fungi associated with marine sponges Clathrina luteoculcitella and Holoxea sp. in the South China Sea. Mar Biotechnol 13:713–721
Frisvad JC, Samson RA (2004) Polyphasic taxonomy of Penicillium subgenus Penicillium: A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud Mycol 49:1–174
Gao Q, Yang ZL (2010) Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the eastern Himalaya. Mycorrhiza 20:281–287
Gao Z, Li B, Zheng C, Wang G (2008) Molecular detection of fungal communities in the Hawaiian marine sponges Suberites zeteki and Mycale armata. Appl Environ Microbiol 74:6091–6101
Gargas A, Trest MT, Christensen M, Volk TJ, Blehert DS (2009) Geomyces destructans sp.nov. associated with bat white-nose syndrome. Mycotaxon 108:147–154
Grasso S, Bruni V, Maio G (1997) Marine fungi in Terra Nova Bay (Ross Sea, Antarctica). New Microbiol 20:371–376
Hentschel U, Usher KM, Taylor MW (2006) Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55:167–177
Kochkina GA, Ivanushkina NE, Akimov VN, Gilichinskiĭ DA, Ozerskaia SM (2007) Halo- and psychrotolerant Geomyces fungi from arctic cryopegs and marine deposits. Mikrobiologiia 76:39–47
Lee Y-M, Li H, Hong J, Cho H-Y, Bae K-S, Kim M-A, Kim D-K, Jung J-H (2010) Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch Pharm Res 33:231–235
Li Q, Wang G (2009) Diversity of fungal isolates from three Hawaiian marine sponges. Microbiol Res 164:233–241
Li Y, Sun B, Liu S, Jiang L, Liu X, Zhang H, Che Y (2008) Bioactive asterric acid derivatives from the Antarctic ascomycete fungus Geomyces sp. J Nat Prod 71:1643–1646
Liu Y, Nair MG (2010) An efficient and economical MTT essay for determining the antioxidant activity of plant natural product extracts and pure compounds. J Nat Prod 73:1193–1195
Liu WC, Li CQ, Zhu P, Yang JL, Cheng KD (2010) Phylogenetic diversity of culturable fungi associated with two marine sponges: Haliclona simulans and Gelliodes carnosa, collected from the Hainan Island coastal waters of the South China Sea. Fungal Div 42:1–15
Loque CP, Medeiros AO, Pellizzari FM, Oliveira EC, Rosa CA, Rosa LH (2010) Fungal community associated with marine macroalgae from Antarctica. Polar Biol 33:641–648
McLaughlin JL, Rogers LL (1998) The use of biological assays to evaluate botanicals. Drug Inform J 32:513–524
Menezes CBA, Bonugli-Santos RC, Miqueletto PB, Passarini MRZ, Silva CHD, Justo MR, Leal RR, Fantinatti-Garboggini F, Oliveira VM, Berlinck RGS, Sette LD (2010) Microbial diversity associated with algae, ascidians and sponges from the north coast of Sao Paulo state, Brazil. Microbiol Res 165:466–482
Montemartini Corte A, Liotta M, Venturi CB, Calegari L (2000) Antibacterial activity of Penicillium spp. strains isolated in extreme environments. Polar Biol 23:294–297
Moore BS (1999) Biosynthesis of marine natural products: microorganisms and macroalgae. Nat Prod Rep 16:653–674
Muraina IA, Suleimanb MM, Eloffb JN (2009) Can MTT be used to quantify the antioxidant activity of plant extracts? Phytomed 16:665–668
Nano GM, Binello A, Bianco M.A, Ugazio G, Burdino S (2002) In vitro tests to evaluate potential biological activity in natural substances. Fitoterapia 73:140–146
Navarrete K, Roa A, Vaca I, Espinosa Y, Navarro C, Chávez R (2009) Molecular characterization of the niaD and pyrG genes from Penicillium camemberti, and their use as transformation markers. Cell Mol Biol Lett 14:692–702
Papagianni M, Ambrosiadis I, Filiousis G (2007) Mould growth on traditional Greek sausages and penicillin production by Penicillium isolates. Meat Sci 76:653–657
Parish CA, de la Cruz M, Smith SK, Zink D, Baxter J, Tucker-Samaras S, Collado J, Platas G, Bills G, Díez MT, Vicente F, Peláez F, Wilson K (2009) Antisense-guided isolation and structure elucidation of pannomycin, a substituted cis-decalin from Geomyces pannorum. J Nat Prod 72:59–62
Paz Z, Komon-Zelazowska M, Druzhinina IS, Aveskamp MM, Shnaiderman A, Aluma Y, Carmeli S, Ilan M, Yarden O (2010) Diversity and potential antifungal properties of fungi associated with a Mediterranean sponge. Fungal Div 42:17–26
Pivkin MV, Aleshko SA, Krasokhin VB, Khudyakova YV (2006) Fungal assemblages associated with sponges of the southern coast of Sakhalin Island. Russian J Mar Biol 32:207–213
Raghukumar C (2008) Marine fungal biotechnology: an ecological perspective. Fungal Div 31:19–35
Schmidt EW, Obraztsova AY, Davidson SK, Faulkner DJ, Haygood MG (2000) Identification of the antifungal peptide-containing symbiont of the marine sponge Theonella swinhoei as a novel δ-proteobacterium, “Candidatus Entotheonella palauensis”. Mar Biol 136:969–977
Sun HH, Mao W-J, Jiao J-Y, Xu J-C, Li H-Y, Chen Y, Qi X-H, Chen Y-L, Xu J, Zhao C-Q, Hou Y-J, Yang Y-P (2011) Structural characterization of extracellular polysaccharides produced by the marine fungus Epicoccum nigrum JJY-40 and their antioxidant activities. Mar Biotechnol 13:1048–1055
Teles HL, Silva GH, Castro-Gamboa I, da Silva Bolzani V, Pereira JO, Costa-Neto CM, Haddad R, Eberlin MN, Young MC, Araújo AR (2005) Benzopyrans from Curvularia sp., an endophytic fungus associated with Ocotea corymbosa (Lauraceae). Phytochem 66:2363–2367
Thirunavukkarasu N, Suryanarayanan TS, Girivasan KP, Venkatachalam A, Geetha V, Ravishankar JP, Doble M (2012) Fungal symbionts of marine sponges from Rameswaram, southern India: species composition and bioactive metabolites. Fungal Div 55:37–46
Thomas TRA, Kavlekar DP, LokaBharathi PA (2010) Marine drugs from sponge-microbe association-a review. Mar Drugs 8:1417–1468
Wang G (2006) Diversity and biotechnological potential of the sponge-associated microbial consortia. J Ind Microbiol Biotechnol 33:545–551
Wang G, Li Q, Zhu P (2008) Phylogenetic diversity of culturable fungi associated with the Hawaiian Sponges Suberites zeteki and Gelliodes fibrosa. Antonie Van Leeuwenhoek 93:163–174
Webster NS, Negri AP, Munro MMHG, Battershill CN (2004) Diverse microbial communities inhabit Antarctic sponges. Environ Microbiol 6:288–300
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and application. Academic Press Inc, San Diego, pp 315–322
Wiese J, Ohlendorf B, Blümel M, Schmaljohann R, Imhoff JF (2011) Phylogenetic identification of fungi isolated from the marine sponge Tethya aurantium and identification of their secondary metabolites. Mar Drugs 9:561–585
Zhou K, Zhang X, Zhang F, Li Z (2011) Phylogenetically diverse cultivable fungal community and polyketide synthase (PKS), non-ribosomal peptide synthase (NRPS) genes associated with the south China Sea sponges. Microb Ecol 62:644–654
Acknowledgments
We thank Antarctic collaborator Carol San Martín, and all the team of Professor Julio Escudero Base in Antarctica for allowing us to use their accommodations and laboratory facilities. We also thank to Mitzy Vega and Patricio Romero for lab assistance. This work was supported by FONDECYT grant 11090192, Instituto Antártico Chileno (INACH) and “Programa Bicentenario de Ciencia y Tecnología” (Chile) project PDA13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henríquez, M., Vergara, K., Norambuena, J. et al. Diversity of cultivable fungi associated with Antarctic marine sponges and screening for their antimicrobial, antitumoral and antioxidant potential. World J Microbiol Biotechnol 30, 65–76 (2014). https://doi.org/10.1007/s11274-013-1418-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1418-x