Abstract
Calcite with laminate structure was successfully prepared by culturing Synechocystis sp. PCC6803 with different concentrations of calcium chloride (CaCl2) in BG11 media. S. PCC6803 was examined by scanning electron microscopy (SEM), transmission electron microscopy (TEM), laser confocal scanning microscope (LCSM) and energy dispersive spectroscopy (EDS). The effects of Ca2+ concentrations and pH values on calcification were investigated and the micro morphs of the CaCO3 crystals were observed by means of SEM. These results showed that CaCO3 crystals could be more easily formed with increasing the concentration of CaCl2 in S. PCC6803 culture solution. S. PCC6803 could largely bind calcium ions, most of which were present in extracellular polymeric substances and on the cell wall. Inside the cells there were a lot of circular areas rich in calcium ions without the crystallization of calcium. Some cells produced a thicker gelatinous sheath outside of the translucent organic thin layer. And the cells inside also produced major changes that the original chloroplasts were almost transformed into starch grains whose sizes were from 0.5 to 1 μm with relatively uniform in sizes. At the same time the cell sizes significantly reduced to only about 8–9 μm almost changing to half of its original diameters. The calcite crystals with a highly preferred orientation induced by S. PCC6803 were observed with X-ray diffraction (XRD). A critical implication was that S. PCC6803 could induce bio-calcification and then mediate the further growth of CaCO3 crystals in the biological system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomineralization is a kind of common phenomenon in nature with the formation or accumulation minerals by organisms especially into biological tissues or structures (as bones, teeth and shells). Typical biominerals are the ingredients of teeth and bones and hydroxy apatite Ca10(PO4)6(OH)2, CaCO3 along with other ingredients can constitute a shell. Biomineralization process means the typical extraction and uptake of chemical elements from the surrounding environments and their integrations into functional structures under the strict controls of biology. Biomineralization is closely related to bone, teeth, calculus, pathology mineralization control. It plays an important role in the design and application of a new type of medical bionic materials, synthesis of artificial bone and dental implant. It also has great effects on archaeology, geology, jewelry and other applications.
Many outstanding scientists like Weiner, Mann and Williams, have conducted their researches to the comprehension of the biomineralization process, such as organic–inorganic interfacial recognition, molecular recognition and molecular geometric matching, etc. They made a great progress of their research theories from microscale to nanoscale (Ameye et al. 2001; Choi and Kim 2000), from structure organization to bio-mimic preparation (Davis 2004), from theoretical analysis to biomimetic synthesis (Hunter 1996; Mann and Ozin 1996), from cell mediation to gene mediation (Hunter 1996), from macroscopic view to microcosmic view (Ogasawara et al. 2000).
The last few decades the mechanism of the mutual effects between polymorphs of calcium carbonates (CaCO3) and given corresponding compound matrices have been further developed (Belcher et al. 1996; Falini et al. 1996; Tong et al. 2004; Mann 1988; Weiner 1983). Elementary analysis conclusions have shown that a small amount of acidic macromolecules such as acidic glycoproteins lie in almost all the inorganic/organic interface of biomineralized substance (tooth, otolith, pearl and mollusk shell, etc.) (Browen and Tang 1996; Marsh 1989). The concentration of these glycoprotein molecules containing lots of acidic amino acids and aspartic acid is about 30–40 % in atomic weight, while the mean concentration of other amino acids is 10 % in atomic weight. Simple biomicromolecules such as aspartic acids and glutamic acids may complete the process of the nucleation and mediate the quick growth of minerals (Falini et al. 1996). The surface formed by multi-carboxyl of the acidic amino acid residues entails the surface of heterogeneous nucleation and to some extent plays an important role in the polymorphs controlling (Tong et al. 2004). Many bacterial species have been shown to produce the precipitation of CaCO3 (Cacchio et al. 2003; Castanier et al. 1999; Fujita et al. 2000; Hammes et al. 2003; Hammes and Verstraete 2002; Milliman et al. 1999). This kind of phenomenon occurs in different environments, for example, travertine deposits in psychrophilic (Sanchez-Moral et al. 2003) and high temperature environments (Pentecost 2003). Microbes can increase surface area for nucleation (Dittrich et al. 2004), and create alkaline environments near the cell surface and increase concentrations of dissolved inorganic carbon through physiological activity of microbes to facilitate these processes (Dittrich et al. 2003).
As primary producers and the final source of atmospheric oxygen, cyanobacteria have played an important role in earth history. The typical cyanobacterium, Synechocystis sp. PCC6803, which is capable of photosynthetic growth, has been used as a model organism to study the oxygenic photosynthesis of higher plants because of its relatively simple genetic systems. It is also an important model organism for the study of biomineralization process in geobiology. However, there is no concrete study about S. PCC6803 involvement in calcium carbonate precipitation and distribution of calcium ion inside and outside of the cells until now.
To our knowledge, there is no information available in literature about the effect of S. PCC6803 on the morphology of CaCO3 (Jansson and Northen 2010; Obst et al. 2009). In present study, S. PCC6803 was used as organic template that induced the nucleation and growth of calcium carbonate. This study was focused on the effect of the cyanobacteria and Ca2+ chelate on the polymorphic character, morphological modification and aggregate state (shape and size) of calcium carbonate in the presence of calcium chloride (CaCl2) with different concentrations. The results could be a better understanding of the principles about governing biomineral formation. In order to obtain the direct evidence that Ca2+ interacted with cyanobacteria, laser confocal scanning microscopy (LCSM) was used to investigate the complex action of Ca2+/cyanobacteria. Transmission electron microscope (TEM) was used to investigate the calcium carbonate crystal existed inside the cyanobacteria cells. The structure of precipitated calcium carbonate was analyzed by a wide angle X-ray diffraction (XRD). TEM and SEM were used to investigate morphologic features of the microscale aggregates of calcium carbonate. The objective of this study is to examine the influences of S. PCC6803 on the crystallization of CaCO3, and then to acquire further understandings of crystal-cyanobacteria interactions and knowledges that could be applied to other bio-systems.
Materials and methods
Cyanobacteria strain and calcium carbonate preparation
S. PCC6803 was cultured in BG11 liquid medium or agar plates (Yeager et al. 2011; Rippka et al. 1979). The strains were grown in 250 ml BG11 medium in 1,000 ml of conical bottle at 28 °C on a shaking table that was illuminated by fluorescent lamps at an intensity of 30 μE (m2/s), 12 h light–12 h dark cycle. Calcium carbonate materials were prepared by the precipitation reaction of BG11 medium with CaCl2 in the presence of S. PCC6803 at room temperature. In a typical synthesis, a solution of CaCl2 was added into an aqueous solution of BG11 medium in 1,000 ml conical flasks. The Ca2+ concentrations of BG11 are 0, 9.8, 100, 200, 300, 400, 500, 600, 700, 800, 900 and 1,000 mg/l, respectively. The above media without S. PCC6803 inoculations were control groups, and they were cultured under the same conditions. At the same time, the effects of pH on calcification were also investigated, herein pH values were 6, 7, 8, 9 and 10, respectively, with 100 mg/l Ca2+ concentration in the media.
Calcium ions concentration analysis
Ca2+ concentration was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES). Each sample was cultured for 7 days, subsequently the amount of Ca2+ was tested every 4 days, and the concentration difference of Ca2+ in the culture was used for calcification.
SEM and LCSM analysis of S. PCC6803
Appropriate amount of microalgae suspension cultured for 14 days was centrifuged at 3,000 rpm for 3 min and then mixed together with phosphate buffer solution containing 2.5 % glutaraldehyde (pH 6.5) for 24 h, then centrifuged and the pellet was washed three times with 0.1 mol/l phosphate buffer (pH 7.4, 0.1 mol/l NaH2PO4 19.0 ml and 0.1 mol/l Na2HPO4 81.0 ml), subsequently was dehydrated gradually with ethanol (50–100 %), followed by natural drying and spraying gold, finally was observed by SEM (JEOL-JSM-6406) at 20 kV and 300 μA. The chemical composition analysis of S. PCC6803 cell micro area was carried out by using energy dispersive spectrometer (EDS).
88.5 μl dimethyl sulfoxide was added to AM ester (Fluo-3-AM, C51H50Cl2N2O23, molecular weight 1,129.85, http://www.bomeike.com) tube, and preserved in dark place. While 25 μl dimethyl sulfoxide was added to F-127 (Pluronic F-127, molecular weight ~12,500) tube, and stored at room temperature. 500 μl S. PCC6803 suspension was centrifuged and washed gently three times with Hank’s balanced salt solution (HBSS, media component: 8 g/l NaCl, 0.4 g/l KCl, 1 g/l glucose, 60 mg/l KH2PO4, 47.5 mg/l Na2HPO4, pH 7.2), then was mixed with 1,000 μl HBSS solution. 6 ml of the as-prepared dimethyl sulfoxide in AM ester tube was taken out after melting at room temperature, and was mixed with 6 ml of that in F-127 tube. 10 ml of the mixture was immediately added to the washed S. PCC6803, and cultured in dark for 30–40 min after mixing uniformly, then the residual dye was washed away with HBSS solution, subsequently 1,000 μl HBSS was added and preserved without light for 15 min, after that S. PCC6803 cells were used for fluorescence measurement with LSCM (Leica TCS-NT, Germany). Finally those were processed with TCS-NT view and photoshop, and fluorescence intensity quantification of Fluo-3/AM was treated by TCS-NT physiology.
TEM analysis
The S. PCC6803 ultrathin slices observation was carried out using a JEOL JEM-2100 TEM at an accelerating voltage of 100 kV. S. PCC6803 cells were cultured in BG11 media for 2 months, of which Ca2+ concentration were 9.8, 100 and 1,000 mg/ml respectively. Then they were fixed with 2 % glutaraldehyde at 4 °C for 2 h, centrifugated at 3,000×g 5 min and then washed the pellet three times with phosphate buffer saline (PBS) solution (everytime 10 min), and subsequently soaked in 4 °C 1 % osmic acid solution for 2 h, then it was carried through a stepwise dehydration process by increasing the concentration of ethanol in the following sequence: 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 % with each time for 10 min. Cells as mentioned above were embedded in epon 618 and ultrathin sections were processed. Ultramicrocuts were mounted on formvar-coated copper grids, electron stained with uranyl acetate–acetate lead and visualized with a TEM. Micro area chemical composition was analyzed with a TEM X-ray EDS.
Mineral composition analysis
The minerals in S. PCC6803 media can be available by natural settlement, or by scraping the conical flask inwall with a spoon, or by centrifugation at 1,000×g 5 min. Then the white minerals at the bottom of centrifugal tube were dried in the oven at 40 °C for one night. The obtained mineral was analyzed by D/Max-RC XRD instrument, at a 2θ angle range of 10°–90°, with a step size of 0.02 and a count time of 8°/min.
Results
Influences of pH values and calcium ion concentrations on calcification
Figure 1a shows that calcium concentrations decreased along with the changes of pH value at the same initial calcium concentration, possessing the similar curves, which proved that pH values had little influence on calcification. Figure 1b indicates that all levels of calcium concentration in the culture medium decreased when pH value was same (pH = 7), which demonstrated that calcium concentration had an effect on the Ca2+ absorption of S. PCC6803. The reduction of the calcium ion could be calculated through the data. The reduction of the control sample (Δt1) was due to natural precipitation, while experiment group (Δt2) was affected by natural precipitation and S. PCC6803 cells activity together. Therefore, the data differences between Δt2 and Δt1 were referred to the reduction of calcium ions influenced by S. PCC6803 (Δt3). That was to say, the calcium ion deposition quantity (Δt3) was the amount of biomineralized utilization.
Figure 1c shows that the amount of calcification reached to a maximum when pH value was 10 and Ca2+ concentration was 100 mg/l, while the others changed little at pH 6, 7, 8 and 9, respectively. Therefore pH values were influenced relatively less by bio-calcification. It maybe originated from kinds of organic acids produced by S. PCC6803 to change the solution pH values. So this conclusion was consistent with the tested culture pH at the later culture stage, of which the final pH values were tending towards stability between 7.6 and 8.0. When pH value maintained 7.6, the amount of biomineralization (Δt3) decreased because of the reduction of the number of calcium ions. This suggested that Ca2+ concentrations had a large effect on S. PCC6803 calcification. The S. PCC6803 inoculation greatly contributed to the consumption of Ca2+ which promoted itself growth as well.
SEM and energy spectrum analysis of S. PCC6803
The morphology features of S. PCC6803 under different CaCl2 concentrations were observed by SEM (Fig. 2a). The results showed that cells grew and contacted closely with each other, which appeared round or oval shape and some were undergoing binary fission and reproduction. Shiny crystals occurred among cells, and there were two calcium peaks by energy spectrum analysis (Fig. 2b), of which one was strong and the other was weak. Shiny minerals were present on some cell walls, whose energy spectrum analysis result were similar to the above with the same two calcium peaks, of which one was obvious and the other was weak as well (Fig. 2c). It suggested that the white shiny minerals contained abundant of calcium. There were no calcium peaks in the image of S. PCC6803 cells cultured without calcium with EDS analysis, which indicated that the shiny minerals were calcium crystals mainly produced by bio-calcification.
From the SEM and EDS analysis results, test data of the same type of S. PCC6803 cells changed with voltage increasing (Table 1). The differences mainly showed that the proportion of magnesium (Mg), Ca, and ferrum (Fe) increased under the voltage of 20 kV obtained from S. PCC6803 cells with EDS analysis, while the proportion of other chemical elements changed littlely. The increase of proportion of Mg was consistent with that of Ca. Fe appeared firstly under high pressure 20 kV which indicated that the energy was strong enough and the laser entered the cell. There were a certain amount of Fe in cells but none in the culture medium. Fe and Mg are essential for cells growth. The increase of Ca was due to calcium ions outside entering the cells. Therefore the percentage of calcium ions inside of the cell could be obtained from the difference of the percentage of Ca under voltage of 10 and 20 kV.
A comparative analysis of sample 1 and sample 2 has been made, which were cultured in different Ca2+ concentration. At the voltage of 10 kV, there was a large amount of calcium ions existed on the cell wall of sample 2 which was cultured in calcium ions solution, while calcium ions were not found in sample 1 which was cultured in the same solution without calcium ions. Fe element analysis result showed that there were no Fe ions tested at 10 kV voltage in both two samples. The increment of Fe ions in sample 2 was nearly the same with that of sample 1 tested at the voltage of 20 kV. Meanwhile calcium element appeared in sample 1 tested at the voltage of 20 kV, and it also increased in sample 2, which was similar to the percentage increment at 10 kV voltage, and the percentage was about 0.38. Comprehensive analysis indicated that the increment was the percentage of calcium inside cells. S. PCC6803 could bind and capture calcium and other mineral ions in the solution in large quantities, of which most were present on the cell wall. The calcium ions inside the cells changed little and were relatively stable, which existed in the cells in the bound and free conditions and played important roles in signal transduction and metabolism processes as messengers or participants.
LCSM test of S. PCC6803
In the experiment, S. PCC6803 cells under different CaCl2 concentrations were stained by Fluo-3/AM. The observed biochemical changes of the cells by using LCSM analysis could be used to determine the location and number of the calcium ions in the cell walls and cell interiors. Since the intrinsic phycobiliprotein fluorescences of S. PCC6803 were stronger, which had the same fluorescence with the calcium calibrated by the probe, and at the same time also emitted strong red signals. So the location and quantity of calcium ions could be judged by comparing the light intensity of green fluorescences emitted by calcium ions (Fig. 3).
Confocal laser scanning microscopy of S. PCC6803. A, B the S. PCC6803 cells cultured with calcium ions; C the S. PCC6803 cells cultured without calcium ions; D the local magnification of B. a the union of green fluorescence of S. PCC6803 cell itself and calcium ion calibrated by Fluo-3/AM; b the red fluorescence of S. PCC6803 cell itself; c the overlap between a and b. (Color figure online)
According to the images, S. PCC6803 in Figure A, B, D emitted green fluorescences, which were strong and mainly located on the cell wall, while cells in Figure C emitted faint green fluorescences under the same test conditions. Deduction of cellular autofluorescence, the rest of the fluorescence signal was strong. The results indicated that the strong fluorescence signal was emitted by calcium ions. The calcium ions added into the culture mainly existed on the cell wall of S. PCC6803 through adsorption and adhesion, which agreed with the SEM analysis.
From Figure A-c and B-c, green fluorescence released from the cell wall could be observed, which was in the state of dot or semi-annular. But there was not this phenomenon in Figure C-c. It was indicated that the green fluorescence was emitted by the dyed calcium ions which were distributed outside of the cell. Subsequently it formed calcium precipitation with some ions, then developed into semi-annular shape, finally grew into annular shape around the cell wall, resulted in the cell death and precipitation. It was mainly because there was a gelatinous layer outside the cell wall of the S. PCC6803 cells. The gelatinous layer possessed strong capability of adsorbing small particles and metal ions. So the layer adsorbed and captured calcium ions and calcium carbonate particles in the solution to form precipitation locally. This conclusion coincided with the SEM and energy spectrum analysis, in which the calcium content of the part was higher.
Figure D-a shows that green fluorescences circled the cells. Comparing faint green fluorescence in Figure C-a with red fluorescence in Figure D-b, after removing the S. PCC6803 autofluorescence, the rest was fluorescence emitted by calcium ions. Calcium ions were distributed within cell wall in the existence of combination and existed for a long time to supply themselves to the cell metabolism at any time. The cell wall was made up of 23.8 % of polysaccharides, which were sticky and carrying plentiful of negative charges, and could provide a lot of functional groups that could bind calcium ions. The calcium ions which were existed in the cell wall formed the organic calcium mainly through combining with the functional groups provided by the polysaccharides.
TEM analysis of S. PCC6803
The microtopography differences of cells inside or outside, S. PCC6803 individuals under different concentrations of calcium ions reflected the different mineralization rate among cells. According to TEM analysis result, it could be seen that outside surface of sample A (Fig. 4a) at a low degree of mineralization was usually smooth, meanwhile, translucent organic thin layer was pervasive inside and outside surface. In addition, there were abundant chloroplasts filled within the whole cell, and almost no or little amyloids existed in the cells. S. PCC6803 cells were comparatively large with diameter of about 15 μm (Fig. 4a). But in the sample B (Fig. 4b) with higher mineralization rate, microtopography outside of cells surface changed greatly. Some cells produced a thick gelatinous sheath outside of the translucent organic thin layer, significantly thicker than that in sample A. And the cells inside also produced major changes, the original chloroplasts were almost transformed into amyloids. It could be observed there were a large amount of amyloids, and whose sizes were from 0.5 to 1 μm, exhibited relatively uniform characteristics. At the same time, the S. PCC6803 individuals were significantly smaller, whose diameters were only about 8–9 μm, almost half of its original diameters (Fig. 4b).
TEM images of S. PCC6803. a S. PCC6803 cultured in 9.8 mg/ml ca2+ BG11 medium for 2 months; b S. PCC6803 cultured in 100 mg/ml Ca2+ BG11 medium for 2 months; c S. PCC6803 cultured in 1,000 mg/ml Ca2+ BG11 medium for 2 months; d a magnified view of micro area, sac marked with an arrowed in c. e Energy spectrum analysis of cell internal bladder cultured at high calcium environment; f selected area electron diffraction of S. PCC6803 internal bladder
Sample C, at a high degree of mineralization whose thick gelatinous sheath of the surface outside became shrunken, was significantly smaller with a diameter of about 4 μm. The volume of sample C (Fig. 4c) reduced 64 fold compared with S. PCC6803 in BG11 medium with 9.8 mg/ml Ca2+. It may be due to external environments with high calcium ion concentrations. High osmotic pressure was easy to cause cell dehydration and atrophy. It also could be found that the cells were filled with amyloids and no chloroplasts were detected. Surprisingly, a bladder similar to the vacuole appeared, which was shown in Figure C by using an arrow, whose diameter was about 0.5 μm. That was the peculiar phenomenon in high calcium ion concentrations, and also a feature different from S. PCC6803 in the low calcium ion concentrations (Fig. 4d).
Vacuoles often existed in cells of plant and animal, contained inorganic salts, amino acids, sugars and a variety of soluble pigments. The vacuole was a place of hoarding metabolism wastes which was deposited in the vacuole in the state of crystals. The elements involved in the vesicle were carbon (C), oxygen (O), Ca, which was proved by the energy spectrum analysis in this region (Fig. 4e). The weight percentage of C, O, Ca was 80.36, 16.08 and 3.56 % respectively, while atomic percentages was 85.95, 12.91 and 1.14 % respectively. Therefore, it was estimated the calcium ions were deposited in the sacs with the existence of metabolic waste crystals inside the cell. The whole vesicle was elected for electron diffraction and no electron diffraction spot appeared (Fig. 4f). Maybe the reason was that calcium ions in the cell were just in the transition stage from amorphous state to crystal state, or the crystal was too small to be observed in these tests.
Surface morphology of minerals aggregated in BG11 medium with different concentrations of calcium ions
In the process of biomineralization, organic molecules were often used to initiate precipitation, influence the continued growth of the precipitate. In BG11 medium calcium ion concentration rose gradually, and was 9.8, 100, 200, 300, 400, 500, 600, 700, 800, 900 and 1,000 mg/l, respectively. After 2 months the brilliantly shining microscale CaCO3 mineral particles were easily isolated from S. PCC6803 solution, and particles even could be seen clearly on the wall of the bottle (Fig. 5b, arrow place). This result proved that biomineralization induced by S. PCC6803 occurred not only in high concentrations of calcium ion environments, but also in low concentrations. But the difference was that mineral particles were obviously generated much more in high Ca2+ concentration environments. So it indicated that high Ca2+ concentration environments could be more favorable to biomineralization. But in contrast under the same conditions there was no brilliantly shining CaCO3 mineral particles on the bottle wall in the control which was BG11 medium with different Ca2+ concentration but without S. PCC6803 (Fig. 5a). There were only yellow nanoscale precipitates at the bottom of bottle and the wall of the bottle was very smooth and transparent (Fig. 5a, c).
Mineral aggregates produced at the same cultivation condition and the image of yellow particles in control observed with TEM. a Control, yellow nanoscale precipitates at the bottom of bottle produced by the medium BG11 with 1,000 mg/l Ca2+ and no S. PCC6803; b brilliantly shining microscale CaCO3 mineral particles on the wall of the bottle produced by the medium BG11 with 1,000 mg/l Ca2+ and S. PCC6803 cells; c the image of yellow particles in control observed with TEM. (Color figure online)
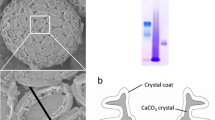
There were great differences of surface morphology between the shining microscale CaCO3 mineral particles induced by S. PCC6803 and yellow nanoscale precipitates at the bottom of bottle by natural mineralization. Paragenesis mineral particles were formed by a plurality of superimposed layers together. In addition there were many concave holes in the mineral particles, whose diameters were generally consistent with S. PCC6803 (Fig. 6a). It was deduced that S. PCC6803 originally adhered to the concave holes, and then cells were detached from the original position during mineral samples drying and SEM processing. And the later research result also confirmed this point. There were visible S. PCC6803 cells in the concave holes of mineral particles. Cells changed into withered ball and deformed by using glutaraldehyde to be fixed (Fig. 6b). Minerals adhered to the bottle wall had neat scaly slice structure and there were indeed no concave holes without S. PCC6803 cells (Fig. 6c). Hence the reasons making the minerals surface morphology obviously different needed to be further studied.
It was well known that bacterial cells were usually surrounded by a matrix of extracellular polymeric substances (EPS). The EPS, produced and secreted by bacteria and other microorganisms, could facilitate to attach and buffer their immediate extracellular environments. There were a wide range of organic molecules had been found in the EPS matrix, and some were structural components, while others were absorbed or localized molecules. The effects making the above two kinds of minerals obviously different were probably relevant to the EPS of S. PCC6803. The detailed mechanism was not very clear and also needed to be further studied.
XRD analysis of minerals produced in the BG11 culture medium with or without inoculation of S. PCC6803 cells
Minerals could be both produced in the cultures medium with or without inoculations of S. PCC6803. But the reasons of mineral production were different. The minerals in the control group precipitated maybe due to sterilization process under high temperature and pressure after adding different concentrations of CaCl2 for preparing culture medium. The filtrate was still turbid after filtration and could be clarified after standing for one night. The precipitation on filter paper was dried under 50 °C, and then further analyzed by XRD. But both the color and the size of the minerals that produced in the culture with inoculation of S. PCC6803 were very different from those of the control. It showed that there were obvious differences between minerals from the cultures with inoculation of S. PCC6803 and the control, according to the XRD analysis results of the minerals (Fig. 7).
According to Fig. 7a, b, it showed that minerals were both CaCO3 crystals with different crystal orientation. Calcium carbonates preferred orientation phenomenon mainly originated from the S. PCC6803 inoculations and their growths. Preferred orientation means that crystal grains are arranged around some special orientations. Preferred orientation growths were attributed to different nucleation mechanisms. According to the results, (012) and (104) of crystal a, the intensity of crystal plane diffraction peak decreased, but in (113(−)), (018) and (1, 2, 10(−)), the result was reverse, which suggested that it occurred preferred orientation of the calcium carbonate crystals inoculated with S. PCC6803. From the SEM analysis results, it was obvious that crystals grew and presented a scalelike structure from the cultures inoculated with S. PCC6803, showing the crystals order in a given direction, which also proved the preferred orientation of the mineral.
Discussion
The calcium elements were mainly enriched in the outside and inside of cell walls in the culture medium. Within the cell wall calcium ions occupied a significant proportion. Polysaccharide, one of the components of the cell wall, was reached up to 23.8 %. Polysaccharides were sticky, rich of negative charges, could provide a large quantity of ion binding sites. So the organic calcium substances were formed by calcium bonding with the provided polysaccharide’s functional groups and existed in the interior of cell wall. The main form of the element calcium outside of the cell wall was inorganic calcium. Because algae changed local water environments through photosynthesis, the carbon dioxide balance system in water changed and produced hydrogen carbonate groups. Hydrogen carbonate groups and calcium ions made reactions and produced calcium carbonate precipitation. The colloid hydration layer around the cell wall outside was polysaccharide which had strong capability of small particles and metal ions adsorption. The calcium ions, the calcium carbonate and calcium sulfate particles etc. could be adsorbed and enriched in the cell wall external.
Inside the cells element calcium was less but stable and the content was approximately 0.38 %, which was in a state of freedom and combination. The free calcium ions with highly biological activity in cells acted as the second messenger, and played regulatory role in the process of cell metabolism, cell division, pinocytosis and exocytosis, etc. The free calcium ions located in protoplasts, thylakoids and endoplasmic reticulum, etc. were in a dynamic balance. Once the balance was lost, it was not to be restored and maybe even cause cell death. So inside the cells calcium ion concentration needed to be kept in a certain range, and ensured the normal growth of cells. If the concentration of calcium ion inside of the cell was too high or too low, it would have a great influence on the life activities of the algae. Only in the cell division or on the spores formation, a large number of calcium ions could be absorbed. According to the present study, the organic calcium of cell wall interior and the inorganic calcium of the surface through the calcium pump or calcium ion channels entered into the cells, participated in the separation activities. After metabolization of cells, organic calcium was decomposed or set of organic matters of the algae were broken down to produce a large number of inorganic carbonic acid (CO3 2−) which were combined with calcium ions absorbed by algae to form calcite crystals in a good shape.
References
Ameye L, De Becker G, Killian C et al (2001) Proteins and saccharides of the sea urchin organic matrix of mineralization: characterization and localization in the spine skeleton. J Struct Biol 134:56–66
Belcher AM, Wu XH, Christensen RJ et al (1996) Control of crystal phase switching and orientation by soluble mollusk-shell proteins. Nature 381:56–58
Browen CE, Tang H (1996) Conchiolin-protein in aragonite shell of mollusks. Comp Biochem Physiol 115A:269–275
Cacchio P, Ercole C, Cappuccio G et al (2003) Calcium carbonate precipitation by bacterial strains isolated from a limestone cave and from a loamy soil. Geomicrobiol J 20:85–95
Castanier S, Le Metayer-Levrel G, Perthuisot J-P (1999) Ca-carbonates precipitation and limestone genesis—the microbiologist point of view. Sediment Geol 126:9–23
Choi CS, Kim YW (2000) A study of the correlation between organic matrices and nanocomposite materials in oyster shell formation. Biomaterials 21:213–222
Davis ME (2004) How life makes hard stuff. Science 305:480–481
Dittrich M, Muller B, Mavrocordatos D et al (2003) Induced calcite precipitation by cyanobacterium Synechococcus. Acta Hydrochim Hydrobiol 31(2):162–169
Dittrich M, Kurz P, Wehrli B (2004) The role of autotrophic picocyanobacteria in calcite precipitation in an oligotrophic lake. Geomicrobiol J 21:45–53
Falini G, Albeck S, Weiner S et al (1996) Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271:67–69
Fujita Y, Ferris FG, Lawson RD et al (2000) Calcium carbonate precipitation by ureolytic subsurface bacteria. Geomicrobiol J 17:305–318
Hammes F, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1:3–7
Hammes F, Boon N, de Villiers J et al (2003) Strain-specific ureolytic microbial calcium carbonate precipitation. Appl Environ Microbiol 69(8):4901–4909
Hunter GK (1996) Interfacial aspects of biomineralization. Curr Opin Solid State Mater 1:430–435
Jansson C, Northen T (2010) Calcifying cyanobacteria—the potential of biomineralization for carbon capture and storage. Curr Opin Biotechnol 21(3):365–371
Mann S (1988) Molecular recognition in biomineralization. Nature 332:119–124
Mann S, Ozin GA (1996) Synthesis of inorganic materials with complex form. Nature 382:313–318
Marsh ME (1989) Self-association of calcium and magnesium complexes of dentin phosphophoryn. Biochemistry 28:339–345
Milliman JD, Troy PJ, Balch WM et al (1999) Biologically mediated dissolution of calcium carbonate above the chemical lysocline? Deep Sea Res 46:1653–1669
Obst M, Dynes JJ, Lawrence JR et al (2009) Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: a STXM study of the influence of EPS on the nucleation process. Geochim Cosmochim Acta 73(4):4180–4198
Ogasawara W, Shenton W, Davis SA et al (2000) Template mineralization of ordered macroporous chitin–silica composites using a cuttlebone-derived organic matrix. Chem Mater 12:2835–2837
Pentecost A (2003) Cyanobacteria associated with hot spring travertines. Can J Earth Sci 40(11):1447–1457
Rippka R, Deruelles J, Waterbury J et al (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Sanchez-Moral S, Canaveras JC, Laiz L et al (2003) Biomediated precipitation of calcium carbonate metastable phases in hypogean environments: a short review. Geomicrobiol J 20(5):491–500
Tong H, Ma W, Wang L et al (2004) Control over the crystal phase, shape, size and aggregation of calcium carbonate via a l-aspartic acid inducing process. Biomaterials 25:3923–3929
Weiner S (1983) Mollusk shell formation: isolation of two organic matrix proteins associated with calcite deposition in the bivalve Mytilus californianus. Biochemistry 22:4139–4145
Yeager CM, Milliken CE, Bagwell CE et al (2011) Evaluation of experimental conditions that influence hydrogen production among heterocystous Cyanobacteria. Int J Hydrogen Energy 36:7487–7499
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (40972043, 41040018, 41210104058, 21176145), Project of Shandong Province Higher Educational Science and Technology Program (J10LC15), Open Funding from the State Key Laboratory of Crop Biology (2010KF06 and 2011KF14), Shandong Agricultural University, Open Project Program of Key Lab of Marine Bioactive Substance and Modern Analytical Technique (MBSMAT-2012-03), State Oceanic Administration, and Open Project Program of the Key Laboratory of Marine Bio-resources Sustainable Utilization (LMB101004), SCSIO, Chinese Academy of Sciences. The authors also express their thank to the standard Synechocystis sp. Stain PCC6803 suppliers, Prof. Xuefeng Lu from Qingdao Institute of Bioenergy and Bioprocess Technology, and Prof. Song Qin from Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Huaxiao Yan and Zuozhen Han contributed equally to this study and they are considered as co-first authors.
Rights and permissions
About this article
Cite this article
Han, Z., Yan, H., Zhou, S. et al. Precipitation of calcite induced by Synechocystis sp. PCC6803. World J Microbiol Biotechnol 29, 1801–1811 (2013). https://doi.org/10.1007/s11274-013-1341-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1341-1